Abstract
Background
Radioiodine is routinely used or proposed for diagnostic and therapeutic purposes: 123I, 125I and 131I for diagnostics and 125I and 131I for therapy. When radioiodine-labelled pharmaceuticals are administered to the body, radioiodide might be released into the circulation and taken up by the thyroid gland, which may then be an organ at risk. The aim of this study was to compare dosimetric properties for 123I, 125I and 131I in previously developed thyroid models for man, rat and mouse.
Methods
Dosimetric calculations were performed using the Monte Carlo code MCNPX 2.6.0 and nuclear decay data from ICRP 107. Only the non-radiative transitions in the decays were considered. The S value was determined for the cell nuclei in species-specific thyroid follicle models for mouse, rat and man for different spatial distributions of radioiodine.
Results
For the species-specific single follicle models with radioiodine homogeneously within the follicle lumen, the highest S value came from 131I, with the largest contribution from the β particles. When radioiodine was homogeneously distributed within the follicle cells or the follicle cell nucleus, the highest contribution originated from 125I, about two times higher than 123I, with the largest contribution from the Auger electrons. The mean absorbed dose calculated for our human thyroid multiple follicle model, assuming homogenous distribution of for 123I, 125I, or 131I within the follicle lumens and follicle cells, was 9%, 18% and 4% higher, respectively, compared with the mean absorbed dose according to Medical Internal Radiation Dose (MIRD) formalism and nuclear decay data. When radioiodine was homogeneously distributed in the follicle lumens, our calculations gave up to 90% lower mean absorbed dose for 125I compared to MIRD (20% lower for 123I, and 2% lower for 131I).
Conclusions
This study clearly demonstrates the importance of using more detailed dosimetric methods and models than MIRD formalism for radioiodine, especially 123I and 125I, in the thyroid. For radioiodine homogeneously distributed in the follicle lumens our calculations for the human multiple follicle models gave up to 90% lower mean absorbed dose compared with MIRD formalism.
Keywords: Monte Carlo, MCNPX, S value, MIRD, Man, Rat, Mouse, Thyroid gland
Background
Radioiodine has been used clinically for about 70 years, both for diagnosis (123I, 125I and 131I) and therapy (125I and 131I) of various diseases. Radioiodine is to a high extent accumulated in the thyroid gland and metabolically built into the thyroid hormones, and the biokinetics of radioiodine is used for measurement of thyroid function [[1]]. 131I, as iodide, is therefore routinely used for diagnosis and treatment of various thyroid disorders such as thyrotoxicosis (hyperthyroidism) and thyroid cancer [[1]-[3]]. Lately, 131I has sometimes been replaced by 123I as iodide for diagnostic purposes to avoid the thyroid stunning phenomenon [[4],[5]]. Radioiodine bound to specific vector molecules is also widely used. For example, 131I- and 123I-MIBG are used for scintigraphy and 131I- and 125I-MIBG for treatment of various neuroendocrine tumours [[6],[7]], 123I-receptor ligands for brain scintigraphy [[8]], 125I methylene blue for sentinel node localization [[9]] and 131I-labelled monoclonal antibodies for treatment of lymphoma [[10]]. When, radioiodine labelled pharmaceuticals are administered to the body, radioiodide might be released into the circulation, e.g. by enzymatic reactions by dehalogenases in tissues, and taken up by the thyroid gland [[11]]. For radiopharmaceuticals or tracers labelled with radioiodine, the thyroid is thus an organ at risk.
Furthermore, 131I is a radionuclide of importance in nuclear accidents, where it is a rest product from the nuclear fission process in nuclear energy plants. After the Chernobyl accident in 1986, contamination with 131I (and other short-lived isotopes such as 132I and 133I) led to an increased incidence of differentiated thyroid cancers in children but not in adults, with a higher incidence with lower age [[12]–[15]].
There is thus a need for accurate dosimetric calculations of the absorbed dose for both patients examined or treated with radioiodine, personnel handling radioiodine and for personnel and the general population in case of accidental exposure to radioiodine.
Dosimetric estimations using the MIRD formalism is mostly utilised due to its simplicity, and the use of mean absorbed dose is of interest if the radionuclides and the energy deposited are homogeneously distributed within each organ/tissue. This assumption is adequate as long as the range of the emitted particles is long compared to the size of the cells. For radionuclides emitting particles with shorter range, e.g. Auger and internal conversion electrons, non-uniform distribution within an organ/tissue will give heterogeneous absorbed dose distribution, and more detailed dosimetric approaches are clearly needed.
The physical properties differ between these radioiodine isotopes. For 131I, emitting relatively high-energy β particles with a range up to 2 mm in tissue [[16]], the energy distribution will be relatively homogeneous within the thyroid, less dependent of the radionuclide distribution, while for 125I, emitting cascades of Auger electrons with ranges from a few nanometres up to around 23 μm in tissue, the energy deposition within the thyroid gland will be more heterogeneous, dependent of the distribution of the radionuclide, electrons emitted, half-life, and the amount of photons emitted [[17],[18]].
To be able to determine more detailed dosimetric parameters for the thyroid cells from heterogeneously distributed radioiodine isotopes in the thyroid tissue, thyroid tissue models are needed. We have recently published thyroid models for man, but also for mouse and rat, and performed microdosimetric studies of the α particle emitting radiohalogen 211At, demonstrating the importance of detailed dosimetry for the thyroid [[19]]. A few thyroid models for radioiodine dosimetry have been previously published for normal and thyrotoxic thyroid follicles [[20]-[24]]. To our knowledge, few dosimetric studies have been published demonstrating the dosimetric properties of these radioiodine nuclides in these models.
The aim of this study was to compare dosimetric calculations for 123I, 125I and 131I using the general purpose Monte Carlo radiation transport code MCNPX 2.6.0 [[25]] with nuclear decay data from ICRP 107 [[26]] and the recently developed thyroid models for man, rat and mouse [[19]].
Methods
The radioiodine isotopes 123I, 125I and 131I
Nuclear decay data from ICRP 107 for 123I, 125I and 131I were used in all the dosimetric calculations, if not stated otherwise [[26]]. Only the non-radiative (NR) transitions from Auger and Coster-Kronig electrons (denoted AE in the rest of the paper), internal conversion electrons (CE) and β particles were considered. The conventional AE spectrum was used, and AE with initial kinetic energies <1 keV were assumed to be fully absorbed within the source volume.
123I has a half-life of 13 h and decays by electron capture (EC) via 123Te (half-life 6.0 × 1014 years and yield 99.9996%) or 123mTe (half-life 119 days and yield 4.4 × 10-5%) to stable 123Sb. 123I emits CE with 246 possible initial kinetic energies and on average 14 AE per decay. The mean emitted energy per decay for AE with initial kinetic energies <1 keV is 1.25 keV [[26]].
125I has a half-life of 59 days and decays by EC to stable 125Te. 125I emits CE with six possible initial kinetic energies and on average 23 AE per decay. The mean emitted energy per decay for AE with initial kinetic energies <1 keV is 2.11 keV [[26]].
