Abstract
Insecticide resistance amongst disease vectors is a growing problem and novel compounds are needed. Biogenic amines are important for neurotransmission and we have recently shown a potential role for these in mosquito fertility. Here, we dissected the relative contribution of different aminergic signalling pathways to biological processes essential for vectorial capacity such as fertility, locomotion and survival by injecting agonists and antagonists and showed that octopaminergic/tyraminergic signalling is essential for oviposition and hatching rate. We show that egg melanisation is regulated by adrenergic signalling, whose disruption causes premature melanisation specifically through the action of tyramine. In addition to this, co-injection of tyramine with DOPA, the precursor of melanin, had a strong cumulative negative effect on mosquito locomotion and survival. Dopaminergic and serotonergic antagonists such as amitriptyline and citalopram recapitulate this effect. Together these results reveal potential new target sites for the development of future mosquito sterilants and insecticides.
As insecticide resistance is spreading in many insect vectors of disease new agents with novel modes of action are needed. This is particularly the case for the principle malaria vector Anopheles gambiae where of the 4 classes of insecticides currently used i.e. organochlorines1, pyrethroids2, organophosphates3 and carbamates3, resistance has been documented for each. These insecticides act either through prolonged opening of sodium channels or inhibition of acetylcholinesterase leading to neuronal overstimulation and death of the vector.
G-protein-coupled receptors (GPCRs) comprise a large family of membrane-bound receptors found in vertebrates and invertebrates which regulate different cell signalling pathways4,5. In insects they regulate major biological processes such as reproduction, development, locomotion as well as feeding6 and thus provide novel targets for insecticide discovery which have not yet been exploited.
One important class of messengers that primarily bind to GPCRs are biogenic amines. These can act as neurotransmitters, neuromodulators and neurohormones7. We were interested in these compounds because recently we showed in An. gambiae a drastic reduction in female fertility by knocking down a key enzyme responsible for tyrosine and subsequent biogenic amine synthesis (dopamine, tyramine and octopamine)8. Life traits such as oogenesis, flight behaviour and longevity are important determinants in the capacity of a mosquito vector to transmit disease. With the goal of dissecting the aminergic pathways responsible for these behaviours we focused on testing a series of biogenic amines and drugs which are known to bind dopaminergic, serotonergic and adrenergic GPCRs in humans but whose function in mosquitoes is unknown.
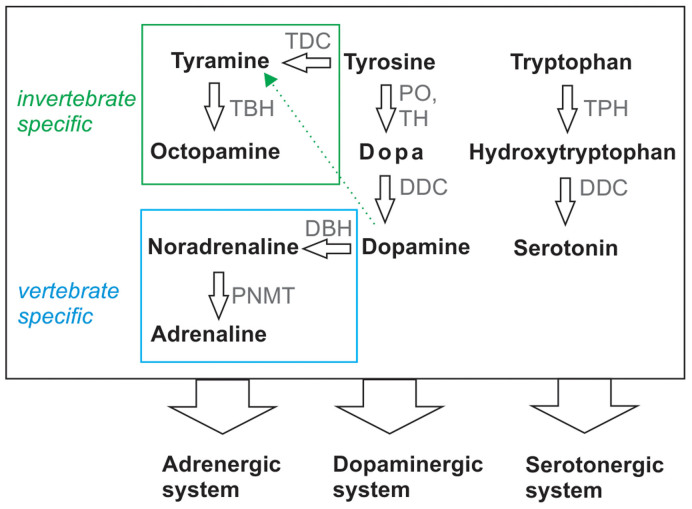
Biogenic amines consist of 5 members that can be found in vertebrates and invertebrates9. They are synthesised from 3 amino acids (tyrosine, tryptophan or histidine) via multiple enzymatic steps. While humans and insects both share dopamine, serotonin (5HT, 5-Hydroxytryptamine) and histamine, some amines are preferentially synthesised in vertebrates or invertebrates (Figure 1). Indeed, the neurotransmitterstyramine and octopamine that are widely distributed and highly abundant in insects10 are present only as traces in the mammalian nervious system compared to other biogenic amines such as adrenaline and noradrenaline11. In adrenergic signalling in insects they are regarded as the functional counterparts of noradrenaline and adrenaline in humans12. Targeting the insect specific tyramine- gated chloride channels13 or octopaminergic and tyraminergic adrenergic-like receptors might represent a window for the development of novel insecticides with minimal off-target effects on non-arthropods.
Figure 1. Biosynthesis pathway of biogenic amines in invertebrates and vertebrates.
DBH- dopamine β- hydroxylase, DDC- Dopa decarboxylase, PNMT- phenyl ethanolamine-N-methyltransferase, PO- phenoloxidase, TDC- tyrosine decarboxylase, TBH- tyramine beta hydroxylase, TPH- tryptophan hydroxylase. The green and blue squares mark the metabolic reactions specific for either invertebrates or vertebrates respectively. The dotted arrow represents a salvage pathway which might exist12. Its physiological relevance is unknown.
The octopamine receptor agonist amitraz has been successfully used as pesticide against ticks14,15,16 as well as parasitic mites17, but its application to mosquitoes has not been explored. Moreover, the potential of tyramine, the metabolic precursor of octopamine, has been largely neglected. However, increases in tyramine levels either by injection of tyramine or knockdown of tyramine beta hydroxylase (TBH), the enzyme that converts tyramine to octopamine, have also been shown to inhibit insect oviposition18,19. Further, administration of tyramine causes opposing effects to octopamine in insect locomotion20,21. Similar to tyramine and octopamine, dopamine and serotonin (5-HT) are important for egg laying and locomotor behaviour22,23,24,25. However, in contrast to the oviposition effect seen in TBH mutants, insects with mutations in dopa decarboxylase (DDC) essential in the synthesis of serotonin and dopamine, die as embryos26 highlighting the differing importance of these amines for separate aspects of insect body function. Recently amitriptyline, a serotonin antagonist has been identified as selective dopamine 2 receptor (Dop2) antagonist in the dengue and yellow fever mosquito Aedes aegypti causing high larval mortality27. Since fertility, locomotion and survival represent important factors that determine the ability of the mosquito to transmit disease-causing parasites, negatively affecting these life history traits through interfering with biogenic amine synthesis or function, could represent a new tool for control of vector-borne diseases. We set out to investigate this possibility through a series of experiments testing a range of different aminergic receptor agonists and antagonists for their effects on the mosquito.
