Abstract
Excess dietary lipid generally leads to fat deposition and impaired glucose homeostasis, but consumption of fish oil (FO) alleviates many of these detrimental effects. The beneficial effects of FO are thought to be mediated largely via activation of the nuclear receptor peroxisomal-proliferator-activated receptor α (PPARα) by omega-3 polyunsaturated fatty acids and the resulting upregulation of lipid catabolism. However, pharmacological and genetic PPARα manipulations have yielded variable results. We have compared the metabolic effects of FO supplementation and the synthetic PPARα agonist Wy-14,643 (WY) in mice fed a lard-based high-fat diet. In contrast to FO, WY treatment resulted in little protection against diet-induced obesity and glucose intolerance, despite upregulating many lipid metabolic pathways. These differences were likely due to differential effects on hepatic lipid synthesis, with FO decreasing and WY amplifying hepatic lipid accumulation. Our results highlight that the beneficial metabolic effects of FO are likely mediated through multiple independent pathways.
The global prevalence of obesity and type 2 diabetes (T2D) is increasing at an alarming rate1,2. Insulin resistance (IR) and disturbances in glucose metabolism are major defects underpinning both of these disorders3. Although the molecular mechanisms are not fully elucidated, the pathophysiology of IR has been strongly associated with the expansion of adipose tissue and accumulation of lipid metabolites in insulin-sensitive tissues, including skeletal muscle and liver4. Dietary lipid oversupply (particularly saturated fats) is known to cause fat deposition and metabolic defects, however previous research has shown that not all dietary fats have deleterious outcomes, with clear lipid species-dependent effects5,6,7,8.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), which are a highly abundant fatty acid type in fish oil (FO), are one of the distinct classes of fatty acids known to reduce lipid abundance and to protect against fat-induced defects in glucose metabolism and insulin action9. The partial or complete substitution of saturated, monounsaturated and omega-6 polyunsaturated fatty acids (n-6 PUFAs) in high-fat diets (the most common fatty acid types in western diets) by n-3 PUFAs has been reported to decrease adiposity and improve insulin sensitivity and glucose homeostasis in rodents6,7,10,11,12,13,14,15,16,17. This is paralleled by the almost universal observation of reduced hepatic steatosis11,14,16,17,18,19,20,21 and decreased triacylglycerol (TAG) accumulation in skeletal muscle5,19,22. In humans, the outcomes of dietary manipulations with FO supplementation have been variable, with insulin-sensitising effects being observed in a number of trials (reviewed in23).
n-3 PUFAs are thought to convey many of their beneficial effects via the activation of the nuclear receptor peroxisome-proliferator-activated receptor α (PPARα) and the consequent remodelling of lipid metabolism to enhance fat catabolism19. PPARα is present at high levels in mammalian liver and influences the expression of an extensive network of downstream genes involved in lipid transport and oxidation24. Subsequently, the consumption of FO in rodents is accompanied by profound induction of hepatic peroxisomal proliferation, elevated expression of key peroxisomal oxidative enzymes and to a lesser extent enhancement of the mitochondrial fatty acid oxidation (FAO) system16,19. Unlike mitochondria, peroxisomal FAO is inefficient and incomplete, with some electrons being passed directly to oxygen to generate hydrogen peroxide, rather than coupled to ATP production as in the mitochondrial respiratory chain25,26. Furthermore, as peroxisomes lack the machinery for the Krebs Cycle and oxidative phosphorylation, the oxidative end-products (acetyl-CoA and chain-shortened fatty acids) are released into the cytosol awaiting complete oxidation by mitochondria25,26. Thus, the substantial enhancement of peroxisomal FAO capacity in response to FO is suggested to promote inefficient lipid catabolism and potentially reduce lipid flux to the mitochondria if the transfer of peroxisomal end-products to mitochondria is incomplete16.
A previous study indicated that the ability of FO to counteract the development of hepatic and whole-body IR in mice was dependent on the presence of functional PPARα20, while other groups have implicated different intracellular targets and pathways as mediators of the beneficial effects of n-3 PUFA27,28,29. Synthetic PPARα agonists, similar to FO, have been shown to diminish lipid abundance and prevent the incidence of IR in both genetic and diet-induced rodent models30,31,32,33. On the other hand, several studies have reported that PPARα null mice displayed a more favourable metabolic profile, including improved insulin sensitivity compared to wildtype littermates under high-fat feeding conditions34,35,36, while transgenic mice overexpressing PPARα show disturbances in lipid metabolism and insulin resistance36,37. In view of the discrepancies in the literature regarding the effects of PPARα activation, the current study aimed to investigate whether the activation of PPARα by the synthetic agonist Wy-14,643 (here on referred to as WY) was sufficient to convey most of the beneficial effects of FO on lipid abundance and glucose homeostasis in mice fed a high-fat diet.
Results
Effects of FO and WY on body weight and adiposity
C57BL6/J mice received either a standard low-fat chow diet, a lard-based high-fat diet (HFD), a HFD supplemented with WY, or HFDs containing FO and lard at ratios of 10:90, 50:50 and 90:10 (10%FO, 50%FO and 90%FO) for 8 weeks. Detailed fatty acid composition of the diets is shown in Table 1. After 8 weeks on the respective diets, mice on the lard-based HFD showed a significant 16% increase in body weight in comparison to chow-fed mice, whereas FO-fed and WY–treated animals were not significantly different from controls (Table 2). The increase in body weight in the HFD group was caused by a marked increase in adiposity (measured by DXA and weight of individual white adipose depots), which was attenuated in the 50%FO and 90%FO groups dose-dependently (Table 2). In contrast, WY supplementation did not result in differences in whole-body adiposity compared to HFD-fed counterparts (Table 2). High ratios of FO (50% and 90%) and WY induced an increase in liver weight, but had little effect on brown adipose tissue mass (Table 2).