131I has a half-life of 8.0 days and decays by emission of β particles directly or via 131mXe (half-life 11.8 days and yield 1.18%) to stable 131Xe. The full energy spectrum for the emission of β particles was considered, including all six independent transition spectra. 131I emits CE with 108 possible initial kinetic energies and on average 0.7 AE per decay. The mean emitted energy per decay for AE with initial kinetic energies <1 keV is 78.3 eV [[26]]. The contribution from 131mXe was not considered in the dosimetric calculations.
Monte Carlo calculations
Calculations were performed using the general purpose Monte Carlo radiation transport code MCNPX 2.6.0 [[25]] on a MacBook Pro with a 2.66 GHz Intel Core 2 Duo processor with Mac OS X version 10.6.8 (Apple Incorporated, Cupertino, CA, USA). The tally *F8 was used, which gives the energy imparted to the target volume. The default settings in MCNPX were used for the sampling frequency, and the cutoff energy was 1 keV. For each geometric setup, 1 to 5 × 107 histories were simulated, depending on geometry, distribution and NR transition. The default particle transport physics were used in the electron transport calculations, which included multiple scattering, straggling for electron energy loss and generation of secondary electrons [[27]].
Single thyroid follicle model
The single thyroid follicle model used has previously been described [[19]]. Briefly, the model consists of a single layer of thyroid follicular cells surrounding a spherical follicle lumen (diameter 10 to 500 μm). The follicle cells have a thickness of 6, 8 or 10 μm with a centrally located spherical nucleus with diameters of 4, 6 and 8 μm, respectively. For the species-specific models, the follicle lumen diameter, follicle cell thickness and nucleus diameter are: (1) 50, 6 and 4 μm for mouse, (2) 70, 8 and 6 μm for rat and (3) 150, 10 and 8 μm for man, respectively. All the follicle models were assumed to consist of liquid water with unit density (1.0 g/cm3). The radioiodine distributions investigated were (A) homogeneous distribution within the follicle lumen, (B) homogeneous distribution on concentric spherical surfaces in the follicle lumens, (C) homogeneous distribution within the follicle cells and (D) homogeneous distribution within the follicle cell nuclei. In all simulations, the targets were the six follicular cell nuclei symmetrically positioned on the Cartesian axes, and the result was the average value for these targets (cf. [[19]]).
Multiple thyroid follicle models
A multiple thyroid follicle model was used to calculate the contribution from surrounding layers of follicles to the follicle cell nuclei in a centrally placed follicle, based on the previously published multiple follicle model [[19]]. Calculations were performed for the models of mouse, rat and man. The neighbouring follicles were modelled as one surrounding layer of follicle cells, one outer layer simulating the follicle lumens with the respective thickness: (1) 6 and 50 μm for the mouse, (2) 8 and 70 μm for the rat and (3) 10 and 150 μm for the human model and another surrounding layer of follicle cells. The number of surrounding follicle layers that contributed depended on the species and radioiodine isotopes and was 2, 1 and 8 for 123I, 125I and 131I, respectively, in the human model.
In this model, two radioiodine distributions were used: (E) homogeneous distribution within the surrounding follicle lumens and (F) homogeneous distribution within the surrounding follicle cells. The targets were the six follicle cell nuclei in the central follicle, similar to the single follicle model (cf. [[19]]).
Dosimetric parameters
MIRD formalism was used to calculate the mean absorbed dose, D(r T ), using the expression
| (1) |
where is the time-integrated activity (according to MIRD pamphlet no. 21, previously named cumulated activity in MIRD primer), in the source volume in units of Bq⋅s, M(r T ) is the mass of the target volume in units of kilogrammes [[28],[29]]. E i ⋅ Y i , is the mean electron energy per transformation (26.7, 16.5 and 191.2 keV for 123I, 125I and 131I, respectively according to web published MIRD decay data [[30]]), and ϕ(r T ← r S ) is the absorbed fraction in the target volume for the emitted electrons per nuclear transformation, and was assumed to be unity for 123I, 125I and 131I in the thyroid gland of man.
The cumulative specific activity, , is expressed as
| (2) |
To achieve a calculated mean absorbed dose of 1 Gy to the thyroid gland according to MIRD formalism (Equation 1), a equal to 234, 378 and 32.6 TBq⋅s/kg was needed for 123I, 125I and 131I, respectively.
The mean absorbed dose can also be calculated using the expression
| (3) |
where S(r T ← r S ) is the mean absorbed dose per unit cumulated activity in the source volume in units of Gy/Bq⋅s [[29]] and will be denoted as the S value in the continuation of the text.
Results
Single follicle model
Radioiodine in species-specific follicle thyroid models
For the mouse, rat and human models, the calculated S values with radioiodine homogeneously distributed within the follicle lumen are shown in Table 1. For the mouse model, the S value was 280% and 110% higher for 131I than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (88%) and a similar contribution from the AE and CE for 123I and 125I. For the rat model, the S value was 340% and 230% higher for 131I than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (90%) and from the CE for 123I (63%) and 125I (55%). For the human model, the S value was 310% and 650% higher for 131I than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (93%) and from the CE for 123I (87%) and 125I (60%).
Table 1.