Results
Evolutionary relationships between selected human aminergic receptors and insect biogenic amine receptors
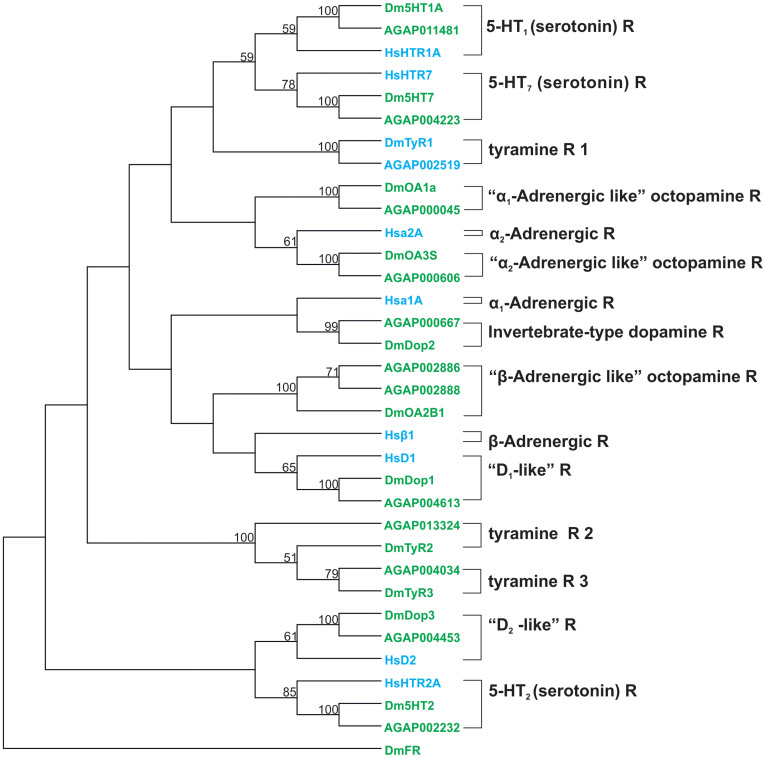
On the assumption that the level of evolutionary relatedness of different GPCRs should positively correlate with conservation of function and ligand partner, we performed a phylogenetic analysis of the different human, fruit fly and malaria mosquito GPCRs (Figure 2). As expected the aminergic GPCRs of two dipteran GPCRs were closer related to each other than to the human GPCRs and formed clusters that have been classified previously28,29,30. Our bootstrapping analysis further supported clades formed by the insect dopaminergic receptors (Dop1, Dop3), serotonergic receptors (5HT1,5,7) and α2 adrenergic-like octopamine receptors (OA3) with the respective human D1, D2, 5-HT and α2 adrenergic receptors. This was in concordance with pharmacological studies investigating GPCRs in insects which highlight potential functional similarities between human and insect GPCRs (Table 1). This close relationship between human and insect receptors could potentially limit the insecticidal application of molecules that target these GPCRs. Interestingly, none of the tyraminergic receptors (Tyr1-3) clustered together with the respective human adrenergic receptors, therefore molecules targeting those receptors might be more suitable to generate insect specific insecticides. Nonetheless, there are examples of clear differences in pharmacological responses of drugs against phylogenetically related receptors and even between different insect genera27. For example tick and Aedes mosquito D1 like receptors can be inhibited by Sch2339027 while no effect is observed on the fruitfly31 or honey bee Dop1 receptor32. This highlights on one hand the opportunity to potentially generate mosquito specific agents but also the need to test empirically each compound on its merit for the species of interest.
Figure 2. Phylogenetic analysis of selected human, fly and mosquito biogenic amine receptors.
Protein sequences of each receptor (“R”) were first aligned in Muscle47 and then a Maximum-Likelihood tree was constructed in MEGA 6 using 1000-fold bootstrap re-sampling. All insect receptors are shown in green, while the human receptors are highlighted in blue. The numbers at the nodes of the branches represent the level of bootstrap support for each branch. The D.melanogaster FMRF amide receptor (DmFR, AAF47700.1) was used as outgroup. The accession numbers for each receptor are listed in Supplementary Table S1.
Table 1. Adrenergic, dopaminergic and serotonergic action of compounds applied in humans and insects.
| compound | function in humans | function in insects | Reference |
|---|---|---|---|
| Tyrosine | Precursor of DOPA, dopamine, adrenaline and noradrenaline | Precursor of DOPA, dopamine, tyramine and octopamine | [48] |
| L-DOPA | D(1-4) dopamine receptor agonist, dopamine precursor | Dopamine and DOPA melanin precursor, | [48,49,50] |
| Dopamine | D(1-4) dopamine receptor agonist, dopamine transport inducer, dopamine beta hydroxylase ligand, | dopamine melanin precursor, Dop1-3 receptor agonist, Oct α2R (OA3) agonist, TyR1 and TyR3 agonist | [27,29,30,38,51,52] |
| Carbidopa | Aromatic amino acid decarboxylase (AADC) inhibitor | DDC inhibitor | [53,54] |
| Tyramine | Trace amine associated receptor (TAAR) agonist, β-adrenergic receptor 1 and 2 (ADBR1,2) antagonist | Oct α2R (OA3) agonist, TyR1-3 agonist, Dop3 (D2 like) agonist | [29,30,38,45,55,56,57] |
| Octopamine | TAAR agonist, ADRB2-antagonist, ADRB1,3-agonist | Oct α2R (OA3) agonist, TyR 1 agonist, OctβR (OA2) agonist, TyR3 agonist | [29,30,38,57,58,59] |
| Clonidine | α2 adrenergic agonist | TyR > OctR agonist, Oct α2R (OA3) agonist, OctR 1 (OA1) agonist | [30,60,61] |
| Yohimbine | α2, 5-HT(1B), 5-HT(1D), and D(2) receptor antagonist, 5HT (1A) agonist | TyR 1 antagonist, 5-HT1,2,7 antagonist, OctR1 (OA1) antagonist, TyR>OctR antagonist | [28,30,45,61,62,63,64] |
| Prazosin | α1 antagonist | TyR1 (Oct/Tyr) antagonist, 5-HT1,7 antagonist, OctR antagonist | [58,61,65,66] |
| Phenylephrine | α1 agonist | TyR agonist, Oct R agonist | [67,68] |
| Clenbuterol | β2 agonist | ND | [69] |
| Dobutamine | β1 and α1 agonist | ND | [70] |
| Acebutolol | β1 antagonist | ND | [71] |
| Sotalol | Non-selective β- blocker | ND | [72] |
| Amitriptyline | Noradrenaline and serotonin transport (SERT) inhibitor, 5HT-2A receptor antagonist, TrkA and TrkB receptor agonist | Dop2 antagonist | [27,73,74,75,76,77] |
| Sch-39166 (Ecopipam) | D1 receptor antagonist | ND | [78] |
| SKF-38393 | D1 receptor agonist | Dopamine 1 receptor (Dop1) agonist | [27,79] |
| Domperidone | D2,D3 receptor antagonist | Dop3 (D2 like) antagonist | [80,81] |
| Bromocriptine | D2,D3, 5-HT receptor agonist, α2-adrenergic, D1 receptor antagonist, inactivates dopamine D4 and 5-HT7 receptors | Dop3 (D2 like) agonist | [55,82,83] |
| Serotonin (5HT) | 5-HT agonist | 5HT1,2,7 agonist, Dop3 (D2 like) agonist | [55,61,63,66] |
| 5-Methyl-N,N-dimethyltryptamine (5-MeO-DMT) | 5-HT agonist | 5-HT2 agonist | [63,84] |
| Ketanserin | 5-HT antagonist | 5-HT2 antagonist | [63,85] |
| Citalopram | SERT inhibitor | ND | [86] |
ND-not determined.
