Abstract
Background
Vegetables and fruits are rich in vitamins, minerals and, dietary fiber and contribute to the prevention and improvement of obesity and metabolic syndrome. However, inadequate intake of vegetable and fruit is a concern in Japan.
We therefore produced a juice mixture of fresh fruit and komatsuna (Brassica rapa L. var. perviridis: B. rapa) with the aim to investigate the effects of this juice mixture on anthropometric data, blood parameters, and dietary intake differences.
Methods
This study was performed as a single blind and randomized controlled trial. Subjects were 16 men (mean age, 46.4 ± 7.1 years), and they were divided into two groups (control group and intervention group). The intervention group consumed the juice mixture of fresh fruit and B. rapa. The control group consumed commercial vegetable juice. Subjects consumed juice twice a day throughout the weekday, for 4 weeks. We prepared both juices with an equivalent energy balance.
Results
Weight and body mass index (BMI) of the control group after 4 weeks were significantly increased compared with baseline values. Serum total cholesterol (T-Chol) and low-density lipoprotein cholesterol (LDL-Chol) of the intervention group after 4 weeks were significantly reduced compared with baseline values. Furthermore, intake of total vegetables and fruits were significantly increased compared with baseline values in both groups.
Conclusions
Both vegetable juices contributed to improved intake of total vegetables and fruit. Compared with the intake of commercial vegetable juice, the intake of fresh fruit and B. rapa juice is highly effective in reducing serum cholesterol. Short-term intake of fresh fruit and B. rapa juice was shown to enhance cholesterol metabolism.
Keywords: Vegetable, Fruit, Serum cholesterol, Middle-aged men, Metabolic syndrome
Background
An increase in LDL-Chol concentration is a risk factor for arteriosclerosis. Arteriosclerosis is closely linked to increases in the incidence of coronary artery disease, stroke, and arteriosclerosis obliterans. A recent study indicates that a high LDL-Chol concentration increases the risk of arteriosclerosis, and a high T-Chol concentration increases the risk of coronary artery disease [1].
The consumption of vegetable and fruit improves lipid composition in blood. Guimares reported that intake of eggplant (Solanum melongena) infusion significantly reduced the blood concentrations of T-Chol, LDL-Chol, and apolipoprotein B in hypercholesterolemia subjects [2]. Futhermore, in a cohort study of 4466 subjects, Djoussé reported that consumption of fruits and vegetables is inversely related to LDL-Chol [3].
Moreover, previous studies show an association between fruit and vegetable consumption and coronary artery disease, ischemic heart disease, and cerebrovascular disease. Increasing the consumption of fruit and vegetables reduces the risk of coronary artery disease, stroke, and hypertension [4]. Compared with people who consume vegetables and fruits once or less a day, those who consume vegetables and fruits thrice or more a day have lower stroke incidence, stroke mortality, ischemic heart disease mortality, and cardiovascular disease mortality [5].
High consumption of vegetables and fruits may have effects on blood lipid metabolism and reduce the risk of diseases stemming from arteriosclerosis.
In Japan, “Healthy Japan 21” was developed as a prophylaxis of life-style related diseases by Health, Labour and the Welfare Ministry in 2000 [6]. Healthy Japan 21 promotes a vegetable intake of more than 350 g/day, green and yellow vegetable intake of more than 120 g/day, and fruit intake of 200 g/day. However, these goals may still be unattainable in all life stages [6].
To improve the current situation, we need to take some drastic measures. With such an aim, we produced fresh of fruit and B. rapa juice mixture as a means of providing easy intake of vegetable and fruit.
There are reports that daily intake of commercial vegetable juice increases dietary vegetable intake [7,8]. However, reports of intervention studies using fresh fruit and vegetable juice are few [9-11].
We conducted an intervention study to investigate whether daily intake of a juice mixture from fresh fruit and B. rapa would affect anthropometric data, blood parameters, and dietary intake.