S values for radioiodine in the species-specific single follicle models
| |
β particles |
CE |
AE |
Total |
β particles |
CE |
AE |
|---|---|---|---|---|---|---|---|
| |
S
value |
Relative contribution |
S
value |
Relative contribution |
S
value |
Relative contribution |
S
value |
| (Gy/Bq·s) | (%) | (Gy/Bq·s) | (%) | (Gy/Bq·s) | (%) | (Gy/Bq·s) | |
| Nucleus ← Lumen | |||||||
| Mouse |
|
|
|
|
|
|
|
| 123I |
- |
- |
1.23E-6 |
47.9 |
1.33E-6 |
52.1 |
2.56E-6 |
| 125I |
- |
- |
2.49E-6 |
53.6 |
2.16E-6 |
46.4 |
4.66E-6 |
| 131I |
8.56E-6 |
88.2 |
1.07E-6 |
11.0 |
7.74E-8 |
0.8 |
9.71E-6 |
| Rat |
|
|
|
|
|
|
|
| 123I |
- |
- |
6.81E-7 |
62.7 |
4.06E-7 |
37.3 |
1.09E-6 |
| 125I |
- |
- |
8.05E-7 |
55.1 |
6.57E-7 |
44.9 |
1.46E-6 |
| 131I |
4.27E-6 |
89.8 |
4.63E-7 |
9.7 |
2.38E-8 |
0.5 |
4.75E-6 |
| Man |
|
|
|
|
|
|
|
| 123I |
- |
- |
2.20E-7 |
86.7 |
3.39E-8 |
13.3 |
2.54E-7 |
| 125I |
- |
- |
8.16E-8 |
59.8 |
5.49E-8 |
40.2 |
1.37E-7 |
| 131I |
9.60E-7 |
93.3 |
6.71E-8 |
6.5 |
2.13E-9 |
0.2 |
1.03E-6 |
| Nucleus ← Follicle cells | |||||||
| Mouse |
|
|
|
|
|
|
|
| 123I |
- |
- |
1.79E-6 |
10.2 |
1.58E-5 |
89.8 |
1.76E-5 |
| 125I |
- |
- |
1.24E-5 |
32.1 |
2.62E-5 |
67.9 |
3.86E-5 |
| 131I |
1.34E-5 |
86.2 |
1.25E-6 |
8.0 |
9.03E-7 |
5.8 |
1.56E-5 |
| Rat |
|
|
|
|
|
|
|
| 123I |
- |
- |
9.64E-7 |
13.0 |
6.47E-6 |
87.0 |
7.43E-6 |
| 125I |
- |
- |
5.18E-6 |
32.6 |
1.07E-5 |
67.4 |
1.59E-5 |
| 131I |
6.87E-6 |
87.4 |
6.23E-7 |
7.9 |
3.66E-7 |
4.7 |
7.86E-6 |
| Man |
|
|
|
|
|
|
|
| 123I |
- |
- |
3.05E-7 |
19.1 |
1.29E-6 |
80.9 |
1.60E-6 |
| 125I |
- |
- |
1.06E-6 |
32.9 |
2.15E-6 |
67.1 |
3.21E-6 |
| 131I |
1.76E-6 |
89.0 |
1.45E-7 |
7.3 |
7.28E-8 |
3.7 |
1.98E-6 |
| Nucleus ← Nucleus | |||||||
| Mouse |
|
|
|
|
|
|
|
| 123I |
- |
- |
3.52E-4 |
1.7 |
2.06E-2 |
98.3 |
2.10E-2 |
| 125I |
- |
- |
1.38E-2 |
28.5 |
3.45E-2 |
71.5 |
4.83E-2 |
| 131I |
3.48E-3 |
70.9 |
2.18E-4 |
4.4 |
1.21E-3 |
24.7 |
4.91E-3 |
| Rat |
|
|
|
|
|
|
|
| 123I |
- |
- |
1.58E-4 |
2.4 |
6.40E-3 |
97.6 |
6.56E-3 |
| 125I |
- |
- |
4.35E-3 |
28.9 |
1.07E-2 |
71.1 |
1.51E-2 |
| 131I |
1.52E-3 |
76.3 |
9.74E-5 |
4.9 |
3.74E-4 |
18.8 |
1.99E-3 |
| Man |
|
|
|
|
|
|
|
| 123I |
- |
- |
9.03E-5 |
3.1 |
2.82E-3 |
96.9 |
2.91E-3 |
| 125I |
- |
- |
1.93E-3 |
29.1 |
4.70E-3 |
70.9 |
6.63E-3 |
| 131I | 8.44E-4 | 79.4 | 5.56E-5 | 5.2 | 1.63E-4 | 15.4 | 1.06E-3 |
The S value and the relative contribution from the non-radiative transitions β particles, Auger electrons (AE) and internal conversion electrons (CE) to the follicle cell nucleus are given for the species-specific models of mouse, rat and man. 123I, 125I and 131I were homogeneously distributed within the follicle lumen, follicle cells and follicle cell nucleus.
For the mouse, rat and human models, the calculated S values with radioiodine homogeneously distributed within the follicle cells are shown in Table 1. For the mouse model, the S value for 131I was 10% and 60% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (86%) and from the AE for 123I (90%) and 125I (68%). For the rat model, the S value for 131I was 6% higher and 50% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (87%) and from the AE for 123I (87%) and 125I (67%). For the human model, the S value for 131I was 20% higher and 40% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (89%) and from the AE for 123I (81%) and 125I (67%).
For the mouse, rat and human models, the calculated S values with radioiodine homogeneously distributed within the follicle cell nucleus are shown in Table 1. For the mouse model, the S value for 131I was 80% and 90% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (71%) and from the AE for 123I (98%) and 125I (72%). For the rat model, the S value for 131I was 70% and 90% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (76%) and from the AE for 123I (98%) and 125I (71%). In the human model, the S value for 131I was 60% and 80% lower than that for 123I and 125I, respectively. The largest contribution originated from the β particles for 131I (79%) and from the AE for 123I (97%) and 125I (71%).
Comparison with previously published cellular S values
Comparison between the calculated S values for 123I, 125I and 131I homogeneously distributed within the follicle cell nuclei with 4, 6 and 8 μm diameter calculated in the present study and previously published cellular S values by Goddu and Budinger [[31]] gave the following results: for 123I, the previously published S values were 1.3%, 2.9% and 2.4% higher, for 125I, 0.07%, 0.99% and 0.53% higher and for 131I, 2.1%, 1.5% and 0.71% higher, respectively.
Radioiodine homogeneously distributed within the follicle lumen in human model
For the 8-μm diameter follicle cell nuclei, the calculated S values with 123I, 125I and 131I homogeneously distributed within the follicle lumen are shown as a function of the follicle lumen diameter in Figure 1. For the smallest follicle lumen diameter (10 μm), 125I gave the highest S value, 30% and 120% higher than that for 131I and 123I, respectively. For larger follicle lumens, 131I gave the highest S value, 350% and 2,000% higher (500 μm) than that for 123I and 125I, respectively (Figure 1a). For 123I, the relative contributions from AE and CE were 42% and 58% and 5% and 95% for 50-μm and 500-μm lumen diameter, respectively (Figure 1b). For 125I, the relative contributions from AE and CE were 42% and 58, and 39% and 61% for 50-μm and 500-μm lumen diameter, respectively (Figure 1c). For 131I, the relative contributions from the β particles, AE and CE were 88%, 11% and 0.6% and 96%, 4% and 0.1% for 50-μm and 500-μm lumen diameter, respectively (Figure 1d).
Figure 1.
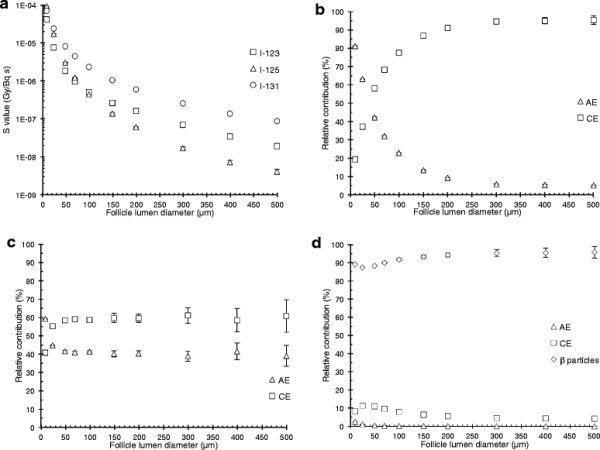
The S value for homogeneous distribution of radioiodine in the lumen of the human single follicle model. The S value and the relative contributions from the non-radiative transitions for homogeneously distributed radioiodine within the follicle lumen. The follicle lumen diameter varied from 10 to 500 μm surrounded by one single layer of follicle cells with a thickness of 10 μm and centrally placed 8 μm diameter follicle cell nucleus. (a) The S value for the follicle cell nucleus for 123I (square), 125I (triangle) and 131I (circle), as a function of the follicle lumen diameter. (b to d) The relative contribution per decay to the follicle cell nucleus from Auger electrons (AE) (triangle), internal conversion electrons (CE) (square), β particles (diamond), as a function of the follicle lumen diameter, for (b)123I, (c)125I and (d)131I. Error bars indicate the standard deviation of the mean value (SD) and are smaller than the symbol when not visible. Note the logarithmic scale on the ordinate in (a).