Table 1. Fatty acid profiles of diets used in the current study.
| Fatty acid | Chow | Lard HFD | 10% Fish oil | 50% Fish Oil | 90% Fish Oil |
|---|---|---|---|---|---|
| 14:0 | 0.7 | 1.4 | 2.0 | 3.9 | 6.0 |
| 16:0 | 13. 5 | 25.0 | 23. 9 | 20.9 | 17.5 |
| 16:1 n7 | 1.0 | 2.1 | 2.8 | 5.2 | 7.6 |
| 18:0 | 5.7 | 17. 8 | 15.7 | 9.5 | 7.1 |
| 18:1 n9 | 39.3 | 33.7 | 31.1 | 25.7 | 15.3 |
| 18:2 n6 | 30.6 | 18.3 | 18.3 | 15.9 | 13.7 |
| 18:3 n3 | 6.6 | 1.0 | 1.0 | 1.0 | 1.0 |
| 20:0 | 0.5 | 0.3 | 1.4 | 1.5 | 2.6 |
| 20:4 n6 | 0.2 | 0.2 | 0.3 | 0.6 | 0.9 |
| 20:5 n3 | 0.4 | 0.1 | 2.0 | 9.0 | 16.5 |
| 22:5 n6 | 0.1 | 0.01 | 0.04 | 0.2 | 0.3 |
| 22:5 n3 | 0.1 | 0.1 | 0.3 | 1.1 | 1.4 |
| 22:6 n3 | 1.4 | 0.1 | 1.3 | 5.5 | 10.2 |
| % Sat | 20.3 | 44.5 | 43.0 | 36.0 | 33.2 |
| % Mono | 40.3 | 35.8 | 33.9 | 30.9 | 22.9 |
| % Poly | 39.4 | 19.7 | 23.1 | 33.3 | 43.8 |
| % n-6 | 30.9 | 18.5 | 18.6 | 16.7 | 14.8 |
| % n-3 | 8.4 | 1.2 | 4.5 | 16.6 | 29.0 |
Fatty acid composition was measured by gas chromatography. Fatty acid profile is shown for chow and high-fat diets where the lipid components are comprised of 12% safflower oil and 88% lard or fish oil and lard at ratios of 10:90, 50:50 and 90:10. Lipid-derived energy contents are 8% and 45% in chow and high-fat diets respectively. Data shown as percentage of total fatty acids.
Table 2. Body weight, adiposity, tissue weights and systemic lipid abundance.
| Chow | Lard HFD | 10% Fish oil | 50% Fish Oil | 90% Fish Oil | WY-14,643 | |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 28.7 ± 0.2 | 27.3 ± 0.4 | 27.3 ± 0.7 | 27.0 ± 0.5 | 27.1 ± 0.5 | 27.5 ± 0.4 |
| End point body weight (g) | 29.5 ± 0.3 | 34.0 ± 0.7a | 32.1 ± 1.3 | 31.0 ± 1.1 | 30.5 ± 0.8 | 32.4 ± 0.6 |
| End point adiposity (% body fat) | 12.5 ± 0.4 | 29.4 ± 1.3c | 24.2 ± 2.0c | 21.7 ± 1.2c,e | 16.0 ± 0.9f,*,‡ | 26.4 ± 1.2c |
| Epididymal fat pad (%body weight) | 1.6 ± 0.2 | 4.3 ± 0.2c | 3.2 ± 0.3c,d | 3.0 ± 0.2c,e | 2.3 ± 0.2f,* | 3.1 ± 0.2c,d |
| Inguinal fat pad (%body weight) | 1.0 ± 0.03 | 2.4 ± 0.2c | 2.0 ± 0.2c | 2.0 ± 0.2c | 1.3 ± 0.09f,* | 1.8 ± 0.06b |
| Liver weight (%body weight) | 4.6 ± 0.2 | 3.5 ± 0.1c | 3.7 ± 0.2c | 4.5 ± 0.2f | 5.3 ± 0.1a,f | 5.8 ± 0.1c,f |
| BAT weight (%body weight) | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.03 | 0.4 ± 0.02 | 0.3 ± 0.02 | 0.4 ± 0.02 |
| Serum NEFA (mM) | 0.7 ± 0.06 | 0.8 ± 0.04 | 0.8 ± 0.06 | 0.6 ± 0.04d | 0.6 ± 0.02e | 0.5 ± 0.01b,f |
| Serum TAG (mM) | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.0 ± 0.07e | 0.7 ± 0.07b,f | 0.5 ± 0.03c,f | 0.5 ± 0.03c,f |
Mice were maintained on chow or high-fat diets containing different proportions of FO or WY-14,643 for 8 weeks. Adiposity was determined by DEXA. Tissue weights and serum samples were obtained at the end of feeding period when the animals were culled. Serum NEFA and TAG were measured enzymatically. Data are the means ± SEMs of 8–10 animals per group.
a,b,cp < 0.05, 0.01, 0.001 vs chow;
d,e,fp < 0.05, 0.01, 0.001 vs lard;
*p < 0.05 compared to 50%FO;
‡p < 0.001 compared to 10%FO.
The FO-based diets displayed significant dose-dependent hypolipidaemic effects, illustrated by marked decreases in serum non-esterified fatty acids (NEFA) and triacylglycerols (TAG) (−30% and −63% respectively in the 90% FO group in comparison to HFD) (Table 2). Interestingly, WY-treatment reduced serum lipid levels to an even greater extent than FO supplementation (−41% and −67% for NEFA and TAG respectively, compared to HFD) (Table 2). Taken together, high-FO diets produced less adiposity and lowered circulating lipid levels, while WY administration exerted potent hypolipidaemic effects, but provided minimal protection against fat-induced obesity.
Effects of FO and WY on glucose homeostasis
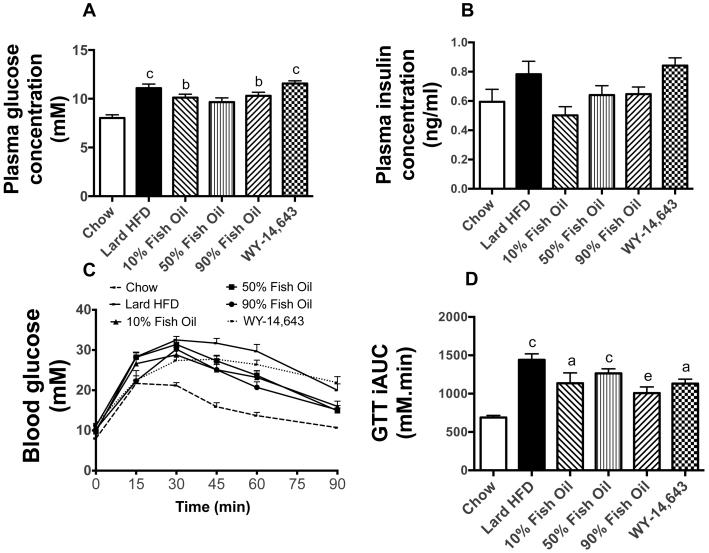
Fasting glucose levels were higher in HFD animals compared to chow controls and were not altered by FO or WY (Figure 1A). Serum insulin levels did not differ significantly amongst the 6 groups (Figure 1B). An intra-peritoneal glucose tolerance test (GTT) revealed a substantial decrease in glucose clearance in mice fed the lard-based HFD (Figure 1C and 1D). This diet-induced glucose intolerance was partly rescued by the inclusion of 90% FO in the diet, but not in the 10%FO and 50%FO groups (Figure 1C and 1D). Supplementation with WY did not protect against the diet-induced deterioration of glucose tolerance.