Perturbation of the adrenergic system and dopamine availability inhibits female fertility
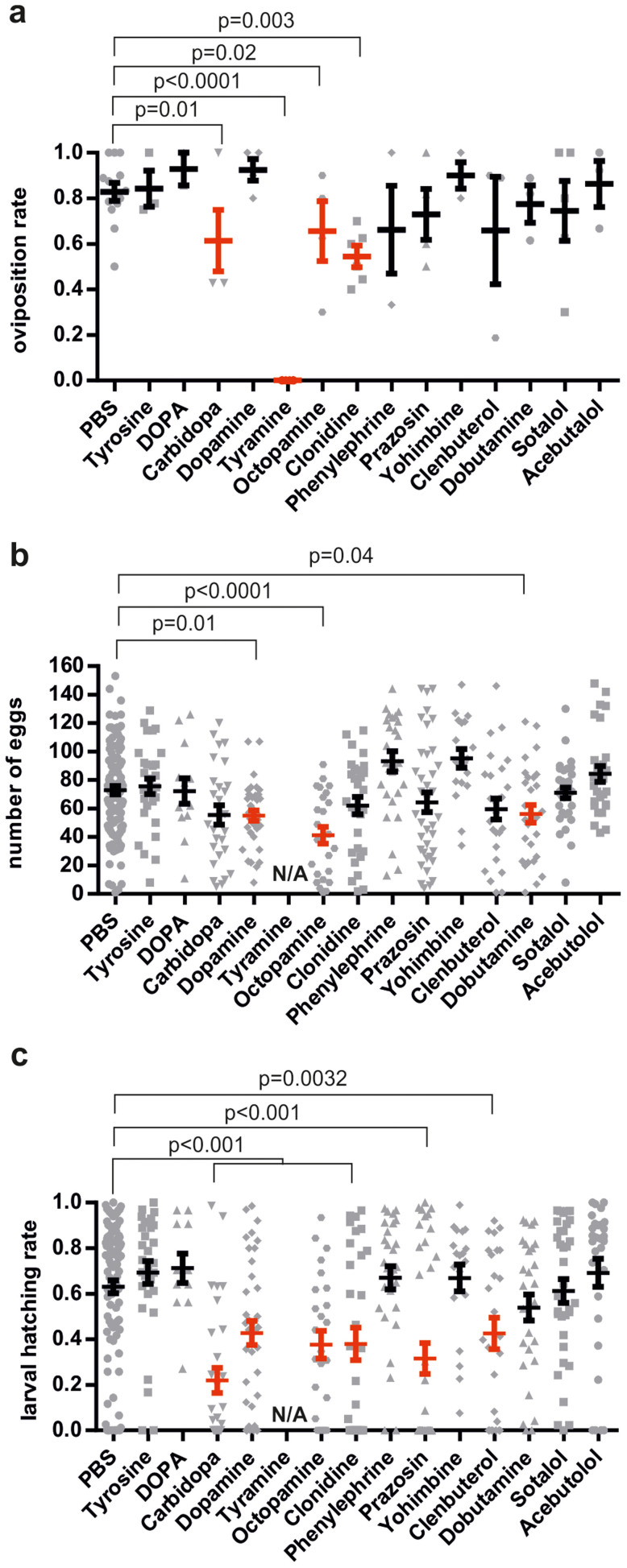
Our recent results showed that phenylalanine hydroxylase activity, which catalyzes the first step of phenylalanine metabolism by converting phenylalanine into tyrosine, is needed for oviposition and egg formation in An. gambiae mosquitoes8. Tyrosine is an essential precursor in the formation of insect neurotransmitters tyramine, octopamine and dopamine. In order to dissect the potential involvement of these biogenic amines in female mosquito fertility, we injected females with different agonists and antagonists of the adrenergic system and compounds involved in dopamine synthesis. The strongest effect on egg laying ability was observed by injection of tyramine, which resulted in complete inhibition of oviposition (Figure 3A). Oviposition rate was also reduced by 16% by the tyramine-derived neurotransmitter octopamine and by 28% by the α2-adrenergic agonist clonidine, compared to the PBS-injected control. We observed that relatively few compounds (octopamine, dobutamine and dopamine) had an effect on the number of eggs laid, possibly due to the fact that injection of compounds coincided with the latter stages of oogenesis, when oocytes would be expected to be fully formed (Figure 3B). However, many compounds (the α2-adrenergic agonist clonidine, α1-antagonist prazosin, the β2-agonist clenbuterol and the DDC inhibitor carbidopa) that had no effect on clutch size had a negative impact on embryo viability as measured by larval hatching rate (Figure 3C). Interestingly this effect seemed to be due to either the specific activation or inactivation of each of the different adrenergic receptors and the injection of compounds with putative opposing function on α2-receptors (yohimbine, antagonist), α1 receptors (phenylephrine, agonist) and β-receptors (acebutolol, sotalol, both antagonists) had no effect. Reduction in embryo viability by carbidopa on the other hand could have been due to the fact that it targets dopa decarboxylase (DDC) which has a likely role in the formation of the embryo serosa, a protective membrane that can enclose the entire embryo33.
Figure 3. Female fertility after injection of adrenergic and dopamine-related compounds.
(a) Mean ± standard error of the mean (SEM) proportion of females that oviposited (N = 10 per experiment). The Fisher's exact Test was used to determine the Likelihood of oviposition of the PBS control vs. compound from a minimum of 3 experiments, red error bars indicate p<0.05. (b) Mean ± SEM number of eggs per ovipositing female (Mann Whitney test, in red p<0.05), N/A not applicable. (c) Mean ± SEM hatching rate of eggs laid per female (Student's t-test, in red p<0.05).
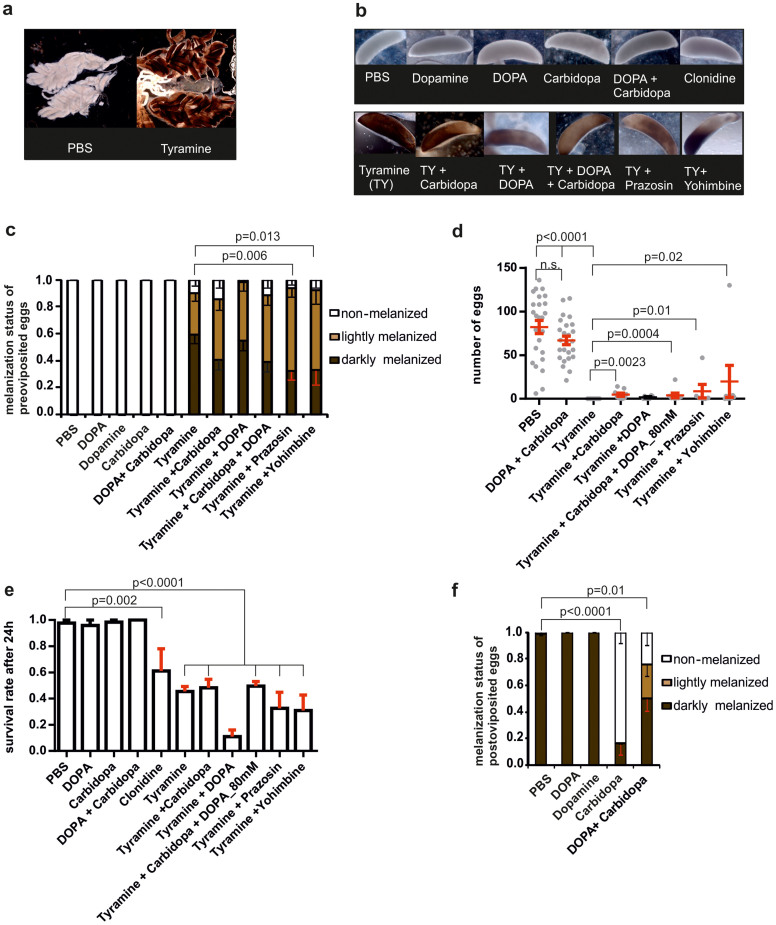
Tyramine injection induced a preoviposition egg melanisation phenotype which can be modulated via α-adrenergic inhibition
Since all tyramine-injected females failed to lay eggs, we dissected their ovaries to examine egg development. Most eggs retained by females appeared to be melanised (Figure 4A). This tyramine-mediated premature melanisation phenotype was very unusual, as An. gambiae eggs ordinarily melanise only after oviposition. It is possible that the reduced oviposition phenotype in tyramine-injected females was directly due to premature melanisation of eggs and concomitant chorion hardening that prevented their physical release from the ovary rather than a behavioural effect on oviposition stimulus. Interestingly, when we injected DOPA or dopamine, known precursors of melanin34 or any other adrenergic compounds, we did not observe this premature melanisation phenotype, suggesting a tyraminergic regulatory mechanism for this process (Figure 4B). To investigate this further we tried to rescue this melanisation effect in two ways: 1) by inhibition of melanin synthesis through injection of carbidopa that inhibits the dopa decarboxylase (DDC) essential for dopamine melanin and sclerotin synthesis; 2) antagonising injected tyramine activity with its antagonists prazosin or yohimbine. Fewer eggs were fully melanised in the presence of prazosin or yohimbine, suggesting a tyraminergic pathway is responsible for this effect (Figure 4C). We observed only a very low proportion of females was able to lay eggs following injection with any of the above compound combinations (Figure 4D) but we cannot exclude the possibility that general toxicity of these combinations contributed to low egg number and oviposition rate (Figure 4E). Carbidopa did not reduce tyramine-induced pre-oviposited egg melanisation but it did inhibit melanisation post-oviposition and this was rescued by the addition of DOPA, suggesting that at that stage melanisation depends highly on dopamine availability (Figure 4F).