Results
Characteristics of subjects
Characteristics of the study subjects are shown in Table 1. The mean anthropometric data of the study subjects did not exceed the reference value for metabolic syndrome. However, BMI and waist circumference tended to be moderately high.
Table 1.
Characteristics of subjects
| Mean ± S.D. | |
|---|---|
| Age (years) |
46.6 ± 7.1 |
| Height (cm) |
172.3 ± 5.7 |
| Weight (kg) |
73.3 ± 16.8 |
| Body fat percentage (%) |
23.2 ± 8.1 |
| Body mass index (kg/m2) |
24.6 ± 5.2 |
| Waist Circumference (cm) |
87.4 ± 13.6 |
| Systolic blood pressure (mmHg) |
126 ± 16 |
| Diastolic blood pressure (mmHg) | 79 ± 12 |
Data shows mean ± S.D.
Nutrient components of commercial vegetable juice and fresh fruit and B. rapa juice mixture
Nutrient components of both juices are shown in Table 2. Nutrition components of commercial vegetable juice (100 g) were as follows: energy, 48 kcal; potassium, 173 mg; and β-carotene, equivalents 2628 μg. In contrast, nutrition components of fresh fruit and B. rapa juice mixture (100 g) were as follows: energy, 44 kcal; potassium, 180 mg; and β-carotene equivalents, 360 μg. Volume of protein, fat, carbohydrate, sodium, iron, folate, vitamin C, and dietary fiber are shown in Table 2.
Table 2.
Nutrient components of commercial vegetable juice and fresh fruit and B. rapa juice mixture
| Commercial vegetable juice ※1) | Fresh fruit and B. rapa juice mixture ※2) | |
|---|---|---|
| Energy (kcal/100 g) |
48 |
44 |
| Protein (g/100 g) |
0.4 |
0.7 |
| Fat (g/100 g) |
0 |
0 |
| Carbohydrate (g/100 g) |
12.3 |
10.3 |
| Sodium (mg/100 g) |
43 |
12 |
| Potassium (mg/100 g) |
173 |
180 |
| Iron (mg/100 g) |
0.2 |
0.2 |
| β-carotene equivalent (μg/100 g) |
2628 |
360 |
| Folate (μg/100 g) |
9 |
8 |
| Vitamin C (mg/100 g) |
9 |
35 |
| Dietary fiber (g/100 g) | 1.0 | 0.5 |
※1) Nutritional labeling of commercial vegetable juice.
※2) Analysis values by Japan Inspection Association of Food and Food Industry Environment.
Abbreviation: B. rapa Brassica rapa L. var. perviridis.
Anthropometric data
Anthropometric data of both groups are shown in Table 3. Weight and BMI after 4 weeks in the control group were significantly increased compared with baseline values (weight: 70.7 ± 14.1 vs 71.9 ± 13.8 kg, p = 0.017; BMI: 23.8 ± 4.9 vs 24.2 ± 4.8 kg/m2, p = 0.042). In contrast, there were no significant differences in the intervention group.
Table 3.
Change of anthropometric data
|
Control group (n = 8) |
|
Intervention group (n = 8) |
|
|||
|---|---|---|---|---|---|---|
|
Baseline |
After 4 weeks |
|
Baseline |
After 4 weeks |
|
|
| Mean ± S.D. | Mean ± S.D. | p | Mean ± S.D. | Mean ± S.D. | p | |
| Height (cm) |
172.3 ± 6.7 |
172.3 ± 6.7 |
0.735 |
172.4 ± 4.9 |
172.4 ± 4.7 |
0.611 |
| Weight (kg) |
70.7 ± 14.1 |
71.9 ± 13.8 |
0.017* |
76.0 ± 19.7 |
76.3 ± 19.4 |
0.362 |
| BFP (%) |
24.3 ± 9.5 |
24.9 ± 9.6 |
0.484 |
22.1 ± 7.0 |
23.1 ± 7.5 |
0.069 |
| BMI (kg/m2) |
23.8 ± 4.9 |
24.2 ± 4.8 |
0.042* |
25.4 ± 5.6 |
25.5 ± 5.6 |
0.259 |
| Waist circumference |
86.6 ± 12.9 |
86.5 ± 12.8 |
0.932 |
88.3 ± 15.2 |
88.2 ± 15.2 |
0.326 |
| Systolic blood pressure (mmHg) |
125 ± 13 |
128 ± 15 |
0.327 |
126 ± 19 |
133 ± 17 |
0.050 |
| Diastolic blood pressure (mmHg) | 79 ± 12 | 80 ± 10 | 0.147 | 79 ± 14 | 82 ± 12 | 0.237 |
Data shows mean ± S.D.