Figure 1. Glucose metabolism measures in chow, lard-HFD, fish oil-fed and WY-treated mice.
Basal plasma glucose (A) and insulin (B) levels. An intraperitoneal glucose tolerance test (C) was performed in mice that were fasted for 6 hours prior to receiving a glucose bolus (2 g/kg). (D) The incremental area under the curve (iAUC) indicates the animal's ability to clear the glucose load from the circulation. Shown are means ± SEMs, with n = 8–10 mice per group; a,b,c p < 0.05, 0.01, 0.001 vs chow; d,e p < 0.05, 0.01 vs. lard-HFD.
Effects of FO and WY on energy output
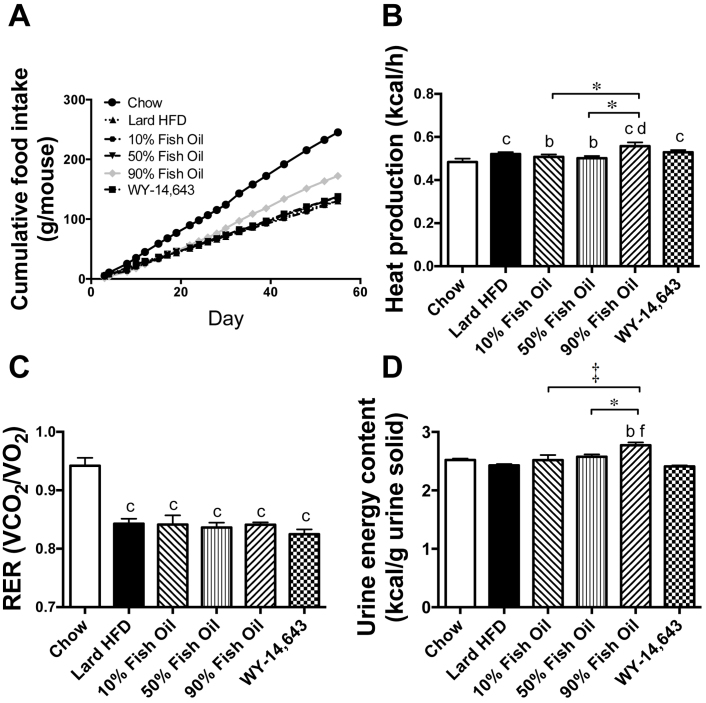
Given the diverse effects of the diets on adiposity and particularly the lipid-lowering effects of the FO, parameters relating to energy balance were investigated. Food intake over the course of the study is shown in Fig. 2A. Chow mice consumed the highest volume of food (4.4 ± 0.5 g/day) and average daily food intake per mouse was found to be similar in the HFD (2.4 ± 0.1 g/day), 10%FO (2.4 ± 0.1 g/day), 50%FO (2.5 ± 0.3 g/day) and WY (2.5 ± 0.1 g/day) groups. However, an accurate measurement of food intake in the 90%FO group was not possible, due to the oily nature of the diet, and the clear deposition of dietary lipid on the inside of the cage and the fur of the animals (i.e food intake was 3.1 ± 0.1 g/day which is likely an overestimate). To examine how the diets affected energy output, we measured whole-body energy expenditure and energy excretion in the urine. Whole-animal energy expenditure of the chow group was lower than all fat-fed groups, while the 90%FO group was significantly higher than the HFD, 10%FO and 50%FO groups (Figure 2B). The ratio of carbohydrate to lipid utilisation (represented by the respiratory exchange ratio (RER)) was significantly higher over the 24 h period in the chow mice compared to the high-fat diet groups, indicating a greater oxidation of carbohydrate as expected (Figure 2C). RER was lower in all high-fat diet groups but unaffected by lipid composition. Urinary energy content was measured by bomb calorimetry and was significantly higher in the 90%FO group in comparison to all other diet groups (Figure 2D).
Figure 2. Food intake, Whole body energy metabolism and urinary energy output in chow, lard-HFD, fish oil-fed and WY-treated mice.
(A) Food intake was measured in every 2–3 days and the average cumulative intake per mouse over the course of the study is shown. Indirect calorimetry was performed in mice and the 24 hr average for energy expenditure (B) and respiratory exchange ratio (C) are presented. Energy output in the urine (D) was assessed by bomb calorimetry. Shown are means ± SEMs, with n = 7–8 per group; b,c p < 0.01, 0.001 vs chow; d,f p < 0.05, 0.001 vs. lard-HFD, *p < 0.05, † p < 0.01, ‡ p < 0.001.