Figure 4. Premature egg melanisation phenotype mediated by tyramine.
(a) Ovary dissection of PBS (control) and tyramine-injected females 3 days post-bloodmeal. (b) Representative examples of eggs dissected from female ovaries ~24 h after aminergic compound injection. (c) Mean ± SEM melanisation ratio of egg batches dissected from ovaries of 31–35 injected females from 3 repeats. (d) Mean ± SEM number of eggs laid by females following injection of tyramine alone or in combination with other compounds (N = 10 per experiment, minimum of 3 experiments, Student's t-test, in red p<0.05). (e) Proportion of females that survived 24 h post-injection with compounds (N = 10, minimum of 3 repeats, Student's t-test, in red p< 0.05). (f) Mean ± SEM melanisation ratio of egg batches laid by injected females (N = 10 per experiment, 3 experiments, Student's t-test, in red p< 0.05).
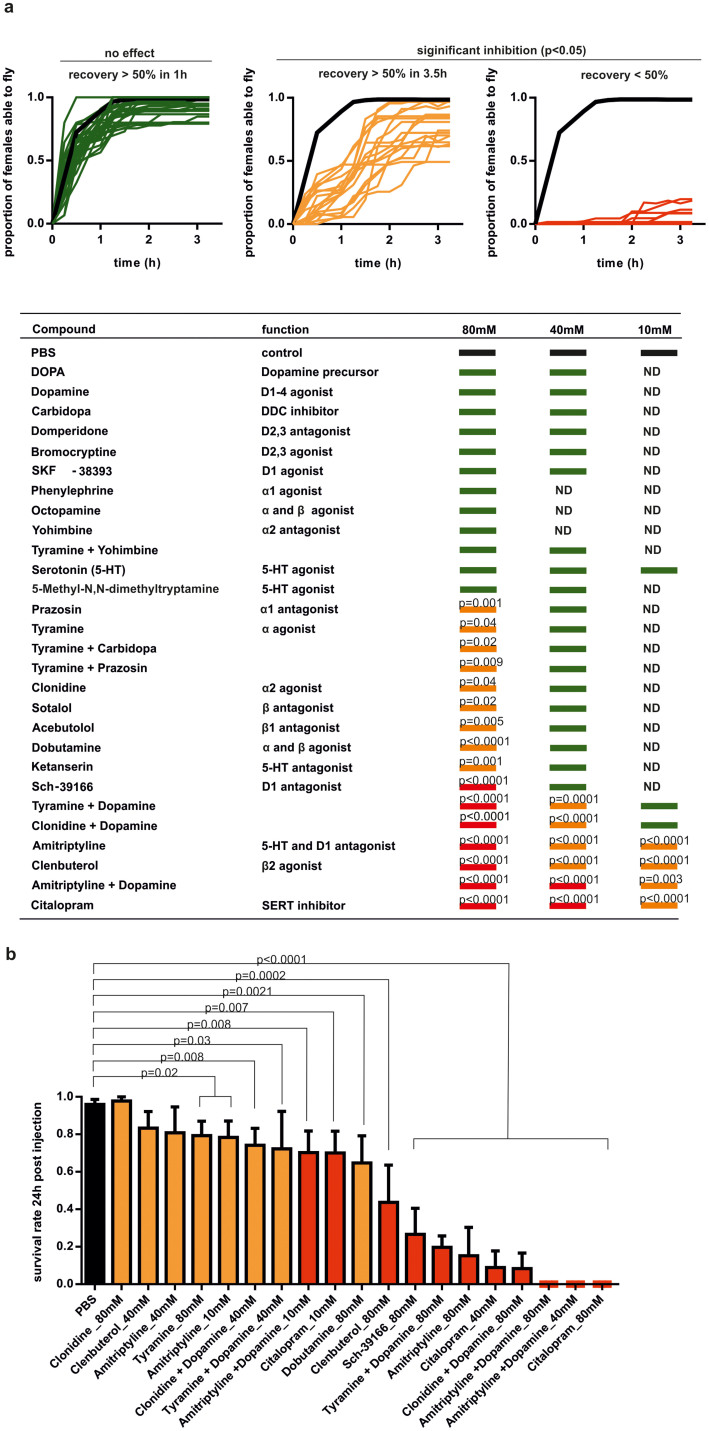
Locomotor activity is severely disturbed by activation of the adrenergic system and inhibition of the dopaminergic and serotonergic system
Normally, following anaesthesia with a brief pulse of CO2, mosquitoes will require several minutes to regain posture and be able to fly again. However, in our oviposition assays we observed that upon injection of tyramine, clonidine, clenbuterol and tyramine + DOPA females required a longer recovery period post-anaesthesia of more than 2 h. In addition tyramine-injected females showed leg tremors and flight inability during that time and most of the tyramine +DOPA injected females died. This suggested that we were able to interfere in neuromuscular transmission which is required for mosquito locomotor behaviour. This is in concordance with previous studies that have shown that biogenic amines can modulate this function in other insect species35,36. To ascertain the relative contribution of the adrenergic and dopaminergic pathways to locomotor activity we injected female mosquitoes with a panel of agonists/antagonists that are known to interfere in these pathways in the human nervous system and measured the post-immobilisation recovery (PIR) time as a proxy for regain of locomotive function. We then classified the outcome in 3 groups (Figure 5A): PIR not statistically different from PBS-injected controls (green line); significant longer PIR but >50% recovery within 3 h (orange line); significant longer PIR but <50% recovery within 3 h (red line). Our results showed that in general drug-mediated dopamine receptor antagonists (Sch-39166, amitriptyline), α-adrenergic (tyramine, clonidine, prazosin) and β-adrenergic (sotalol, acebutolol, dobutamine and clenbuterol) agonists and antagonists prolonged PIR significantly. The antagonist yohimbine was able to reverse the negative effect of tyramine on the locomotory behaviour. Although amitriptyline has been recently identified as dopamine receptor (Dop2) antagonist in Ae. aegypti, this compound has been characterised in humans mainly as inhibitor of the re-uptake of serotonin and noradrenaline37. In order to test whether the prolonged PIR seen in amitriptyline-injected females could be also caused by interference in the serotonergic system we tested other serotonergic agonists and antagonists. Indeed, compounds that are known to inhibit serotonin receptors (ketanserin) or serotonin re-uptake (citalopram) in human nervous system caused a significant increase in PIR. Citalopram and combinatory injections of amitriptyline with dopamine showed the strongest effect of all tested aminergic molecules even, in the case of citalopram, at concentrations as low as 0.25 mM (data not shown). The fact that dopamine co-injection did not rescue the effect of amitriptyline suggests that amitriptyline affects non-dopaminergic signalling in An. gambiae mosquitoes. Moreover, dopamine actually prolonged the PIR in coinjection with amitriptyline as well as with the alpha agonists tyramine and clonidine. Potentially these effects could be caused either by an increased imbalance between the different aminergic systems or by dopamine binding also to the respective adrenergic or serotonergic receptors thereby aggravating the effect caused by tyramine, clonidine or amitriptyline alone. The latter hypothesis is supported by studies which found that dopamine is an Oct α2R (OA3), TyR1 and TyR3 agonist in the insect nervous system30,38. However, it also seems plausible that a balance of these systems is essential as often different biogenic amines have opposing effects on behaviours such as egg laying and locomotion in insects25,39.