Abbreviations: BFP Body fat percentage, BMI Body mass index.
Baseline vs After 4 weeks, Wilcoxon signed rank test, *p < 0.05.
Blood parameters
Blood parameters of both groups are shown in Table 4. T-Chol and LDL-Chol after 4 weeks in the intervention group were significantly decreased compared with baseline values (T-Chol: 220 ± 28 vs 211 ± 27 mg/dL, p = 0.017; LDL-Chol: 143 ± 27 vs 134 ± 23 mg/dL, p = 0.017).
Table 4.
Change of blood parameter
|
Control group (n = 8) |
|
Intervention group (n = 8) |
|
|||
|---|---|---|---|---|---|---|
|
Baseline |
After 4 weeks |
|
Baseline |
After 4 weeks |
|
|
| Mean ± S.D. | Mean ± S.D. | p | Mean ± S.D. | Mean ± S.D. | p | |
| Glucose (mg/dL) |
95 ± 6 |
96 ± 6 |
0.359 |
100 ± 7 |
96 ± 10 |
0.106 |
| Hemoglobin A1c (%) |
5.3 ± 0.3 |
5.3 ± 0.3 |
0.589 |
5.7 ± 0.8 |
5.6 ± 0.4 |
0.526 |
| Triacylglycerol (mg/dL) |
121 ± 82 |
160 ± 118 |
0.080 |
122 ± 47 |
121 ± 49 |
1.000 |
| Free fatty acid (μEq/L) |
586 ± 526 |
451 ± 132 |
0.779 |
497 ± 184 |
379 ± 54 |
0.069 |
| T-Chol (mg/dL) |
210 ± 38 |
210 ± 33 |
0.833 |
220 ± 28 |
211 ± 27 |
0.017* |
| HDL-Chol (mg/dL) |
50 ± 12 |
51 ± 15 |
0.570 |
53 ± 15 |
53 ± 13 |
0.799 |
| LDL-Chol (mg/dL) |
136 ± 36 |
127 ± 23 |
0.262 |
143 ± 27 |
134 ± 23 |
0.017* |
| Insulin (μIU/mL) |
8.72 ± 11.40 |
10.80 ± 14.77 |
0.161 |
7.39 ± 8.42 |
9.42 ± 13.03 |
0.327 |
| Vitamin C (μg/mL) |
2.38 ± 1.14 |
1.82 ± 0.83 |
0.441 |
1.76 ± 0.49 |
1.80 ± 0.44 |
0.726 |
| Leptin (pg/mL) |
236 ± 301 |
214 ± 255 |
0.401 |
129 ± 99 |
161 ± 185 |
1.000 |
| Adiponectin (μg/mL) |
13.2 ± 13.8 |
13.3 ± 13.9 |
1.000 |
8.2 ± 5.9 |
8.3 ± 5.7 |
0.866 |
| HOMA-IR |
2.10 ± 2.83 |
2.69 ± 3.87 |
0.655 |
1.92 ± 2.38 |
2.44 ± 3.71 |
0.144 |
| Arteriosclerotic Index | 3.49 ± 1.58 | 3.55 ± 1.87 | 0.779 | 3.48 ± 1.59 | 3.16 ± 1.05 | 0.327 |
Data shows mean ± S.D.