Radioiodine heterogeneously distributed within the human thyroid follicle model
The S values were calculated for 123I, 125I and 131I homogeneously distributed on concentric spherical shells in the human model to investigate effects of a heterogeneous distribution (Figure 2). The S values were almost constant when 123I was situated inside the central follicle lumen due to the CE (Figure 2a). When 123I was situated on the apical follicle cell surface, the contributions from the AE and CE were similar and 200% higher than when 123I was situated in the centre of the central follicle lumen. The contribution from 123I outside the central follicle decreased with increasing radius. When 125I was situated in the centre of the follicle lumen, the S values were zero until about 20 μm from the apical follicle cell surface due to the short range of emitted AE and CE (Figure 2b). When 125I was situated on the apical follicle cell surface, the contribution from the AE was slightly higher, with 56% compared with 44% from the CE. The S value increased somewhat when 131I was situated peripherally in the lumen compared to that in the centre of the follicle lumen (Figure 2c). The highest contribution originated from the β particles irrespectively of location and was 120% higher when 131I was situated on the apical cell surface compared when centrally situated in the follicle lumen. The contribution from 131I outside the follicle decreased with increased radius.
Figure 2.
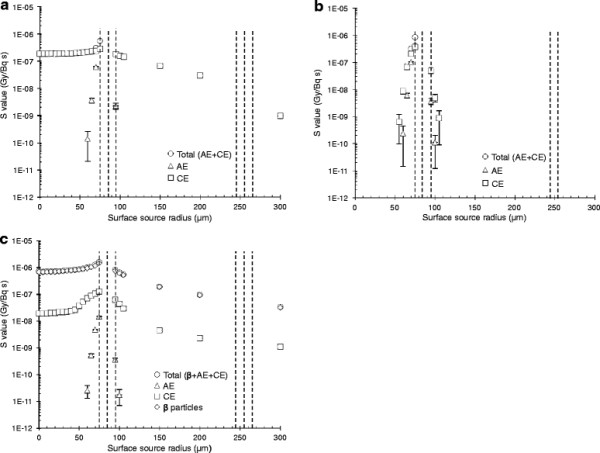
The S value for heterogeneous distribution of radioiodine in the human follicle model. The S value for the follicle cell nuclei from the non-radiative transitions are given for homogeneously distributed (a)123I, (b)125I and (c)131I on concentric spherical shells in the human thyroid model. The contribution from Auger electrons (AE) (triangle), internal conversion electrons (CE) (square) and β particles (diamond) are shown separately together with the total value (circle). The surface source radius 0 μm indicates the centre of the follicle lumen, and the dashed lines represent the apical and basal follicle cell surfaces for the respective models. Error bars indicate the standard deviation of the mean value (SD) and are smaller than the symbol when not visible. Note the logarithmic scale on the ordinate.
Multiple thyroid follicle models
Contribution from surrounding follicle layers in the human model
The calculated S values and the relative contributions to the mean absorbed dose to the inner follicle cell nuclei are presented in Table 2 with 123I, 125I and 131I homogeneously distributed within various source compartments (the follicle lumens and cells) in the human multiple thyroid follicle model (S values for the mouse and rat models, see Additional file 1). When 123I was homogeneously distributed in the entire model, the contribution to the mean absorbed dose from the central follicle was 37%. When 125I was homogeneously distributed in the entire model, the contribution to the mean absorbed dose from the central follicle was 90%. Due to the short range of the AE and CE, only one surrounding follicle layer contributed. When 131I was homogeneously distributed in the entire model, the contribution to the mean absorbed dose from the central follicle was 11%. The central follicle and eight surrounding follicle layers contributed with approximately 99% of the absorbed dose, with the largest contribution of approximately 30% from the first surrounding follicle layer.
Table 2.
S values for radioiodine in the human multiple thyroid models
|
Source compartment |
r
1
|
r
2
|
S
value |
Relative contribution
a
|
Relative contribution
b
|
Relative contribution
c
|
|---|---|---|---|---|---|---|
| (μm) | (μm) | (Gy/Bq·s) | (%) | (%) | (%) | |
| 123I | ||||||
| Lumen 1 |
0 |
75 |
2.54E-07 |
9.6 |
15.0 |
- |
| Cell layer 1 |
75 |
85 |
1.60E-06 |
27.6 |
- |
77.3 |
| Cell layer 2a |
85 |
95 |
2.77E-07 |
6.1 |
- |
16.9 |
| Lumen layer 2 |
95 |
245 |
4.23E-08 |
52.7 |
82.0 |
- |
| Cell layer 2b + 3a |
245 |
265 |
5.86E-09 |
2.1 |
- |
5.8 |
| Lumen layer 3 |
265 |
415 |
3.97E-10 |
1.9 |
2.9 |
- |
| Cell layer 3b |
415 |
425 |
7.50E-12 |
0.0 |
- |
0.0 |
| |
|
|
|
100 |
100 |
100 |
|
125I | ||||||
| Lumen 1 |
0 |
75 |
1.37E-07 |
7.7 |
93.6 |
- |
| Cells layer 1 |
75 |
85 |
3.21E-06 |
82.5 |
- |
89.9 |
| Cells layer 2a |
85 |
95 |
2.85E-07 |
9.3 |
- |
10.1 |
| Lumen layer 2 |
95 |
245 |
2.86E-10 |
0.5 |
6.4 |
- |
| Cell layer 2b |
245 |
255 |
0.00E + 00 |
0.0 |
- |
0.0 |
| |
|
|
|
100 |
100 |
100 |
|
131I | ||||||
| Lumen 1 |
0 |
75 |
1.03E-06 |
5.7 |
6.9 |
- |
| Cells layer 1 |
75 |
85 |
1.98E-06 |
5.0 |
- |
30.0 |
| Cells layer 2a |
85 |
95 |
1.02E-06 |
3.3 |
- |
19.6 |
| Lumen layer 2 |
95 |
245 |
1.44E-07 |
26.4 |
31.6 |
- |
| Cells layer 2b + 3a |
245 |
265 |
5.14E-08 |
2.6 |
- |
15.8 |
| Lumen layer 3 |
265 |
415 |
2.36E-08 |
16.4 |
19.7 |
- |
| Cells layer 3b + 4a |
415 |
435 |
1.35E-08 |
1.9 |
- |
11.6 |
| Lumen layer 4 |