Figure 5. Effect of aminergic compounds on flying ability and survival.
(a) Flying ability of females in response to injection of aminergic compounds at various concentrations after CO2 knockdown (N = 15). Effects were grouped in 3 classes: in green - no effect; in orange- significant effect, but 50% of females recovered within 3 h; in red- significant effect, recovery lower than 50% within 3 h. The experiment was performed in a minimum of 3 independent repeats. Curves were analysed by non-linear regression (one phase association, constraint: plateau level lower than 0.7, extra sum of squares F- test, p<0.05 is significant). ND-not determined (b) Survival rate of 15 females injected with compounds that caused significant effects on flight recovery (N = 15 per experiment, 3 repeats, Student's t-test, p<0.05 is significant).
Most of the females which were not able to resume flying within 3 h died within 24 h (Figure 5B).
Together, these results showed that disruption of the β-adrenergic signalling, activation of the α2-adrenergic system, inactivation of the α1-adrenergic system, inhibition of the D1-aminergic receptor or serotonin transport/receptors can each cause defects in the female locomotor behaviour which can result further in adult death. A detailed analysis of the locomotor behaviour following injection showed that interferences in the different aminergic systems caused distinct behavioural phenotypes (Supplementary Figure S1).
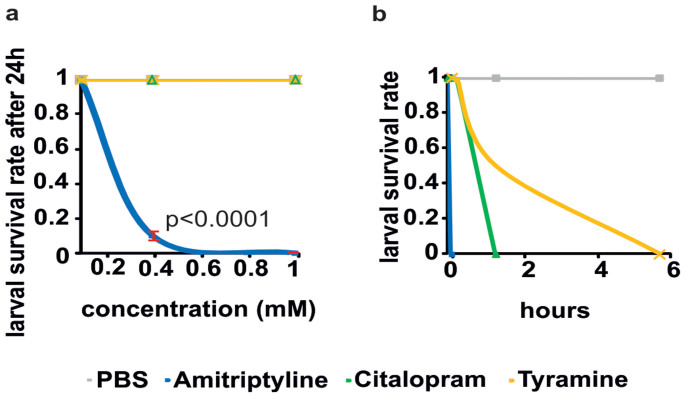
Amitriptyline, citalopram and tyramine are toxic for mosquito larvae
Finally, we tested a range of the same compounds that showed adult toxicity for their effect in larval stages. The most potent of these compounds was amitriptyline, which at a concentration of 0.4 mM killed more than 90% of larvae within 24 h (Figure 6A). This is comparable to its activity in the yellow fever mosquito Ae. aegypti27. Tyramine and citalopram were able to kill larvae but required significantly higher concentrations (40 mM) (Figure 6B).
Figure 6. Larval survival in the presence of dissolved tyramine, citalopram and amitriptyline.
(a) In 5 repeats the survival of 10 larvae per treatment (final concentration: 40 mM, 1 mM, 400 uM, 100 uM) was monitored and compared to the PBS control over a period of 24 h. (Student's t-test, p<0.05 is significant). (b) Larval survival rate (N = 10) within 6 h after rearing in 40 mM compound solution.
Discussion
Biogenic amines are responsible for the regulation of major physiological processes. They have been extensively studied in humans and recent progress has been made to evaluate their function in insects12. We recently showed that the knockdown of a key enzyme involved in phenylalanine/tyrosine metabolism caused reduced fertility in the malaria mosquito8. Because this pathway also regulates the synthesis of 3 of the 5 insect biogenic amines we investigated how amines and drugs known to bind aminergic receptors can affect behaviours that determine reproductive capacity and survival in An. gambiae mosquitoes. The injection of tyramine, the insect equivalent of the human noradrenaline/adrenaline caused complete egg retention by the gravid female and premature egg melanisation. It is possible that rigidity of the chorion following melanisation could have attributed to the observed complete sterility; however, because other adrenergic compounds (clonidine and octopamine) also reduced oviposition but did not cause premature egg melanisation other factors that regulate oviposition must be important. Potentially, these could be linked to an inability to contract the oviduct muscle, which has been found to be the primary cause for sterility in Drosophila mutants unable to convert tyramine into octopamine due to defective tyramine beta hydroxylase19,40,41,42. When applied to isolated locust oviduct muscles, octopamine has been shown to cause reduced muscle contractions that are mediated via octopamine receptors43,44. Octopamine and clonidine might bind preferentially different adrenergic receptors compared to tyramine in mosquitoes. In fact clonidine is an α2- agonist which has been shown in some studies to bind with higher affinity to the α2- adrenergic like octopamine receptor rather than the adrenergic TyR1 receptor in insects30,45. Assuming these compounds bind to different adrenergic receptors, this would explain the lack of premature egg melanisation observed upon octopamine and clonidine injection. The regulation of melanin production via adrenergic compounds has been also recently observed in ticks whereby injection of the α2-adrenergic agonist guanabenz acetate caused whole body melanisation18. In our case, the melanisation induced by tyramine seemed to be limited to the eggs. Given these results, we therefore propose that oviposition is regulated via the octopaminergic and tyraminergic system, but that egg melanisation is mediated via the tyraminergic/alpha 2 adrenergic system in An. gambiae mosquitoes.
In line with tyraminergic regulation of egg chorion melanisation the injection of the adrenergic antagonists prazosin and yohimbine reduced the level of premelanisation of eggs. We could not confirm this for the dopamine synthesis inhibitor carbidopa, although we previously showed that its injection can cause reduced melanisation of oviposited eggs. Interestingly, injection of DOPA or dopamine in itself did not result in premature egg melanisation, indicating again that these melanin precursors are insufficient to cause melanisation alone and that regulatory mechanisms for this process must exist.