Abbreviation: T-Chol Total cholesterol, HDL-Chol High-density lipoprotein cholesterol, LDL-Chol Low-density lipoprotein cholesterol, HOMA-IR Homeostasis model of assessment of insulin resistance.
Baseline vs After 4 weeks, Wilcoxon signed rank test, *p < 0.05.
Energy and nutrition intake
Energy and nutrition intake of both groups are shown in Table 5. Potassium intake after 4 weeks in both groups were significantly increased compared with baseline values (control group: 2,550 ± 1,105 vs 3,049 ± 1,068 mg, p = 0.012; intervention group: 2,815 ± 978 vs 3,486 ± 766 mg, p = 0.017). β-carotene equivalent intake after 4 weeks in the intervention group was significantly increased compared with the baseline value (3,815 ± 2,357 vs 4,795 ± 1,881 μg, p = 0.036). Magnesium intake after 4 weeks in the intervention group tended to increase compared with the baseline value (286 ± 69 vs 312 ± 64 mg, p = 0.069).
Table 5.
Change of energy and nutrition intakes
|
Control group (n = 8) |
|
Intervention group (n = 8) |
|
|||
|---|---|---|---|---|---|---|
|
Baseline |
After 4 weeks |
|
Baseline |
After 4 weeks |
|
|
| Mean ± S.D. | Mean ± S.D. | p | Mean ± S.D. | Mean ± S.D. | p | |
| Energy (kcal) |
1,829 ± 401 |
1,955 ± 460 |
0.327 |
2,190 ± 293 |
2,247 ± 364 |
0.401 |
| Protein (g) |
73.1 ± 24.8 |
74.9 ± 26.7 |
0.484 |
77.3 ± 17.0 |
76.7 ± 16.7 |
0.889 |
| Fat (g) |
52.3 ± 15.0 |
50.1 ± 16.1 |
0.484 |
69.8 ± 14.7 |
61.0 ± 15.9 |
0.069 |
| Carbohydrate (g) |
248.0 ± 52.5 |
291.9 ± 77.0 |
0.327 |
288.0 ± 26.3 |
325.7 ± 54.0 |
0.025* |
| Sodium (mg) |
4,251 ± 1,233 |
4,576 ± 1,519 |
0.123 |
4,339 ± 918 |
4,564 ± 868 |
0.401 |
| Potassium (mg) |
2,550 ± 1,105 |
3,049 ± 1,068 |
0.012* |
2,815 ± 978 |
3,486 ± 766 |
0.017* |
| Calcium (mg) |
611 ± 249 |
572 ± 283 |
0.401 |
610 ± 190 |
608 ± 159 |
0.674 |
| Magnesium (mg) |
266 ± 99 |
278 ± 101 |
0.263 |
286 ± 69 |
312 ± 64 |
0.069 |
| Phosphorus (mg) |
1,105 ± 392 |
1,126 ± 426 |
0.575 |
1,172 ± 282 |
1,173 ± 258 |
0.889 |
| Iron (mg) |
8.4 ± 3.5 |
8.9 ± 3.4 |
0.123 |
9.0 ± 2.6 |
9.8 ± 2.5 |
0.093 |
| Zinc (mg) |
8.7 ± 2.8 |
8.9 ± 2.9 |
0.484 |
9.1 ± 1.8 |
8.9 ± 1.9 |
0.833 |
| Manganese (mg) |
3.5 ± 1.1 |
3.5 ± 1.1 |
0.401 |
3.6 ± 0.9 |
3.4 ± 1.0 |