Effects of FO and WY on lipid metabolism in the liver
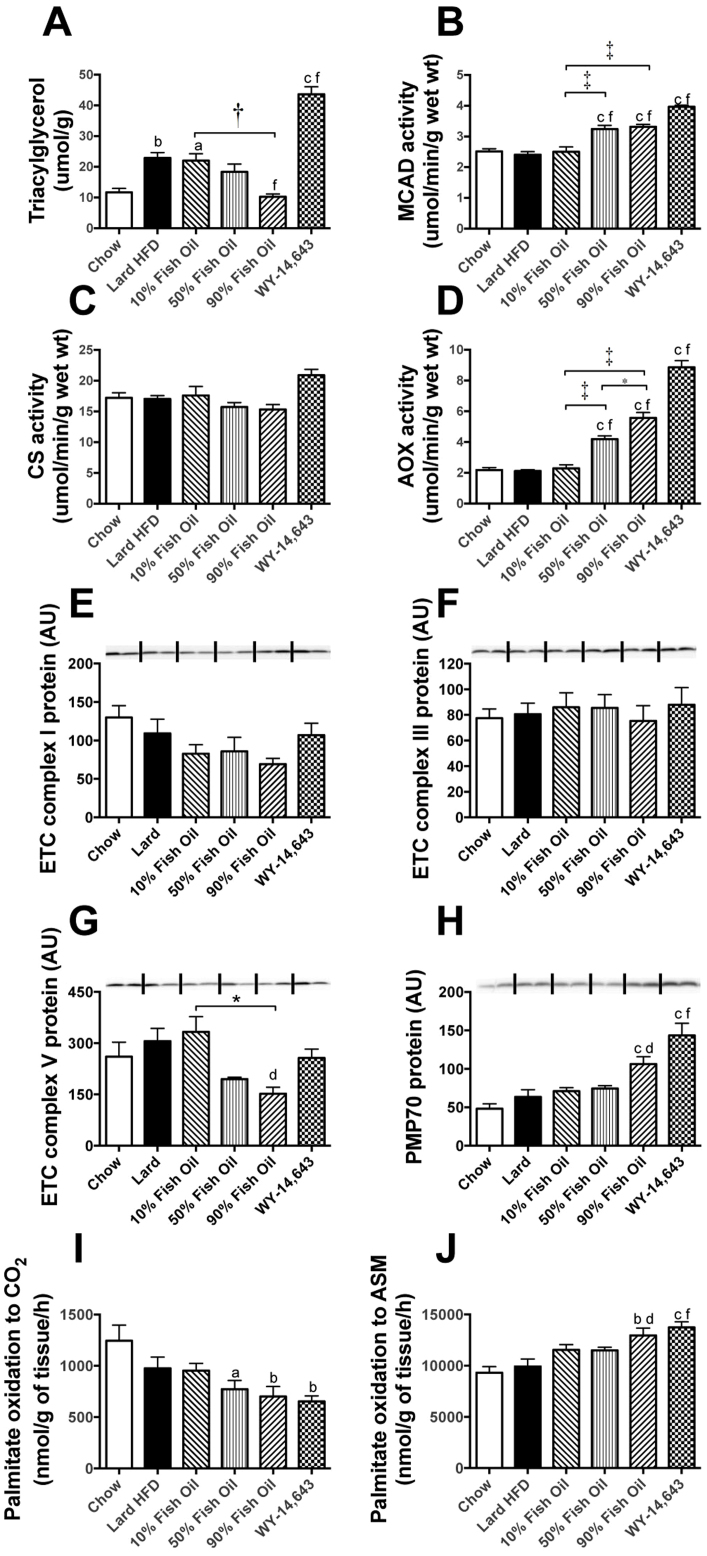
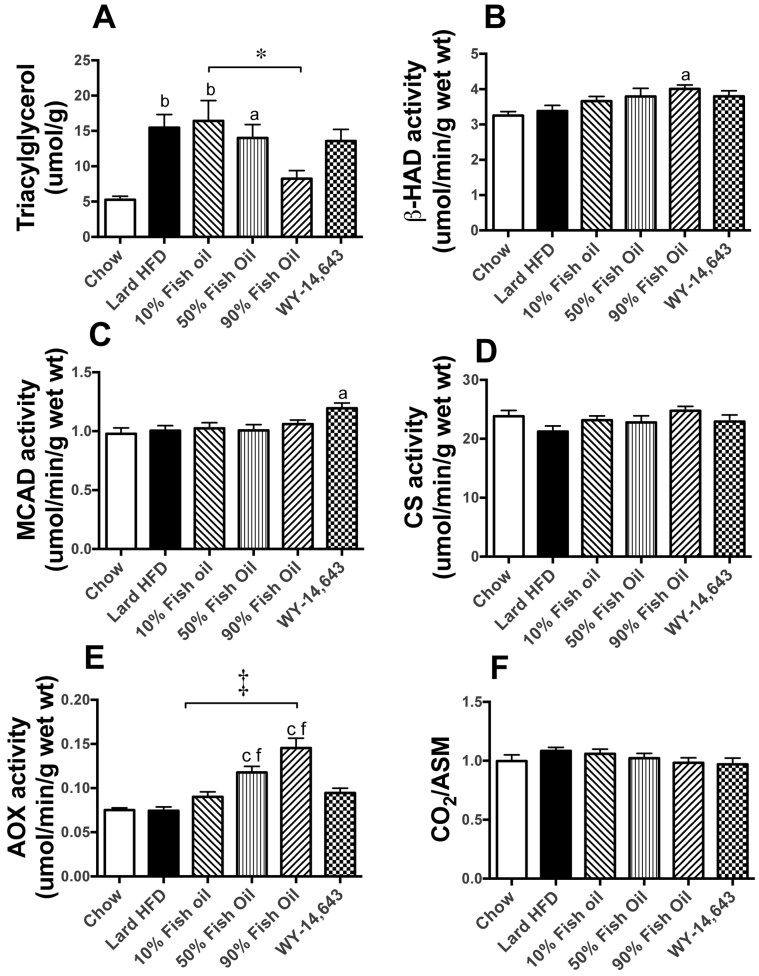
The liver plays an essential role in whole-body lipid metabolism and is thought to be the main site of action of FO and WY due to the high levels of PPARα expression19. HFD-fed mice displayed a significant 2-fold increase in hepatic TAG content compared to LFD mice (Fig. 3A). FO feeding dose-dependently counteracted the accumulation of hepatic fat, while, interestingly, WY treatment induced an exaggeration of the fatty liver phenotype compared to HFD mice (Fig. 3A).
Figure 3. Markers of lipid metabolism in liver.
Triacylglycerol content (A) was used as a measure of lipid accumulation. The activity of medium chain acyl coenzyme A dehydrogenase (MCAD, B), citrate synthase (CS, C) and acyl-CoA oxidase (AOX, D) were determined in liver homogenates as described previously16. Protein levels of oxidative and peroxisomal markers in liver were measured by immunoblotting (E–H) as described in8,53. Oxidation of [1-14C]-palmitic acid to CO2 (I) and acid-soluble metabolites (ASM, J) was assessed in liver homogenates. Shown are means ± SEMs; with n = 8–10 mice per group. Representative immunoblots show n = 2, with densitometry calculated for n = 8–10 per group. a,b,c p < 0.05, 0.01, 0.001 vs chow; d, f p < 0.05, 0.001 vs lard-HFD; * p < 0.05, † p < 0.01, ‡ p < 0.001. Complex I, III and V represent sub-units of the complexes of the electron transport chain (ETC), PMP70 = peroxisomal membrane protein 70. AU – Arbitrary units.