Some of the compounds, such as clonidine, prazosin, and clenbuterol did not lead to a reduction in egg numbers in contrast to the mentioned previous study in ticks18, however these compounds reduced embryo viability significantly. Changes in the availability of dopamine via DOPA, dopamine or carbidopa injection also affected embryo viability. Reasons for the lack of effect in egg numbers compared to the previous study in ticks could be due to the time of injection relative to egg development. While ticks continuously produce egg batches, so that any egg laying defect caused by injection will be seen immediately, An. gambiae mosquitoes lay eggs in discrete batches only every 2–3 days after blood meal therefore the timing of injection may be crucial. In order to observe a maximal effect on egg laying ability and considering the short half-life of some of the compounds, we injected mosquitoes ~2–3 hours before we allowed oviposition. At that stage mosquito eggs are well developed, therefore any effect on egg synthesis by these compounds might have been diminished. Thus, potentially more of these compounds could affect the number of eggs laid if the females were injected at an earlier stage. We also found that several adrenergic, dopaminergic and serotononergic compounds affect the adult locomotor behaviour, which similar to female reproduction is obviously a determining factor for mosquito vectorial capacity. Interference in the adrenergic system by α2-agonists, α1-antagonists, β-agonists and antagonists led to reduced flight recovery. The β2-agonist clenbuterol was particularly effective, but over 40% of females recovered within 24 h. In contrast to this, at the same concentration inhibition of the dopaminergic and serotonin transport/receptor system by amitriptyline and citalopram led to a severe effect on flight recovery but also increased adult mortality to 80–100 percent within 24 h. Interestingly, in combination with dopamine, which in itself did not have an effect on locomotor activity or survival, the inhibitory effect of tyramine, clonidine and amitriptyline was accentuated. This could have been caused by dopamine binding to the respective adrenergic or serotonergic receptors or imbalances between the different aminergic systems. Dopamine has been found to bind various octopamine and tyramine receptors in insects and activation of different aminergic systems can cause opposing effects, highlighting the importance of a critical balance between these systems to maintain body function25,39. Combinatory insecticides which activate one system but inhibit another could be therefore more effective in killing mosquitoes. Our compound concentrations used were comparable to other studies22 validating dopaminergic compounds. It remains to be seen how these correlate to concentrations that would be required in aerosol for our compounds to affect processes, such as oviposition, locomotion or respiration.
We finally tested whether adrenergic, dopaminergic and serotonergic molecules would be effective larvicides. We found that although tyramine, citalopram and amitriptyline were able to significantly reduce larval survival rapidly, the latter was the most toxic and killed larvae at a dosage that was equivalent to other pesticides14. This was in concordance with a previous finding which showed that amitriptyline can be effective at killing Ae aegypti larvae27.
Overall we conclude that adrenergic compounds, in particular those activating the adrenergic system, are effective mosquito sterilants. Using compounds that mimic the action of tyramine could be advantageous because tyramine only occurs at low concentrations in vertebrates but at relatively high concentrations in insects, reflecting its greater usage as a neurotransmitter in insects. However, adrenergic compounds in general were less effective at limiting mosquito locomotion and larval/adult survival than dopamine receptor antagonists or serotonin transport-inhibiting reagents. We further showed that the behavioural effects can be various and distinct for these compounds.
Injection of putative agonists and antagonists of neurotransmitter signalling is an approach that has been used in the initial screening of compounds with potential insecticidal properties in a wide range of insects and arachnids18,22,46. In order to develop the aminergic compounds tested here further research investigating their pharmacological properties and their specific binding to mosquito receptors by using heterologous expression systems is needed. Nonetheless we provide a useful experimental framework to study the effect of these compounds on important life history traits as a first step in the future development of aminergic insecticides.
Methods
Ethics Statement
All animal work was conducted according to UK Home Office Regulations and approved under Home Office License PPL 70/6453.
Anopheles Rearing
The Anopheles gambiae G3 strain was maintained on 10% glucose solution at 28°C and 80% humidity with 12/12 h day-night light cycle.
Oviposition assay
In order to investigate the effect of adrenergic and dopamine related compounds on ovipostion and egg maturation, females were blood-fed and then injected at a late stage of oogenesis (~53 h post blood meal) into the thorax either with PBS solution or compound concentration of160 mM in PBS. Compounds were purchased from Sigma and resuspended in PBS prior to injection. The females were then allowed to recover from injection for 2 h before being placed into oviposition cups for egg laying in a minimum of 3 independent experiments. The likelihood of oviposition was analysed by Fisher's exact-test, the number of eggs and hatching rate was compared to the respective PBS control samples by Mann Whitney test and t-test of arc-sine transformed data with Welsh's correction respectively using the GraphPad Prism software. The proportion of females that survived was determined 24 h post-injection and analysed by t-test in 3 independent experiments (N = 10 per experiment).
Ovary dissection
In order to investigate premature egg melanisation, females were prevented from egg laying and dissected 24 h after injection. Ovaries were removed by pulling out the last 2 abdominal segments and inspected for melanisation under the dissection microscope. The arc-sine transformed proportion of dark melanised eggs was analysed between tyramine and coinjected females with tyramine with DOPA, DOPA + carbidopa, carbidopa, yohimbine and prazosin by using a Student's t-test.
Flight recovery assay
Fifteen 1–2 day old adult females were anaesthetized on a CO2 pad and injected in their thorax with 69 nl of the compound at various concentrations (80 mM, 40 mM or 10 mM). The total time of anaesthesia was ~2–3 min. Females were then allowed to recover in paper cup. Every 15 min. the paper cap was tapped at the bottom 3 times to induce flying behaviour. The proportion of females able to fly per cup/condition was recorded for 3 h. After 24 h we recorded the number of females alive. The flight recovery responses were analysed in GraphPad Prism by non-linear regression comparing each compound vs. the respective PBS repeats (exponential one-phase association equation with plateau constraint level of less than 0.7 and comparison of curves by using extra sum of squares F- test).
Behavioural analysis
After compound injection and anaesthesia 3 × 5 females were placed on their back (which we refer to as basal stage) into a round glass bowl radius 8 cm, height 3.5 cm which served as observation arena. The bottom of this bowl was covered with white filter paper (Whatman) to prevent females from sticking to the glass while on top we placed a transparent petri dish lid. Females were allowed to acclimate for 15 min. before the bottom of the bowl was tapped 3 times and their behavioural response was video recorded for 1 min. This procedure was repeated after 30 min, 45 min, 1 h and 3 h. We then analyzed the video by tracking each individual female for the entire minute and scoring the following behaviours as mutually exclusive: 1) the stationary behaviours: on back, upright (no movement) and 2) other behaviours: leg and wing movement without taxis, walking, flying, jumping, grooming, falling over and seizures. Jumping refers to a sudden leap (max. 1 sec) by the mosquito without movement of wings. When mosquitoes rubbed their legs we considered this behaviour as grooming. Falling over described a situation in which the female loses balance and overturns. Females with seizures showed convulsions with rapid uncontrolled movements of the body. A behavioural profile corresponding to the injected compound was determined by measuring how much time injected females spent in each behaviour, adding up to a total of 100%. We analysed the mean behavioural profile of 15 females per compound by Student's t-test. All observations were performed at the mosquito day cycle and the multiple treatment groups were performed in a randomized order.
Larval survival assay
10 L2–L3 larvae were placed per well of a 24well Nunc plate (VWR). Amitriptyline, tyramine and citalopram were dissolved in PBS and added at a final concentration of 40 mM, 1 mM, 400 µM or 100 µM to the well (total volume: 2 ml). 5 repeats were performed for the all but the 40 mM concentration. Larval movement was recorded daily by gentle tapping on the Nunc plate. Non-moving larvae were recorded as dead when touched with a pipette.
Author Contributions
S.F. and E.R. performed experiments. S.F. analysed data and prepared figures. S.F., T.N. and A.C. wrote the paper.
Supplementary Material
Supplemetary Information
Acknowledgments
This Research was funded by grants from European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements N° 242095 (EviMalaR) and N° 228421 (INFRAVEC) and the Foundation for the National Institutes of Health through the Vector-Based Control of Transmission: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also would like to thank Ann Hall for rearing of the mosquitoes.