0.208 |
| β-carotene equivalent (μg) |
3,926 ± 2,674 |
3,945 ± 1,639 |
0.674 |
3,815 ± 2,357 |
4,795 ± 1,881 |
0.036* |
| Retinol equivalent (μg) |
753 ± 555 |
808 ± 471 |
0.779 |
929 ± 459 |
855 ± 370 |
0.674 |
| α-tocopherol (mg) |
7.0 ± 2.5 |
7.6 ± 2.4 |
0.161 |
8.9 ± 2.9 |
9.7 ± 2.4 |
0.161 |
| Vitamin C (mg) |
101.6 ± 50.9 |
188.0 ± 67.0 |
0.012* |
121.2 ± 67.3 |
223.9 ± 46.9 |
0.012* |
| Saturated fat (g) |
13.6 ± 3.9 |
13.2 ± 5.0 |
0.674 |
18.7 ± 3.5 |
16.4 ± 3.9 |
0.093 |
| Monounsaturated fat (g) |
18.4 ± 5.4 |
17.7 ± 5.4 |
0.575 |
25.0 ± 5.3 |
21.5 ± 6.1 |
0.093 |
| Polyunsaturated fat (g) |
13.5 ± 4.0 |
12.4 ± 4.0 |
0.263 |
17.2 ± 4.1 |
14.9 ± 3.8 |
0.069 |
| Cholesterol (mg) |
417 ± 205 |
424 ± 193 |
0.889 |
434 ± 127 |
441 ± 153 |
0.779 |
| Total dietary fiber (g) |
12.2 ± 5.2 |
11.6 ± 4.0 |
0.484 |
13.6 ± 3.8 |
14.1 ± 3.9 |
0.484 |
| Soluble dietary fiber (g) |
3.1 ± 1.5 |
3.2 ± 1.3 |
0.673 |
3.5 ± 1.1 |
3.9 ± 1.1 |
0.093 |
| Insoluble dietary fiber (g) |
8.7 ± 3.5 |
8.1 ± 2.6 |
0.263 |
9.6 ± 2.5 |
9.6 ± 2.6 |
1.000 |
| Sodium chloride equivalent (g) | 10.8 ± 3.1 | 11.6 ± 3.8 | 0.123 | 10.9 ± 2.3 | 11.5 ± 2.2 | 0.401 |
Data shows mean ± S.D.
Baseline vs After 4 weeks, Wilcoxon signed rank test, *p < 0.05.
Vitamin C intake after 4 weeks in both groups were increased compared with baseline values (control group: 101.6 ± 50.9 vs 188.0 ± 67.0 mg, p = 0.012; intervention group: 121.2 ± 67.3 vs 223.9 ± 46.9 mg, p = 0.012).
Food intake
Food intakes of both groups are shown in Table 6. Total vegetable and fruit intake after 4 weeks in both groups were increased compared with baseline values (total vegetable value of control group: 246.0 ± 149.0 vs 324.6 ± 121.3 g, p = 0.012; total vegetable value of intervention group: 280.7 ± 168.3 vs 396.9 ± 121.5 g, p = 0.012; fruits value of control group: 70.1 ± 63.0 vs 438.3 ± 181.3 g, p = 0.017; fruits value of intervention group: 106.2 ± 91.8 vs 524.0 ± 44.2 g, p = 0.012). Furthermore, green and yellow vegetables intake after 4 weeks in both groups were increased compared with baseline values (control group: 105.5 ± 71.8 vs 177.8 ± 60.4 g, p = 0.012; intervention group: 108.1 ± 78.5 vs 225.2 ± 54.6 g, p = 0.012).
Table 6.