To assess alterations in hepatic FAO capacity, the activities of key enzymes involved in mitochondrial and peroxisomal oxidative pathways were measured. The activity of the mitochondrial β-oxidation enzyme medium-chain acyl-CoA dehydrogenase (MCAD) was significantly increased in the 50%FO and 90%FO groups as well as after WY treatment (35, 38 and 65% respectively compared to HFD) (Fig. 3B). Beta-hydroxy acyl-CoA dehydrogenase (β-HAD) showed a similar, however non-significant trend towards an increased activity in the FO and WY groups (data not shown), whereas the TCA cycle marker citrate synthase (CS) remained unaffected by diet (Figure 3C). The activity of acyl-CoA oxidase (AOX), the rate-limiting enzyme in the peroxisomal β-oxidation pathway, displayed more profound increases, with a 100%, 165% and 320% increase in the 50%FO, 90%FO and WY groups, respectively (Figure 3D). Immunoblotting confirmed the preferential upregulation of peroxisomes over mitochondria, with protein levels of subunits of the complexes of the mitochondrial electron transport chain (ETC) remaining mostly unchanged across all diet groups, while the content of the peroxisomal membrane protein PMP70 was increased markedly by the 90%FO diet and WY treatment (67% and 126%, respectively) (Figure 3E–H).
In addition to enzyme activities and protein levels, the capacity of liver homogenates to oxidise fatty acids (using 14C-palmitate) was measured. The amount of palmitate converted to CO2 (complete oxidation) can be considered a marker for mitochondrial fatty acid oxidative capacity, while the concentration of acid soluble metabolites (ASMs) (end products of incomplete oxidation) provides an index of peroxisomal oxidation. Consistent with a potent upregulation of peroxisomes, but not mitochondria, the 90%FO diet and WY treatment both resulted in a decreased CO2 release (Figure 3I) and a corresponding increase in ASM production (Figure 3J), with the CO2/ASM ratio therefore displaying a step-wise decrease.
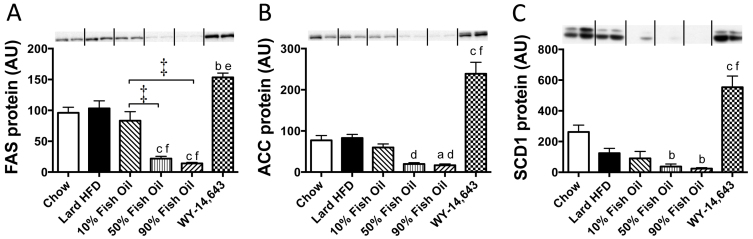
Apart from oxidative capacity, another factor contributing to lipid accumulation in the liver is lipogenesis. The protein levels of acetyl-CoA carboxylase (ACC), fatty acid synthae (FAS) and stearoyl-CoA desaturase (SCD1), as determined by immunoblotting, were downregulated profoundly by FO consumption (Figure 4A–C). In contrast, WY treatment led to substantial elevations in all three lipogenic enzymes (Figure 4A–C), potentially explaining the differences in hepatic lipid content.
Figure 4. Protein levels of fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) and stearoyl-CoA desaturase 1 (SCD1) in liver measured by immunoblotting.
Representative immunoblots show n = 2, with densitometry (means ± SEMs) calculated for n = 8 per group. a,b,c p < 0.05, 0.01, 0.001 vs chow; d, f p < 0.05, 0.001 vs lard-HFD; * p < 0.05, † p < 0.01, ‡ p < 0.001. AU – Arbitrary units.
Effects of FO and WY on lipid metabolism in skeletal muscle
Another major tissue regulating whole-body metabolism and glucose homeostasis is skeletal muscle. Consistent with our observations in the liver, the lard-based HFD led to a significant increase in TAG levels, with FO dose-dependently lowering muscle lipid accumulation. In contrast to the liver, WY supplementation did not potentiate TAG accumulation compared to the HFD group (Figure 5A).
Figure 5. Markers of lipid metabolism in skeletal muscle.
Triacylglycerol content (A) in quadriceps muscle was used as a measure of lipid accumulation. Beta-hydroxyacyl dehydrogenase (β-HAD; B), medium chain acyl coenzyme A dehydrogenase (MCAD, C) citrate synthase (CS, D) and acyl-CoA oxidase (AOX, E) activities were measured in muscle homogenates. Oxidation of [1-14C]-palmitic acid was assessed in muscle homogenates, with the ratio of CO2:acid soluble metabolites (ASM) shown (F). Shown are means ± SEMs; with n = 8–10 mice per group. a,b,c p < 0.05, 0.01, 0.001 vs chow; f, p < 00.001 vs lard; * p < 0.05, ‡ p < 0.001.
In skeletal muscle, lipogenesis has negligible activity and hence the FAO pathway has a major impact on intramuscular lipid levels. There were only minor increases in the activities of the mitochondrial β-oxidation markers β-HAD and MCAD with 90%FO feeding and WY administration (Figure 5B and C), whereas CS remained unchanged across diet groups (Figure 5D). The activity of AOX was increased in the 50%FO and 90%FO groups, but not by WY treatment (Figure 5E). In muscle homogenates, neither CO2 nor ASM production were significantly altered, suggesting minimal effect of the dietary treatments on fatty acid oxidative pathways in the tibialis muscle (Figure 5F).
Discussion
Diet-induced obesity and ectopic lipid accumulation in non-adipose tissues are strong pre-disposing factors for the development of IR and T2D38. Previous research has indicated that unlike most types of lipids, the consumption of FO, rich in n-3 PUFAs, counteracts the development of obesity and the deterioration of insulin sensitivity and glucose homeostasis15,16. The results of the current study were consistent with the many reports of protective effects of FO on whole-body and tissue-specific lipid metabolism11,14,16,17,18,19,20,21, with FO-enriched diets resulting in less adipose mass, lower systemic lipid abundance and less TAG accumulation in skeletal muscle and liver. FO-enriched diets also partially protected from lipid-induced glucose intolerance. These effects were most pronounced in the 90%FO group, with only minor effects observed with the lowest dose of dietary FO.
The protective effects of FO have been suggested to be dependent on the activation of hepatic PPARα and the resulting augmentation of peroxisomal FAO16,19,20. Indeed, we observed dose-dependent induction of several peroxisomal markers in the liver of mice provided with dietary FO. To contrast the effects of FO with direct PPARα activation, a group of animals on the HFD received WY for 4 weeks. The effectiveness of WY administration was evident by the induction of liver hypertrophy, lowering of circulating lipids and potent upregulation of several peroxisomal markers. The addition of WY to a lard-based HFD, however, offered little protection against lipid-induced obesity and glucose intolerance.