435 |
585 |
7.68E-09 |
11.9 |
14.3 |
- |
| Cells layer 4b + 5a |
585 |
605 |
5.39E-09 |
1.5 |
- |
9.0 |
| Lumen layer 5 |
605 |
755 |
3.28E-09 |
9.0 |
10.8 |
- |
| Cells layer 5b + 6a |
755 |
775 |
2.08E-09 |
1.0 |
- |
5.8 |
| Lumen layer 6 |
775 |
925 |
1.31E-09 |
5.6 |
6.8 |
- |
| Cells layer 6b + 7a |
925 |
945 |
9.70E-10 |
0.7 |
- |
4.0 |
| Lumen layer 7 |
945 |
1,095 |
6.81E-10 |
4.2 |
5.1 |
- |
| Cells layer 7b + 8a |
1,095 |
1,115 |
3.67E-10 |
0.4 |
- |
2.1 |
| Lumen layer 8 |
1,115 |
1,265 |
3.30E-10 |
2.8 |
3.3 |
- |
| Cells layer 8b + 9a |
1,265 |
1,285 |
2.21E-10 |
0.3 |
- |
1.7 |
| Lumen layer 9 |
1,285 |
1,435 |
1.12E-10 |
1.2 |
1.5 |
- |
| Cell layer 9b |
1,435 |
1,445 |
6.30E-11 |
0.1 |
- |
0.3 |
| 100 | 100 | 100 | ||||
The S values for the innermost follicle cell nuclei from radioiodine homogeneously distributed in the different source compartments in the human multiple thyroid follicle models (spherical compartments with r1 = inner radius and r2 = outer radius). The relative contributions to the mean absorbed dose to the innermost follicle cell nuclei from each compartment are shown for three radionuclide distributions: for 123I with two surrounding layers of follicles contributing, 125I with one surrounding layer of follicles contributing, and 131I with eight surrounding layers of follicles contributing. Follicle layers beyond these contributed with <1%. aRadioiodine homogeneously distributed within the follicle lumens and follicle cells (the entire thyroid model). bRadioiodine homogeneously distributed within the follicle lumens. cRadioiodine homogeneously distributed within the follicle cells.
Mean absorbed dose for the multiple thyroid follicle model of man – comparison with MIRD formalism
To obtain a mean absorbed dose of 1 Gy to the cell nuclei from homogeneously distributed for 123I, 125I and 131I, in the human multiple follicle model using MIRD formalism and nuclear decay data, the cumulative specific activities equal to 234, 378 and 32.6 TBq⋅s/kg, respectively, are needed. Using the same in the MC simulations, the mean absorbed dose to the central follicle cell nuclei with radioiodine homogeneously distributed within the follicle lumens and follicle cells was 1.09, 1.18 and 1.04 Gy for 123I, 125I and 131I, respectively, in our multiple thyroid follicle models.
The mean absorbed dose to the central follicle cell nuclei for various fractions of radioiodine distributed in the cells versus lumens are shown in Figure 3. The mean absorbed dose was 0.80 Gy ( = 267 TBq⋅s/kg) when 123I was homogeneously distributed within the follicle lumens only, while it was 3.1 Gy ( = 1,860 TBq⋅s/kg) when 123I was located within the follicle cells only. For 125I, the corresponding results were 0.11 Gy ( = 440 TBq⋅s/kg) and 7.8 Gy ( = 2,700 TBq⋅s/kg), and for 131I 0.98 Gy ( = 37 TBq⋅s/kg) and 1.5 Gy ( = 276 TBq⋅s/kg).
Figure 3.
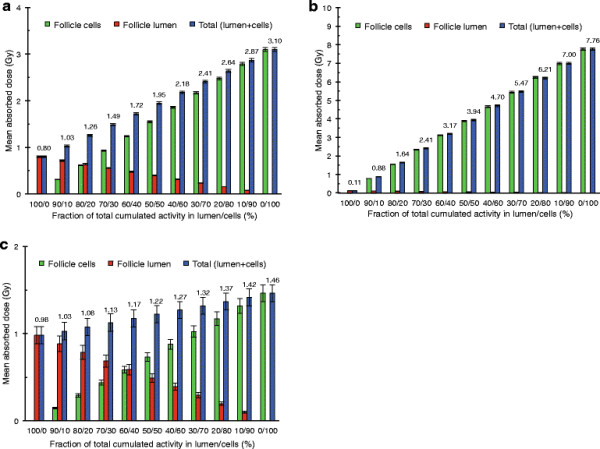
Mean absorbed dose for different distributions of radioiodine in the human multiple follicle model. The mean absorbed dose to the central follicle cell nuclei in the human multiple thyroid follicle model for different relations in radioiodine concentration (homogeneous in each compartment) between follicle lumens and/or follicle cells for (a)123I, (b)125I and (c)131I. The values indicate total mean absorbed dose (blue bars, numbers), and the contribution to the mean absorbed dose from the follicle lumen (red bars) and from the follicle cells (green bars). The error bars indicate the standard deviation of the mean value (SD).
Discussion
In general, the S values determined in the present study were in good agreement with the few corresponding data available in the literature. Our results for 123I, 125I and 131I homogeneously distributed within the follicle cell nuclei in the species-specific models were in excellent agreement with published cellular S values calculated with an analytical method [[31]] based on the experimental range-energy relationship for electrons by Cole [[32]].
The mean absorbed dose to the follicle cells for 131I homogeneously distributed within the follicle lumens was determined for a similar human thyroid follicle model as ours [[24]]. Their model included interstitial tissue (51% of the total tissue volume), and 12 surrounding follicles layers, compared with no interstitial tissue and 8 follicle layers in our human model (since we found that the contribution from surrounding layers beyond the 8th was <1%). The contribution from the central follicle was 7% in our model compared with 17% in their model, and the contribution from the surrounding follicles in our model was slightly higher, e.g. 32% for the first surrounding layer compared with 29%. These differences could mainly be explained by the difference in consideration of interstitial tissue. Despite these model differences we found in both studies that the follicle cells received 0.98 Gy when the mean absorbed dose was 1 Gy to thyroid tissue.