Change of food intake
|
Control group (n = 8) |
|
Intervention group (n = 8) |
|
|||
|---|---|---|---|---|---|---|
|
Baseline |
After 4 weeks |
|
Baseline |
After 4 weeks |
|
|
| Mean ± S.D. | Mean ± S.D. | p | Mean ± S.D. | Mean ± S.D | p | |
| Cereals (g) |
459.1 ± 125.5 |
490.8 ± 190.9 |
0.735 |
456.9 ± 102.6 |
428.8 ± 129.7 |
0.310 |
| Potatoes (g) |
53.2 ± 38.6 |
47.0 ± 23.2 |
0.600 |
52.3 ± 46.0 |
48.4 ± 16.3 |
0.600 |
| Nuts and pulses (g) |
87.0 ± 57.8 |
72.1 ± 57.3 |
0.237 |
79.3 ± 34.8 |
73.4 ± 37.9 |
0.600 |
| Total vegetables (g) |
246.0 ± 149.0 |
324.6 ± 121.3 |
0.012* |
280.7 ± 168.3 |
396.9 ± 121.5 |
0.012* |
| Green and yellow vegetables (g) |
105.5 ± 71.8 |
177.8 ± 60.4 |
0.012* |
108.1 ± 78.5 |
225.2 ± 54.6 |
0.012* |
| Other vegetables (g) |
140.6 ± 78.9 |
146.8 ± 74.8 |
0.484 |
172.6 ± 95.1 |
171.7 ± 68.9 |
0.674 |
| Fruits (g) |
70.1 ± 63.0 |
438.3 ± 181.3 |
0.017* |
106.2 ± 91.8 |
524.0 ± 44.2 |
0.012* |
| Fish and shellfish (g) |
75.4 ± 56.9 |
74.9 ± 53.5 |
0.866 |
71.1 ± 31.5 |
75.3 ± 31.5 |
0.674 |
| Meat (g) |
65.6 ± 30.3 |
82.6 ± 44.5 |
0.263 |
77.8 ± 29.1 |
72.5 ± 27.1 |
0.398 |
| Eggs (g) |
51.5 ± 32.0 |
53.2 ± 27.5 |
0.798 |
41.7 ± 24.7 |
48.6 ± 26.7 |
0.310 |
| Dairy products (g) |
191.8 ± 103.1 |
156.4 ± 129.0 |
0.176 |
174.7 ± 82.8 |
150.4 ± 55.0 |
0.176 |
| Fat and oil (g) |
10.4 ± 6.5 |
10.3 ± 6.0 |
0.735 |
14.6 ± 5.3 |
12.9 ± 4.3 |
0.575 |
| Confectionery (g) |
28.8 ± 23.0 |
20.1 ± 17.7 |
0.263 |
73.6 ± 42.1 |
64.7 ± 34.0 |
0.463 |
| Beverage preference (g) | 621.4 ± 325.3 | 508.8 ± 337.3 | 0.069 | 850.0 ± 406.0 | 790.7 ± 281.9 | 0.401 |
Data shows mean ± S.D.
Baseline vs After 4 weeks, Wilcoxon signed rank test, *p < 0.05.
Discussion
We performed an intervention pilot study on the effects of short-term intake of either a juice mixture of fresh fruit and B. rapa or commercial vegetable juice in middle-aged men. The aim of the present study was to compare the effects of a juice mixture of fresh fruit and B. rapa with those of commercial vegetable juice on anthropometric data, blood parameters, and dietary intake.
T-Chol and LDL-Chol after 4 weeks in the intervention group were significantly decreased compared with baseline values. However, T-Chol and LDL-Chol after 4 weeks in the control group were unchanged compared with baseline values. Short-term intake of juice mixture of fresh fruit and B. rapa is thought to enhance cholesterol metabolism. Subjects with hypercholesterolemia who were given two cans of a beverage of mixed green vegetable and fruit beverage (containing broccoli and cabbage for 12 weeks) had reduced LDL-Chol after 3 weeks compared with baseline values [12]. Our results showed a similar result, which infers that reductions in LDL-Chol may involve increases in fecal bile acid by S-methylcysteine sulfoxide contained in Brassica vegetables [12].