The findings regarding PPARα activation and metabolic parameters have been inconsistent. Ye et al (2001) observed that one-week administration of WY to 3-week HFD-fed rats resulted in significant reductions in visceral fat weight and intramuscular TAG accumulation, accompanied by improvements in insulin sensitivity31. Similar results were observed in rats in a different study where the lowering of hepatic TAG content and marked induction of a peroxisomal oxidative enzyme were also noted32. In addition, a further study reported that PPARα activation by three other synthetic PPARα agonists diminished whole-body adiposity and preserved insulin action in both diet-induced and genetic obese rodent models30. On the other hand, transgenic over-expression of PPARα has been documented to cause metabolic defects and the genetic ablation of PPARα has been reported to preserve insulin sensitivity under high-fat feeding conditions, without reducing adiposity34,35,36,37. These studies differ in a number of aspects including rodent species, genetic background, feeding duration, specific agonist employed, dosage of agonist, fat content and in particular fatty acid profile of the high-fat diet, with all of these potentially being contributing factors to the disparate results observed.
Contrasting effects of FO and WY were observed in liver, where ectopic storage of lipids has been linked with reduced insulin sensitivity and impaired glucose tolerance. While the 50%FO and 90%FO diets resulted in lower levels of liver TAG accumulation, administration of WY strikingly exaggerated hepatic steatosis on top of the lard-based high-fat diet. Both FO and WY increased lipid oxidation pathways, but analysis of enzymes involved in de novo lipogenesis showed that while the 50%FO and 90%FO diets downregulated the expression of ACC, FAS and SCD-1, WY resulted in marked elevations of these lipogenic enzymes. Collectively these findings suggest that increased hepatic lipid synthesis may be largely responsible for the excess TAG accumulation in the liver observed with WY, which in turn leads to deterioration of whole-body metabolic parameters. It is well documented that n-3 PUFAs suppress lipid synthesis pathways in the liver, through reductions in both the mRNA level of the pro-lipogenic transcription factor, sterol response element binding protein-1 (SREBP-1), and the nuclear abundance of the mature (active) form of SREBP139,40,41,42. Importantly, these effects of n-3 PUFAs have been shown to be independent of PPARα, as the suppression of SREBP1 and lipogenic gene expression by FO still occurs in liver of PPARα knockout mice40,41. In contrast to the effects of FO, other studies have reported that pharmacological PPARα activation promotes the expression of lipogenic enzymes43,44,45, and the findings of the current study, as well as those in a recent report comparing the PPARα agonist fenofibrate and FO, demonstrate that n-3 PUFA and PPARα activating compounds have opposing effects on a number of biosynthetic pathways, including FA synthesis46. Thus the lipid-lowering effect of FO in liver is not solely due to acceleration of fatty acid oxidation by PPARα activation and is likely also due to the influence of n-3 PUFAs on other signalling pathways. Furthermore, our results highlight that the composition of dietary lipid is an important consideration for studies using PPARα agonists, as the lard-based high fat diet in the current study unmasked a pro-lipogenic effect for WY in vivo, which has not been observed in studies where the high fat diet was rich in n-6 PUFAs31,32, which are a class of FA that also downregulate SREBP-1 levels39.
Besides profound effects on lipid accumulation in the liver, FO also reduced TAG content in skeletal muscle. In agreement with a previous report by Ballie et al47, we observed that the activity of AOX, which catalyses the rate-limiting step of peroxisomal β-oxidation, was elevated by the 50%FO and 90%FO diets. Given the scarcity of PPARα in skeletal muscle, the increase in AOX activity is probably due to the activation of PPARδ, the most abundant PPAR in skeletal muscle, which can be activated by n-3 PUFAs48 and has been shown to have overlapping functions with PPARα, including the upregulation of AOX expression49. Nonetheless, since peroxisomal oxidative capacity is more than one magnitude lower than mitochondrial oxidative capacity in skeletal muscle (based on maximal enzyme activities measured in the current study, Figure 5) and is much lower than peroxisomal capacity in liver, the extent to which decreases in myocellular TAG can be explained by increased peroxisomal FA oxidation requires further exploration.
At the whole body level, high doses of FO diets resulted in lower adiposity despite similar food intake, suggesting alterations on the output side of the energy balance equation. Increased energy expenditure was observed in mice provided with the highest dose of FO. This is in contrast to recent work from our group, where we observed lower whole-body oxygen consumption in Swiss mice provided with a different high FO diet, in which 60% of energy is derived from FO and soybean oil at a 90:10 ratio16. The reason for the discrepancy between mouse strains is not clear, but at least in C57BL/6 mice, part of the adipose lowering effects of high FO are related to enhanced energy expenditure. Most of the peroxisomal oxidative intermediates (e.g. acetate) are water-soluble and require transfer to the mitochondria for oxidation. Thus, we hypothesised that under conditions of increased lipid flux through peroxisomes, as would occur with diets enriched in FO, there may be spillover of lipid-derived carbon units into the bloodstream and excretion in urine, providing another avenue for energy output. Indeed there was a stepwise increase in urinary energy content with increasing doses of FO, with significantly higher excretion of energy observed in 90%FO-fed mice. Despite more pronounced upregulation of the peroxisomal machinery in WY-treated mice, we observed no elevation in urinary energy content, which may be a reflection of the fact that the majority of fatty acid species in the lard-based diet are not normally processed in peroxisomes26. Excretion of glucose in the urine is currently used as a treatment for diabetes50, and disposal of excess calories via the urine may represent a novel approach for enhancing weight loss51.
In conclusion, the results of the current study indicate that in contrast to the beneficial effects of FO feeding, the addition of the synthetic PPARα agonist WY to a lard-based high-fat diet resulted in negligible protection against fat-induced obesity and glucose intolerance, but rather amplified hepatic steatosis, despite profound augmentation of the peroxisomal oxidative system in the liver. The negative effects of WY were attributed to the marked induction of hepatic lipogenic pathways and potentially a lack of suitable substrates that can be utilised in the peroxisomal β-oxidation pathway. This study highlights the difference between nutritional and pharmacological interventions and suggests that the metabolic benefits are greater when the catabolic and anabolic pathways are co-ordinately regulated, as occurred with the high FO diets.