There are some simplifications and assumptions made in the calculations, both for the mathematical models and for physical data. The use of unit density water (1.0 g/cm3) in the models instead of the density of the thyroid gland, which according to ICRP publication 23 is 1.05 g/cm3 [[33]]. Dosimetric calculations performed with a 3% mass concentration of 127I (stable iodine) homogenously distributed within the follicle lumens only showed a small difference for 211At [[19]], and after normalisation of the mass for the follicle cell nuclei this difference would be even less. Other assumptions regarding the thyroid models have previously been discussed [[19]]. In general, we estimate that the assumptions in the dosimetrical calculations, such as limitations in nuclear decay data and transport physics used by the Monte Carlo code only contribute to a minor extent to the results. The conventional AE spectrum from ICRP 107 was used in the calculations [[26]] and have been regarded adequate when calculating absorbed doses to regions with diameters larger than 1 μm [[34]], and AE with initial kinetic energy lower than 1 keV (the lowest cutoff energy for electrons in MCNPX 2.6.0) were assumed to be fully absorbed within the source volume. This assumption is realistic since experiments have shown that electrons with a kinetic energy of 1 keV have a range of about 61 nm in unit density matter [[32]], verified by calculations: absorbed fraction very close to unity for unit density water spheres with radius of 2 μm for monoenergetic 1 keV electrons [[35]]. Bremsstrahlung generated by the electrons was not accounted for in the Monte Carlo calculations, but the contribution was low in this application (for 1 MeV electrons, only about 0.7% of the kinetic energy is transferred to bremsstrahlung in liquid water, and this fraction is even less for lower kinetic energies [[36]]). The contribution from 131mXe was not considered in the dosimetric calculations for 131I, which would result in an underestimation of the S value to the cell nucleus of about 3.6% for 131I homogenously distributed in an 8-μm cell nucleus and about 1.5% for a homogenous distribution in a 150-μm diameter lumen (unpublished data). Furthermore, the contribution from the xenon daughter is probably less significant due to the short retention in the thyroid gland because of its gaseous state [[23]].
For 123I, the contributions from the 123Te and 123mTe daughters were not included in the dosimetric calculations due to a very long half-life and low yield, respectively. Furthermore, the effects of the charge of the tellurium atoms (average charge of about +9 [[37]] due to multiple ionisation when 123I and 125I emit cascades of AE) were not considered. Otherwise, such charged atoms may produce ionizations and excitations in the immediate vicinity of the decay site [[37]], which could enhance the biological effect when covalently bound to the DNA [[38]].
With 123I and 125I homogeneously distributed within the follicle cell nucleus, the S value was 2.3 times higher for 125I than that for 123I in the mouse, rat and human models, a result in accordance with similar calculations for a 10-μm diameter tissue sphere, excluding charge neutralisation [[37]].
Biodistribution studies performed on mice, rats and guinea pigs have shown that the highest uptake of radioiodide was in the thyroid gland, with the highest concentration occurring around 18 to 24 h after injection [[39]-[41]], while the maximal concentration is obtained after approximately 1 to 2 days in normal humans [[42]]. Preclinical studies have shown that radioiodide is rapidly transported through the follicle cell cytoplasm. At early time-points, radioiodine appears as rings peripherally in the follicle lumen close to the apical cell surface [[43]-[46]], and thereafter the radioiodine is more homogeneously distributed in the follicle lumen [[45],[46]]. However peripheral rings have been observed as long as 99 days after injection [[46]]. The specific activity was initially highest in the smallest follicles but became independent of follicle size with time [[45]]. In the human model, the S value for 123I, 125I and 131I distributed on the apical follicle cell surface was 2.2, 5.9 and 1.5 times higher than for a homogeneous distribution within the follicle lumen, respectively. Due to the much shorter half-life of 123I (13 h), the fraction of decays in the follicle cells and at the apical surface would be highest for 123I, indicating a possible higher absorbed dose when biokinetic data are considered.
The MIRD formalism assumes a homogeneous distribution of the radionuclide within the source compartment when determining the mean absorbed dose. Compared with our results, the mean absorbed dose calculated according to MIRD formalism (Equation 1) and nuclear decay data was lower, with the largest difference of 18% for 125I, and the smallest of 4% for 131I. This comparison together with the dosimetric data obtained for inhomogeneous distribution shows the importance of taking the range of the emitted particles into account. For 125I, the emitted low-energy AE and CE, with a range of up to 23 μm in water [[36]], could contribute to a heterogeneous absorbed dose distribution, and about 90% of the absorbed dose originates from the follicle itself. The high-energy β particles emitted by 131I, with a range of up to 2.1 mm in water [[24]], contribute to a cross-fire effect with contributions from eight surrounding layers of follicles, which results in a more homogeneous absorbed dose distribution, and only about 11% of the mean absorbed dose originates from the follicle itself. For 123I, the emitted low-energetic AE and somewhat higher-energetic CE, the mean absorbed dose was about 9% higher than that according to MIRD formalism. The absorbed dose is then a combination between heterogeneous absorbed dose distribution from the very short-ranged AE and the more homogeneous absorbed dose distribution from the more long-ranged CE, and about 38% of the mean absorbed dose originates from the follicle itself. Furthermore, in the peripheral regions of the thyroid gland where fewer surrounding follicles contribute, the absorbed dose may be lower and more heterogeneous. This effect would be largest for 131I with eight surrounding follicle layers contributing and could lead to a reduced mean absorbed dose by up to about 45%.
For radioiodine homogeneously distributed only within the follicle lumens, the mean absorbed dose was 0.80, 0.11 and 0.98 Gy, respectively, for 123I, 125I and 131I, compared with 1 Gy calculated with MIRD formalism and nuclear decay data for radioiodine homogenously distributed within both follicle cells and lumens. Thus, the MIRD formalism overestimates the mean absorbed dose for 123I and 125I.
Conclusions
This study clearly demonstrates the importance of using more detailed dosimetric methods and models than MIRD formalism for radioiodine within the thyroid. For radioiodine homogeneously distributed in the follicle cells and lumens, calculations for our human multiple follicle model gave up to 18% higher mean absorbed dose. For radioiodine homogeneously distributed in the follicle lumens only, our calculations gave up to 90% lower mean absorbed dose for 125I (20% lower for 123I, and 2% lower for 131I).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AJ designed the study, performed the Monte Carlo simulations and drafted the manuscript. Both authors contributed to the scientific and intellectual discussion and interpretation of the data and revision of the manuscript. Both authors read and approved the final manuscript.
Authors’ information
AJ is a PhD student in medical science at the Department of Radiation Physics, the Sahlgrenska Academy at the University of Gothenburg. AJ is also a licenced medical physicist. EFA is professor and Head of the Department of Radiation Physics, the Sahlgrenska Academy at the University of Gothenburg and senior medical physicist at Sahlgrenska University Hospital in Gothenburg.
Additional file
Supplementary Material
S values for radioiodine in the mouse and rat multiple thyroid models. Table S1.S values for radioiodine in the mouse multiple thyroid models. The S values for the innermost follicle cell nuclei from radioiodine homogeneously distributed in the different source compartments in the mouse multiple thyroid follicle models (spherical compartments with r1 = inner radius and r2 = outer radius), for 123I with five surrounding layers of follicles contributing, 125I with one surrounding layer of follicles contributing and 131I with ten surrounding layers of follicles contributing. Table S2.S values for radioiodine in the rat multiple thyroid models. The S values for the innermost follicle cell nuclei from radioiodine homogeneously distributed in the different source compartments in the rat multiple thyroid follicle models (spherical compartments with r1 = inner radius and r2 = outer radius), for 123I with four layers of follicles contributing, 125I with one surrounding layer of follicles contributing and 131I with sixteen surrounding layers of follicles contributing.