References
- Prapanthadara L., Hemingway J. & Ketterman A. J. Ddt-Resistance in Anopheles-Gambiae (Diptera, Culicidae) from Zanzibar, Tanzania, Based on Increased Ddt-Dehydrochlorinase Activity of Glutathione S-Transferases. Bull Entomol Res 85, 267–274 (1995). [Google Scholar]
- Chandre F. et al. Pyrethroid cross resistance spectrum among populations of Anopheles gambiae s.s. from Cote d'Ivoire. J Am Mosq Control Assoc 15, 53–9 (1999). [PubMed] [Google Scholar]
- Corbel V. et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop 101, 207–16 (2007). [DOI] [PubMed] [Google Scholar]
- Hill C. A. et al. G protein-coupled receptors in Anopheles gambiae. Science 298, 176–8 (2002). [DOI] [PubMed] [Google Scholar]
- Marinissen M. J. & Gutkind J. S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 22, 368–76 (2001). [DOI] [PubMed] [Google Scholar]
- Taneja-Bageshwar S. et al. Biostable agonists that match or exceed activity of native insect kinins on recombinant arthropod GPCRs. Gen Comp Endocrinol 162, 122–8 (2009). [DOI] [PubMed] [Google Scholar]
- Orchard I. Octopamine in Insects - Neurotransmitter, Neurohormone, and Neuromodulator. Can J Zool 60, 659–669 (1982). [Google Scholar]
- Fuchs S., Behrends V., Bundy J. G., Crisanti A. & Nolan T. Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PLoS One 9, e84865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenau W. & Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48, 13–38 (2001). [DOI] [PubMed] [Google Scholar]
- Evans P. D. Biogenic amines in the insect nervous system. Adv. Insect Physiol 15, 317–473 (1980). [Google Scholar]
- Usdin E. & Sandler M. Trace amines and the brain : proceedings of a study group held at the fourteenth Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico. (Dekker, New York; Basel, 1976). [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50, 447–77 (2005). [DOI] [PubMed] [Google Scholar]
- Pirri J. K., McPherson A. D., Donnelly J. L., Francis M. M. & Alkema M. J. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron 62, 526–38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh A. J. B. & Gichang M. M. The Activity of Amitraz against Infestations of Rhipicephalus-Appendiculatus. Pestic Sci 11, 674–678 (1980). [Google Scholar]
- Elfassy O. J., Goodman F. W., Levy S. A. & Carter L. L. Efficacy of an amitraz-impregnated collar in preventing transmission of Borrelia burgdorferi by adult Ixodes scapularis to dogs. J Am Vet Med Assoc 219, 185–9 (2001). [DOI] [PubMed] [Google Scholar]
- Pound J. M., Miller J. A. & George J. E. Efficacy of amitraz applied to white-tailed deer by the ‘4-poster' topical treatment device in controlling free-living lone star ticks (Acari: Ixodidae). J Med Entomol 37, 878–84 (2000). [DOI] [PubMed] [Google Scholar]
- Baxter J. R. et al. Amitraz or coumaphos efficacy tests in Guatemala for control of Varroa jacobsoni Mesostigmata : Varroidae in honey bees? Southwest Entomol 24, 309–313 (1999). [Google Scholar]
- Cossio-Bayugar R., Miranda-Miranda E., Padilla V. N., Olvera-Valencia F. & Reynaud E. Perturbation of tyraminergic/octopaminergic function inhibits oviposition in the cattle tick Rhipicephalus (Boophilus) microplus. J Insect Physiol 58, 628–633 (2012). [DOI] [PubMed] [Google Scholar]
- Monastirioti M., Linn C. E. Jr & White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci 16, 3900–11 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S., Fox L. E., Soll D. R. & Wu C. F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol 58, 425–41 (2004). [DOI] [PubMed] [Google Scholar]
- Fussnecker B. L., Smith B. H. & Mustard J. A. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J Insect Physiol 52, 1083–92 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard J. A., Pham P. M. & Smith B. H. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J Insect Physiol 56, 422–430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper I., Kurshan P. T., McBride E., Jackson F. R. & Kopin A. S. Locomotor activity is regulated lay D2-like receptors in Drosophila: An anatomic and functional analysis. Dev Neurobiol 67, 378–393 (2007). [DOI] [PubMed] [Google Scholar]
- Kume K., Kume S., Park S. K., Hirsh J. & Jackson F. R. Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25, 7377–7384 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber J., Kloppenburg P. & Scheidler A. Neuromodulation by Serotonin and Octopamine in the Honeybee - Behavior, Neuroanatomy and Electrophysiology. Experientia 49, 1073–1083 (1993). [Google Scholar]
- Wright T. R., Bewley G. C. & Sherald A. F. The genetics of dopa decarboxylase in Drosophila melanogaster. II. Isolation and characterization of dopa-decarboxylase-deficient mutants and their relationship to the alpha-methyl-dopa-hypersensitive mutants. Genetics 84, 287–310 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. M. et al. A “genome-to-lead” approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D(1)-like dopamine receptors. PLoS Negl Trop Dis 6, e1478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D. & Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci 5, 111–8 (2005). [DOI] [PubMed] [Google Scholar]
- Bayliss A., Roselli G. & Evans P. D. A comparison of the signalling properties of two tyramine receptors from Drosophila. J Neurochem 125, 37–48 (2013). [DOI] [PubMed] [Google Scholar]
- Wu S. F. et al. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J Neurochem (2013). [DOI] [PubMed] [Google Scholar]
- Sugamori K. S., Demchyshyn L. L., McConkey F., Forte M. A. & Niznik H. B. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett 362, 131–8 (1995). [DOI] [PubMed] [Google Scholar]
- Blenau W., Erber J. & Baumann A. Characterization of a dopamine D1 receptor from Apis mellifera: Cloning, functional expression, pharmacology, and mRNA localization in the brain. J Neurochem 70, 15–23 (1998). [DOI] [PubMed] [Google Scholar]
- Goltsev Y. et al. Developmental and evolutionary basis for drought tolerance of the Anopheles gambiae embryo. Dev Biol 330, 462–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. R. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet 24, 127–222 (1987). [PubMed] [Google Scholar]
- Cooper R. L. & Neckameyer W. S. Dopaminergic modulation of motor neuron activity and neuromuscular function in Drosophila melanogaster. Comp Biochem Physiol B Biochem Mol Biol 122, 199–210 (1999). [DOI] [PubMed] [Google Scholar]
- Evans P. Biogenic Amine Receptors and Their Mode of Action in Insects. in Insect Neurochemistry and Neurophysiology · 1986 (eds. Bor̆kovec, A. & Gelman, D.) 117–141 (Humana Press, 1986). [Google Scholar]
- Bendtsen L., Jensen R. & Olesen J. Amitriptyline, a combined serotonin and noradrenaline re-uptake inhibitor, reduces exteroceptive suppression of temporal muscle activity in patients with chronic tension-type headache. Electroencephalogr Clin Neurophysiol 101, 418–22 (1996). [PubMed] [Google Scholar]
- Reale V., Hannan F., Midgley J. M. & Evans P. D. The expression of a cloned Drosophila octopamine/tyramine receptor in Xenopus oocytes. Brain Res 769, 309–320 (1997). [DOI] [PubMed] [Google Scholar]
- Brembs B., Christiansen F., Pfluger H. J. & Duch C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J Neurosci 27, 11122–31 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valentin R. et al. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol 209, 183–198 (2006). [DOI] [PubMed] [Google Scholar]
- Orchard I. & Lange A. B. Evidence for octopaminergic modulation of an insect visceral muscle. J Neurobiol 16, 171–81 (1985). [DOI] [PubMed] [Google Scholar]
- Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol 264, 38–49 (2003). [DOI] [PubMed] [Google Scholar]
- Orchard I. & Lange A. B. Neuromuscular transmission in an insect visceral muscle. J Neurobiol 17, 359–72 (1986). [DOI] [PubMed] [Google Scholar]