Methods
Research design
Male 9–12 weeks old C57BL6/J mice were obtained from the Australian BioResources Centre (Moss Vale, Australia). They were housed at 22 ± 1°C with a controlled 12:12 h light-dark cycle and had ad libitium access to food and water. Weight-matched mice were allocated to receive either a standard chow low-fat diet (LFD) (71, 8 and 21% calories derived from carbohydrate, fat and protein, respectively; Gordon's Specialty Stock Feeds, Yanderra, Australia) (n = 8) or high fat diets (n = 10 per group) containing 45% of calories as fat (based on rodent diet no. D12451, Research Diets, USA), with the lipid components consisting of 12% safflower oil and 88% lard, or FO (Healthmax® Fish Oil, Priceline Pharmacy, Australia, DHA:EPA 12:18) and lard at ratios of 10:90, 50:50 and 90:10 for 8 weeks. The FA profiles of each diet were determined by gas chromatography as described in52 and are shown in Table 1, with the PUFA balance52 showing a stepwise increase from 0.07 in the HFD (mainly comprised of n-6 PUFA) to 1.95 in the 90%FO diet (29% n-3 PUFA,). A separate group of 8 mice were fed the same lard-based high-fat diet for 4 weeks and the same diet in which the PPARα agonist WY was added at a dose resulting in an average intake of 2.3 mg/kg body weight/day for another 4 weeks. Blood and tissue samples were collected at 9–10 am without prior fasting period unless otherwise indicated. All experiments were carried out with the approval of the Garvan Institute/St Vincent's Hospital Animal Experimentation Ethics Committee and were in compliance with code of practice for the care and use of animals for scientific purposes set by the National Health and Medical Research Council of Australia.
Body composition and circulating lipids
Lean and fat mass were measured using a Piximus2 Dual Energy X-ray Absorptiometry (DEXA) scanner according to the manufacturer's instructions (GE Lunar, Madison, USA). Non-esterified fatty acids (NEFA) and TAG were determined using the NEFA-C kit (Wako Pure Chemical Industries, Japan) and TAG kit (Roche, Switzerland), respectively, as described previously32.
Glucose tolerance test and insulin levels
Mice were fasted for 6 hours before receiving intraperitoneal injections of glucose (2 g/kg). Blood glucose levels were monitored at the 0, 15, 30, 45, 60 and 90 min following glucose injection using a Glucometer (Accu-check II, Roche Diagnostics, Australia). Plasma insulin levels were determined by a sensitive rodent insulin radioimmunoassay kit (Millipore, Missouri, USA).
Energy output
Energy content of the urine was determined using a bomb calorimeter (Model 1356, PARR Instrument Company, USA) using benzoic acid as standard. A 24-hour urine collection was carried out by housing the animals in metabolic cages (Tecniplast, Australia) and urine was stored at −20°C. In addition, urine was collected three times after fresh urination from each mouse and one final sample was collected from the bladder at the time of culling. Frozen urine samples (2 ml) were freeze-dried (Telstar Cryodos −80°C freeze dryer, Progen Scientific, UK) without prior evaporation to obtain urinary solid before combustion. Whole-body oxygen consumption rate (VO2) and respiratory exchange ratio (RER) of individual mice were measured using an eight-chambered indirect calorimeter (Oxymax series; Columbus Instruments, Columbus, OH) as previously described53.
Tissue triacylglycerol content
Lipids were extracted from liver or quadriceps muscle and triglyceride content was determined using a colorimetric assay kit (Triglycerides GPO-PAP; Roche Diagnostics, Indianapolis, IN) as previously described32.
Palmitate oxidation
Palmitate oxidation was measured in liver and tibialis muscle homogenates as described in detail previously16. Briefly, tissue homogenates were incubated in assay medium containing 0.2 mM palmitate and 0.2 μCi [1-14C]-palmitic acid. After 90 min of incubation at 30°C, the reaction was terminated via the addition of 1 M perchloric acid and the CO2 released was absorbed in 1 M NaOH solution over 2 hours. The CO2 and acid soluble metabolites (ASM) produced were quantified by counting 14C content in the NaOH solution and acidified assay buffer supernatant, respectively.
Enzyme activity measurements
Liver and quadriceps muscle were manually homogenized 1:19 (wt/vol) in 50 mM Tris-HCl, 1 mM EDTA and 0.1% Triton X-100, pH 7.2 and the homogenates centrifuged for 10 min at 5,000 g. Supernatants were used to determine the activity of β-hydroxyacyl CoA dehydrogenase (βHAD), medium chain acyl-CoA dehydrogenase (MCAD), citrate synthase (CS) and peroxisomal acyl-CoA oxidase (AOX) activity at 30°C, as described previously16 using a temperature-controlled plate-reader (Spectra Max 300, Molecular Devices, Sunnyvale, CA).
SDS-PAGE and Immunoblotting
Liver and quadriceps muscle were homogenised in RIPA buffer, solubilised for 2 h at 4°C, centrifuged for 10 min at 13,000 g at 4°C. Protein concentration of the supernatant was quantified using the Bio-Rad protein assay kit (Bio-Rad, USA). Equal amounts of samples were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were then cut into 4 or 5 individual strips for immunoblotting of specific proteins using procedures and antibodies described previously8,16,53.
Statistical analysis
All results are expressed as means ± standard error of the mean (SEM). Results were analysed by one-way ANOVA and a Bonferroni post-hoc test was conducted to identify differences between groups. For energy expenditure results, an ANCOVA analysis was applied using [lean mass + (0.2 × fat mass)] as a covariate54. Differences with p < 0.05 were deemed statistically significant.
Author Contributions
M.L. performed experiments and wrote the manuscript. M.K.M., C.E.F., B.O. and G.J.C. performed experiments and edited the manuscript. K.B.A. interpreted and analysed data and edited the manuscript. N.T. conceived the study, performed experiments and wrote the manuscript.
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council of Australia (NHMRC). ML is supported by a University International Postgraduate Award from the University of New South Wales. BO is supported by a NHMRC PhD scholarship. MKM and GJC are supported by NHMRC research fellowships and NT is supported by an Australian Research Council Future Fellowship. We thank the Biological Testing Facility at the Garvan Institute (Sydney, Australia) for assistance with animal care.