Contributor Information
Anders Josefsson, Email: anders.josefsson@radfys.gu.se.
Eva Forssell-Aronsson, Email: eva.forssell_aronsson@radfys.gu.se.
Acknowledgements
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, BioCARE, a National Strategic Research Program at University of Gothenburg, the Swedish Radiation Safety Authority, the King Gustav V Jubilee Clinic Cancer Research Foundation and the Assar Gabrielsson Cancer Research Foundation. The work was performed within the EC COST Action BM0607.
References
- Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American thyroid association and American association of clinical endocrinologists. Endocr Pract. 2011;4:456–520. doi: 10.4158/ep.17.3.456. [DOI] [PubMed] [Google Scholar]
- Bonnema SJ, Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;4:920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- Luster M, Hanscheid H, Freudenberg LS, Verburg FA. Radioiodine therapy of metastatic lesions of differentiated thyroid cancer. J Endocrinol Invest. 2012;4:21–29. [PubMed] [Google Scholar]
- Park HM, Perkins OW, Edmondson JW, Schnute RB, Manatunga A. Influence of diagnostic radioiodines on the uptake of ablative dose of iodine-131. Thyroid. 1994;4:49–54. doi: 10.1089/thy.1994.4.49. [DOI] [PubMed] [Google Scholar]
- Postgard P, Himmelman J, Lindencrona U, Bhogal N, Wiberg D, Berg G, Jansson S, Nystrom E, Forssell-Aronsson E, Nilsson M. Stunning of iodide transport by (131) I irradiation in cultured thyroid epithelial cells. J Nucl Med. 2002;4:828–834. [PubMed] [Google Scholar]
- Forssell-Aronsson E, Bernhardt P, Wangberg B, Kolby L, Nilsson O, Ahlman H. Aspects on radionuclide therapy in malignant pheochromocytomas. Ann N Y Acad Sci. 2006;4:498–504. doi: 10.1196/annals.1353.052. [DOI] [PubMed] [Google Scholar]
- Forssell-Aronsson E, Schuler E, Ahlman H. Advances in the diagnostic imaging of pheochromocytomas. Rep Med Imaging. 2011;4:19–37. doi: 10.2147/RMI.S5571. [DOI] [Google Scholar]
- Hauser RA, Grosset DG. [123I] FP-CIT (DaTscan) SPECT brain imaging in patients with suspected parkinsonian syndromes. J Neuroimaging. 2012;4:225–230. doi: 10.1111/j.1552-6569.2011.00583.x. [DOI] [PubMed] [Google Scholar]
- Harkrider WW, Diebold AE, Maloney T, Espenan G, Wang YZ, Stafford SJ, Camp A, Frey D, Chappuis C, Woltering EA. An extended phase II trial of iodine-125 methylene blue for sentinel lymph node identification in women with breast cancer. J Am Coll Surg. 2013;4:599–605. doi: 10.1016/j.jamcollsurg.2012.12.044. discussion 605–596. [DOI] [PubMed] [Google Scholar]
- Tomblyn M. Radioimmunotherapy for B-cell non-Hodgkin lymphomas. Cancer Control. 2012;4:196–203. doi: 10.1177/107327481201900304. [DOI] [PubMed] [Google Scholar]
- Valverde C, Orozco A, Becerra A, Jeziorski MC, Villalobos P, Solis JC. Halometabolites and cellular dehalogenase systems: an evolutionary perspective. Int Rev Cytol. 2004;4:143–199. doi: 10.1016/S0074-7696(04)34004-0. [DOI] [PubMed] [Google Scholar]
- Williams D. Twenty years' experience with post-Chernobyl thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2008;4:1061–1073. doi: 10.1016/j.beem.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Cardis E, Howe G, Ron E, Bebeshko V, Bogdanova T, Bouville A, Carr Z, Chumak V, Davis S, Demidchik Y, Drozdovitch V, Gentner N, Gudzenko N, Hatch M, Ivanov V, Jacob P, Kapitonova E, Kenigsberg Y, Kesminiene A, Kopecky KJ, Kryuchkov V, Loos A, Pinchera A, Reiners C, Repacholi M, Shibata Y, Shore RE, Thomas G, Tirmarche M, Yamashita S, Zvonova I. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;4:127–140. doi: 10.1088/0952-4746/26/2/001. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Gorski AI, Maksioutov MA, Vlasov OK, Godko AM, Tsyb AF, Tirmarche M, Valenty M, Verger P. Thyroid cancer incidence among adolescents and adults in the Bryansk region of Russia following the Chernobyl accident. Health Phys. 2003;4:46–60. doi: 10.1097/00004032-200301000-00004. [DOI] [PubMed] [Google Scholar]
- Hindie E, Leenhardt L, Vitaux F, Colas-Linhart N, Grosclaude P, Galle P, Aurengo A, Bok B. Non-medical exposure to radioiodines and thyroid cancer. Eur J Nucl Med Mol Imaging. 2002;4(Suppl 2):S497–S512. doi: 10.1007/s00259-002-0912-4. [DOI] [PubMed] [Google Scholar]
- Champion C, Zanotti-Fregonara P, Hindie E. CELLDOSE: a Monte Carlo code to assess electron dose distribution–S values for 131I in spheres of various sizes. J Nucl Med. 2008;4:151–157. doi: 10.2967/jnumed.107.045179. [DOI] [PubMed] [Google Scholar]
- Uusijarvi H, Bernhardt P, Ericsson T, Forssell-Aronsson E. Dosimetric characterization of radionuclides for systemic tumor therapy: influence of particle range, photon emission, and subcellular distribution. Med Phys. 2006;4:3260–3269. doi: 10.1118/1.2229428. [DOI] [PubMed] [Google Scholar]
- Uusijarvi H, Bernhardt P, Forssell-Aronsson E. Translation of dosimetric results of preclinical radionuclide therapy to clinical situations: influence of photon irradiation. Cancer Biother Radiopharm. 2007;4:268–274. doi: 10.1089/cbr.2006.317. [DOI] [PubMed] [Google Scholar]
- Josefsson A, Forssell-Aronsson E. Microdosimetric analysis of 211At in thyroid models for man, rat and mouse. Eur J Nucl Med Mol Imag Res. 2012;4:29. doi: 10.1186/2191-219X-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unak T, Unak P. Microscopic energy absorption in the colloid of thyroid follicles from Auger electrons of iodine-125. Int J Rad Appl Instrum A. 1991;4:291–295. doi: 10.1016/0883-2889(91)90091-E. [DOI] [PubMed] [Google Scholar]
- Gillespie FC, Orr JS, Greig WR. Microscopic dose distribution from 125-I in the toxic thyroid gland and its relation to therapy. Br J Radiol. 1970;4:40–47. doi: 10.1259/0007-1285-43-505-40. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Kaul A. Microscopic dose distributions due to iodine isotopes in thyroid. Radiat Environ Biophys. 1978;4:229–239. doi: 10.1007/BF02176792. [DOI] [PubMed] [Google Scholar]
- Van Best J. Dose calculations for 123I, 124I, 125I and 131I in the thyroid gland of the mouse, rat and man and comparison with thyroid function for mice and rats. Phys Med Biol. 1981;4:1035. doi: 10.1088/0031-9155/26/6/005. [DOI] [PubMed] [Google Scholar]
- Hindie E, Champion C, Zanotti-Fregonara P, Rubello D, Colas-Linhart N, Ravasi L, Moretti JL. Calculation of electron dose to target cells in a complex environment by Monte Carlo code “CELLDOSE”. Eur J Nucl Med Mol Imaging. 2009;4:130–136. doi: 10.1007/s00259-008-0893-z. [DOI] [PubMed] [Google Scholar]
- Pelowitz DB. MCNPX, user’s manual version 2.6.0. LA-CP-07-1473. Los Alamos National Laboratory, Los Alamos, USA; 2008. [Google Scholar]
- Valentin J. ICRP Publication 107: nuclear decay data for dosimetric calculations. Published for the International Commission on Radiological Protection by Elsevier, Oxford; 2009. [Google Scholar]
- MCNP - a general Monte Carlo N-particle transport code, version 5 - volume I: overview and theory. LA-UR-03-1987. Los Alamos National Laboratory, Los Alamos, USA; 2003. [Google Scholar]
- Loevinger R, Budinger TF, Watson EE. MIRD primer for absorbed dose calculations. The Society of Nuclear Medicine New York, New York; 1991. [Google Scholar]
- Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry–standardization of nomenclature. J Nucl Med. 2009;4:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- http://www.nndc.bnl.gov/mird/ International Network of Nuclear Structure and Decay Data Evaluators: Nuclear decay data in the MIRD format. Brookhaven, USA: Brookhaven National Laboratory.