- Cook B. J. & Wagner R. M. Some Pharmacological Properties of the Oviduct Muscularis of the Stable Fly Stomoxys-Calcitrans. Comp Biochem Physiol C Toxicol Pharmacol 102, 273–280 (1992). [DOI] [PubMed] [Google Scholar]
- Saudou F., Amlaiky N., Plassat J. L., Borrelli E. & Hen R. Cloning and Characterization of a Drosophila Tyramine Receptor. Embo Journal 9, 3611–3617 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez V. M., Giurfa M., Devaud J. M. & Farina W. M. Latent inhibition in an insect: the role of aminergic signaling. Learn Mem 19, 593–7 (2012). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–7 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y. & Christensen B. M. Involvement of L-Tyrosine and Phenol Oxidase in the Tanning of Aedes-Aegypti Eggs. Insect Biochem and Molec Biol 23, 739–748 (1993). [Google Scholar]
- Koller W. C. & Rueda M. G. Mechanism of action of dopaminergic agents in Parkinson's disease. Neurology 50, S11–4; discussion S44–8 (1998). [DOI] [PubMed] [Google Scholar]
- Birkmayer W. & Hornykiewicz O. [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien Klin Wochenschr 73, 787–8 (1961). [PubMed] [Google Scholar]
- Ostadali M. R. et al. The Detection of Dopamine Gene Receptors (DRD1-DRD5) Expression on Human Peripheral Blood Lymphocytes by Real Time PCR. Iran J Allergy Asthma Immunol 3, 169–74 (2004). [PubMed] [Google Scholar]
- Garland E. M., Black B. K., Harris P. A. & Robertson D. Dopamine-beta-hydroxylase in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293, H684–H690 (2007). [DOI] [PubMed] [Google Scholar]
- Vickers S. et al. Metabolism of carbidopa (1-(-)-alpha-hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid monohydrate), an aromatic amino acid decarboxylase inhibitor, in the rat, rhesus monkey, and man. Drug Metab Dispos 2, 9–22 (1974). [PubMed] [Google Scholar]
- Sideri M., Tsakas S., Markoutsa E., Lampropoulou M. & Marmaras V. J. Innate immunity in insects: surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly haemocytes. Immunology 123, 528–37 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn M. G. et al. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc Natl Acad Sci U S A 99, 14554–9 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzamali G., Klaerke D. A. & Grimmelikhuijzen C. J. A new family of insect tyramine receptors. Biochem Biophys Res Commun 338, 1189–96 (2005). [DOI] [PubMed] [Google Scholar]
- Kleinau G. et al. Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists. PLoS One 6, e27073 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb S. et al. Agonist-Specific Coupling of a Cloned Drosophila Octopamine Tyramine Receptor to Multiple 2nd Messenger Systems. Embo Journal 13, 1325–1330 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpene C. et al. Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch Pharmacol 359, 310–21 (1999). [DOI] [PubMed] [Google Scholar]
- Wang X. M., Zhang Z. J., Bains R. & Mokha S. S. Effect of antisense knock-down of alpha(2a)- and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain 98, 27–35 (2002). [DOI] [PubMed] [Google Scholar]
- Hiripi L., Juhos S. & Downer R. G. Characterization of tyramine and octopamine receptors in the insect (Locusta migratoria migratorioides) brain. Brain Res 633, 119–26 (1994). [DOI] [PubMed] [Google Scholar]
- Millan M. J. et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse 35, 79–95 (2000). [DOI] [PubMed] [Google Scholar]
- Colas J. F., Launay J. M., Kellermann O., Rosay P. & Maroteaux L. Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci U S A 92, 5441–5 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotte C. et al. Molecular characterization and localization of the first tyramine receptor of the American cockroach (Periplaneta americana). Neuroscience 162, 1120–33 (2009). [DOI] [PubMed] [Google Scholar]
- Scheer A., Fanelli F., Costa T., De Benedetti P. G. & Cotecchia S. The activation process of the alpha1B-adrenergic receptor: potential role of protonation and hydrophobicity of a highly conserved aspartate. Proc Natl Acad Sci U S A 94, 808–13 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F., Boschert U., Amlaiky N., Plassat J. L. & Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J 11, 7–17 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S. & Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci 21, 595–606 (1977). [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol 318, 99–122 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances H., Poncelet M., Danti S., Goldschmidt P. & Simon P. Psychopharmacological Profile of Clenbuterol. Drug Develop Res 3, 349–356 (1983). [Google Scholar]
- Williams R. S. & Bishop T. Selectivity of dobutamine for adrenergic receptor subtypes: in vitro analysis by radioligand binding. J Clin Invest 67, 1703–11 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson T. Characterization of the beta 1-adrenoceptor stimulatory effects of the partial beta 1-agonists acebutolol, xamoterol, H142/08 and H201/70. Eur J Pharmacol 164, 121–8 (1989). [DOI] [PubMed] [Google Scholar]
- Juberg E. N., Minneman K. P. & Abel P. W. Beta 1- and beta 2-adrenoceptor binding and functional response in right and left atria of rat heart. Naunyn Schmiedebergs Arch Pharmacol 330, 193–202 (1985). [DOI] [PubMed] [Google Scholar]
- Vaishnavi S. N. et al. Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol Psychiatry 55, 320–2 (2004). [DOI] [PubMed] [Google Scholar]
- Gould G. G., Altamirano A. V., Javors M. A. & Frazer A. A comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol Psychiatry 59, 408–14 (2006). [DOI] [PubMed] [Google Scholar]
- Cusack B., Nelson A. & Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl) 114, 559–65 (1994). [DOI] [PubMed] [Google Scholar]
- Jang S. W. et al. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem Biol 16, 644–56 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi M., Groshan K., Blakely R. D. & Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 340, 249–258 (1997). [DOI] [PubMed] [Google Scholar]
- Chipkin R. E. et al. Pharmacological Profile of Sch39166 - a Dopamine D1 Selective Benzonaphthazepine with Potential Antipsychotic Activity. J Pharmacol ExpTher 247, 1093–1102 (1988). [PubMed] [Google Scholar]
- Setler P. E., Sarau H. M., Zirkle C. L. & Saunders H. L. The central effects of a novel dopamine agonist. Eur J Pharmacol 50, 419–30 (1978). [DOI] [PubMed] [Google Scholar]
- Freedman S. B. et al. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268, 417–26 (1994). [PubMed] [Google Scholar]
- Heykants J. et al. On the pharmacokinetics of domperidone in animals and man. IV. The pharmacokinetics of intravenous domperidone and its bioavailability in man following intramuscular, oral and rectal administration. Eur J Drug Metab Pharmacokinet 6, 61–70 (1981). [DOI] [PubMed] [Google Scholar]
- Kvernmo T., Houben J. & Sylte I. Receptor-binding and pharmacokinetic properties of dopaminergic agonists. Curr Top Med Chem 8, 1049–67 (2008). [DOI] [PubMed] [Google Scholar]
- Davis J. P. & Pitman R. M. Characterization of receptors mediating the actions of dopamine on an identified inhibitory motoneurone of the cockroach. J Exp Biol 155, 203–17 (1991). [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Forler C., Middlemiss D. N. & Fozard J. R. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol 117, 15–24 (1985). [DOI] [PubMed] [Google Scholar]
- Van Nueten J. M. et al. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. J Pharmacol Exp Ther 218, 217–30 (1981). [PubMed] [Google Scholar]
- Chen F. et al. Characterization of an allosteric citalopram-binding site at the serotonin transporter. J Neurochem 92, 21–8 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemetary Information