References
- Kelly T., Yang W., Chen C. S., Reynolds K. & He J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 32, 1431–1437 (2008). [DOI] [PubMed] [Google Scholar]
- Chen L., Magliano D. J. & Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat. Rev. Endocrinol. 8, 228–236 (2012). [DOI] [PubMed] [Google Scholar]
- Savage D. B., Petersen K. F. & Shulman G. I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87, 507–520 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V. T., Petersen K. F. & Shulman G. I. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien L. H. et al. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40, 280–289 (1991). [DOI] [PubMed] [Google Scholar]
- Hill J. O. et al. Lipid-accumulation and body-fat distribution is influenced by type of dietary-fat fed to rats. Int. J. Obes. 17, 223–236 (1993). [PubMed] [Google Scholar]
- Ikemoto S. et al. High-fat diet-induced hyperglycemia and obesity in mice: Differential effects of dietary oils. Metabolism 45, 1539–1546 (1996). [DOI] [PubMed] [Google Scholar]
- Turner N. et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes 58, 2547–2554 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudyal H., Panchal S. K., Diwan V. & Brown L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 50, 372–387 (2011). [DOI] [PubMed] [Google Scholar]
- Buettner R. et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 36, 485–501 (2006). [DOI] [PubMed] [Google Scholar]
- Levy J. R., Clore J. N. & Stevens W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology 39, 608–616 (2004). [DOI] [PubMed] [Google Scholar]
- Samane S. et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metabolism 58, 909–919 (2009). [DOI] [PubMed] [Google Scholar]
- Kim J.-Y. et al. High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R2057–R2065 (2000). [DOI] [PubMed] [Google Scholar]
- Jelenik T. et al. AMP-activated protein kinase α2 subunit Is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids. Diabetes 59, 2737–2746 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien L. H. et al. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237, 885–888 (1987). [DOI] [PubMed] [Google Scholar]
- Fiamoncini J. et al. Enhanced peroxisomal β-oxidation is associated with prevention of obesity and glucose intolerance by fish oil-enriched diets. Obesity 21, 1200–1207 (2013). [DOI] [PubMed] [Google Scholar]
- Rossmeisl M. et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta – Mol. Cell Biol. Lipid 1841, 267–278 (2014). [DOI] [PubMed] [Google Scholar]
- Kim H.-J., Takahashi M. & Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. J. Biol. Chem. 274, 25892–25898 (1999). [DOI] [PubMed] [Google Scholar]
- Neschen S. et al. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am. J. Physiol. Endocrinol. Metab. 282, E395–E401 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neschen S. et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator–activated receptor-α–dependent manner. Diabetes 56, 1034–1041 (2007). [DOI] [PubMed] [Google Scholar]
- Ruiz-Gutiérrez V., Pérez-Espinosa A., Vázquez C. M. & Santa-María C. Effects of dietary fats (fish, olive and high-oleic-acid sunflower oils) on lipid composition and antioxidant enzymes in rat liver. Br. J. Nutr. 82, 233–241 (1999). [PubMed] [Google Scholar]
- Jucker B. M., Cline G. W., Barucci N. & Schulman G. I. Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: A (13 C) nuclear magnetic resonance study. Diabetes 48, 134–140 (1999). [DOI] [PubMed] [Google Scholar]
- Fedor D. & Kelley D. S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 12, 138–146 (2009). [DOI] [PubMed] [Google Scholar]
- Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53, S43–S50 (2004). [DOI] [PubMed] [Google Scholar]
- Wanders R. J. A. Peroxisomes, lipid metabolism, and peroxisomal disorders. Mol. Genet. Metab. 83, 16–27 (2004). [DOI] [PubMed] [Google Scholar]
- Wanders R. J. A. & Waterham H. R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332 (2006). [DOI] [PubMed] [Google Scholar]
- Neschen S. et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator–activated receptor-γ–dependent mechanism in mice. Diabetes 55, 924–928 (2006). [DOI] [PubMed] [Google Scholar]
- Oh D. Y. et al. GPR120 is an Omega-3 atty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Arita M., Taguchi R., Kang J. X. & Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat–fed mice. Diabetes 59, 3066–3073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre-Millo M. et al. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 275, 16638–16642 (2000). [DOI] [PubMed] [Google Scholar]
- Ye J.-M. et al. Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats. Diabetes 50, 411–417 (2001). [DOI] [PubMed] [Google Scholar]
- Ye J.-M. et al. PPARα/γ ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am. J. Physiol. Endocrinol. Metab. 284, E531–E540 (2003). [DOI] [PubMed] [Google Scholar]
- Chan S. M. et al. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 62, 2095–2105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre-Millo M. et al. PPAR-α–null mice are protected from high-fat diet–induced insulin resistance. Diabetes 50, 2809–2814 (2001). [DOI] [PubMed] [Google Scholar]
- Tordjman K. et al. PPARα deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J. Clin. Invest. 107, 1025–1034 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck B. N. et al. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell. Metab. 1, 133–144 (2005). [DOI] [PubMed] [Google Scholar]
- Finck B. N. et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109, 121–130 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V. & Shulman G. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. D. Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br. J. Nutr. 83, 59–66 (2000). [DOI] [PubMed] [Google Scholar]
- Mater M. K., Thelen A. P., Pan D. A. & Jump D. B. Sterol response element-binding protein 1c (SREBP1c) is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. J. Biol. Chem. 274, 32725–32732 (1999). [DOI] [PubMed] [Google Scholar]
- Ren B., Thelen A. P., Peters J. M., Gonzalez F. J. & Jump D. B. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 272, 26827–26832 (1997). [DOI] [PubMed] [Google Scholar]
- Xu J., Cho H., O'Malley S., Park J. H. & Clarke S. D. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanisms. J. Nutr. 132, 3333–3339 (2002). [DOI] [PubMed] [Google Scholar]
- Oosterveer M. H. et al. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. J. Biol. Chem. 284, 34036–34044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. L. et al. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem. J. 389, 413–421 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshandehroo M. et al. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007, 26839 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Boekschoten M. V., Wopereis S., Müller M. & Kersten S. Comparative transcriptomic and metabolomic analysis of fenofibrate and fish oil treatments in mice. Physiol. Genomics 43, 1307–1318 (2011). [DOI] [PubMed] [Google Scholar]
- Baillie R. A., Takada R., Nakamura M. & Clarke S. D. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostag. Leukotr. Ess. 60, 351–356 (1999). [DOI] [PubMed] [Google Scholar]
- de Lange P. et al. Peroxisome proliferator-activated receptor delta: A conserved director of lipid homeostasis through regulation of the oxidative capacity of muscle. PPAR Res. 2008, 172676 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-X. et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113, 159–170 (2003). [DOI] [PubMed] [Google Scholar]
- Vasilakou D. et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 159, 262–274 (2013). [DOI] [PubMed] [Google Scholar]
- Barnett A. H. Impact of sodium glucose cotransporter 2 inhibitors on weight in patients with type 2 diabetes mellitus. Postgrad. Med. 125, 92–100 (2013). [DOI] [PubMed] [Google Scholar]
- Turner N., Mitchell T. W., Else P. L. & Hulbert A. J. The omega-3 and omega-6 fats in meals: a proposal for a simple new label. Nutrition 27, 719–726 (2011). [DOI] [PubMed] [Google Scholar]
- Montgomery M. K. et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56, 1129–1139 (2013). [DOI] [PubMed] [Google Scholar]
- Even P. C. & Nadkarni N. A. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R459–476 (2012). [DOI] [PubMed] [Google Scholar]