- Goddu SM, Budinger TF. MIRD cellular S. values: self-absorbed dose per unit cumulated activity for selected radionuclides and monoenergetic electron and alpha particle emitters incorporated into different cell compartments. Society of Nuclear Medicine Reston, VA, Reston, USA; 1997. [Google Scholar]
- Cole A. Absorption of 20-eV to 50,000-eV electron beams in air and plastic. Radiat Res. 1969;4:7–33. doi: 10.2307/3572707. [DOI] [PubMed] [Google Scholar]
- Snyder WS, Cook M, Nasset E, Karhausen L, Howells GP, Tipton I. ICRP Publication 23: report of the task group on reference man. Pergamon Oxford, Oxford; 1975. [Google Scholar]
- Howell RW. Radiation spectra for auger-electron emitting radionuclides: report no. 2 of AAPM nuclear medicine task group no. 6. Med Phys. 1992;4:1371–1383. doi: 10.1118/1.596927. [DOI] [PubMed] [Google Scholar]
- Goddu SM, Howell RW, Rao DV. Cellular dosimetry: absorbed fractions for monoenergetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartments. J Nucl Med. 1994;4:303–316. [PubMed] [Google Scholar]
- ICRU report 37: stopping power for electrons and positrons. International Commission on Radiation Units and Measurements, Bethesda, MD; 1984. [Google Scholar]
- Makrigiorgos GM, Kassis AI, Baranowska-Kortylewicz J, McElvany KD, Welch MJ, Sastry KS, Adelstein SJ. Radiotoxicity of 5-[123I] iodo-2′-deoxyuridine in V79 cells: a comparison with 5-[125I] iodo-2′-deoxyuridine. Radiat Res. 1989;4:532–544. doi: 10.2307/3577411. [DOI] [PubMed] [Google Scholar]
- Charlton DE, Pomplun E, Booz J. Some consequences of the Auger effect: fluorescence yield, charge potential, and energy imparted. Radiat Res. 1987;4:553–564. doi: 10.2307/3576939. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Soley MH. A comparison of the metabolism of iodine and of element 85 (Eka-iodine) Proc Natl Acad Sci U S A. 1940;4:483–489. doi: 10.1073/pnas.26.8.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetz J, Rudqvist N, Forssell-Aronsson E. Biodistribution and dosimetry of free 211At, 125I- and 131I- in rats. Cancer Biother Radiopharm. 2013;4:657–664. doi: 10.1089/cbr.2013.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh C, Lindencrona U, Schmitt A, Nilsson M, Forssell-Aronsson E. Biodistribution of free 211At and 125I- in nude mice bearing tumors derived from anaplastic thyroid carcinoma cell lines. Cancer Biother Radiopharm. 2006;4:591–600. doi: 10.1089/cbr.2006.21.591. [DOI] [PubMed] [Google Scholar]
- Johansson L, Leide-Svegborn S, Mattsson S, Nosslin B. Biokinetics of iodide in man: refinement of current ICRP dosimetry models. Cancer Biother Radiopharm. 2003;4:445–450. doi: 10.1089/108497803322285206. [DOI] [PubMed] [Google Scholar]
- Ekholm R, Wollman SH. Site of iodination in the rat thyroid gland deduced from electron microscopic autoradiographs. Endocrinology. 1975;4:1432–1444. doi: 10.1210/endo-97-6-1432. [DOI] [PubMed] [Google Scholar]
- Wollman SH, Wodinsky I. Localization of protein-bound I131 in the thyroid gland of the mouse. Endocrinology. 1955;4:9–20. doi: 10.1210/endo-56-1-9. [DOI] [PubMed] [Google Scholar]
- Loewenstein JE, Wollman SH. Distribution of organic 125-I and 127-I in the rat thyroid gland during equilibrium labeling as determined by autoradiography. Endocrinology. 1967;4:1074–1085. doi: 10.1210/endo-81-5-1074. [DOI] [PubMed] [Google Scholar]
- Loewenstein JE, Wollman SH. Diffusion of thyroglobulin in the lumen of rat thyroid follicle. Endocrinology. 1967;4:1086–1090. doi: 10.1210/endo-81-5-1086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S values for radioiodine in the mouse and rat multiple thyroid models. Table S1.S values for radioiodine in the mouse multiple thyroid models. The S values for the innermost follicle cell nuclei from radioiodine homogeneously distributed in the different source compartments in the mouse multiple thyroid follicle models (spherical compartments with r1 = inner radius and r2 = outer radius), for 123I with five surrounding layers of follicles contributing, 125I with one surrounding layer of follicles contributing and 131I with ten surrounding layers of follicles contributing. Table S2.S values for radioiodine in the rat multiple thyroid models. The S values for the innermost follicle cell nuclei from radioiodine homogeneously distributed in the different source compartments in the rat multiple thyroid follicle models (spherical compartments with r1 = inner radius and r2 = outer radius), for 123I with four layers of follicles contributing, 125I with one surrounding layer of follicles contributing and 131I with sixteen surrounding layers of follicles contributing.