Potassium and Vitamin C intake after 4 weeks in both groups were increased compared with baseline values. β-carotene equivalent intake after 4 weeks in the intervention group was increased compared with baseline values. These findings are related to reports on mineral and lipids metabolism from previous studies. Potassium increases activity of LPL through high insulin concentrations [8,13,14]. Magnesium is the essential mineral for the enzyme that is involved in lipid metabolism, capable of decreasing T-Chol concentrations [15,16]. Magnesium intake after 4 weeks in the intervention group tended to increase compared with the baseline value in the present study. Furthermore, β-carotene reduces the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase and inhibits the synthesis of cholesterol [17,18].
Antioxidant vitamins are related to lipid metabolism. LDL-Chol binds to the LDL receptor and is translocated into the cell. However, when LDL-Chol is oxidized, receptor binding affinity is decreased. Antioxidant vitamins prevent oxidation of LDL-Chol and supports lipid metabolism [11]. Vitamin C intake after 4 weeks in both groups were significantly increased compared with baseline values in the present study. Furthermore, β-carotene equivalent intake after 4 weeks in the intervention group was increased compared with the baseline value. These results show that intake of fresh fruit and B. rapa juice mixture increased antioxidant vitamins.
Weight and BMI after 4 weeks in the control group were significantly increased compared with baseline values in the present study. However, there was no difference in dietary energy after 4 weeks compared with the baseline value. We used brief and self-administered diet history questionnaires (BDHQ) as a dietary survey method in our study. BDHQ has been used and validated in many studies [19-21]. However, BDHQ is not as accurate as weighed dietary records. Hence, it was not clear whether dietary changes in the present study caused the increases in weight and BMI in the control group.
Commercial juice contains 7 times more carotene than the juice mixture comprising fresh fruit and B. rapa. However, β-carotene intake in the control group did not change from baseline. BDHQ does not enable the identification of the types of vegetables included in the vegetable juice. Therefore, the fresh fruit and B. rapa juice mixture and commercial juice were calculated as vegetable juice. For these reasons, we were not able to confirm an effect of the β-carotene equivalent of the commercial juice using the dietary survey.
The limitation of the present study is the short-term intervention. We also did not have a control group that did not drink juice. In future, we will perform a long-term intervention of fresh fruit and B. rapa juice mixture, and also examine the effects on lipid metabolism.
Conclusions
We performed an intervention pilot study to compare the effects of a short-term intake of fresh fruit and B. rapa juice mixture with those of commercial vegetable juice in middle-aged men.
Regular consumption of vegetable juices contributed to increased intake of total vegetables and fruits. Interestingly, compared with the intake of commercial vegetable juice, the intake of fresh fruit and B. rapa juice mixture is highly effective in reducing serum cholesterol.
Short-term intake of fresh fruit and B. rapa juice mixture most likely enhances cholesterol metabolism.
Methods
Preparation of fresh fruit and B. rapa juice mixture and commercial juice
Figure 1 shows the protocol adopted for the preparation of fresh fruit and B. rapa juice mixture. The ingredients of the juice are B. rapa (120 g), banana (100 g), and commercial 100% pure apple juice (200 g). We beat B. rapa, banana and commercial 100% pure apple juice with an electric mixer (Vitamix Model No. VM0111, VITA-MIX CORP., USA) at high speed until smooth (1 min., 37,000 rpm). Preparation of this juice mixture was on the basis of the “sanitary management of large scale cooking facilities manual [22]”. Vegetable juice of the control group was a commercialized product. We prepared fresh fruit and B. rapa juice mixture every day. The commercial juice is a blend of several fruits (grape, banana, apple, lemon, and acerola) and various vegetables (carrots, sweet potatoes, lettuce, red pepper, kidney beans, kale, green pepper, Chinese cabbage, broccoli, celery, asparagus, pumpkin, komatsuna, Angelica keiskei, parsley, watercress, cabbage, radish, spinach, and Japanese honewort). It was produced by ITO EN, Ltd (trade name: ITO EN Jujitsu Yasai Banana Mix, Ito En, Ltd, Tokyo, Japan).
Figure 1.
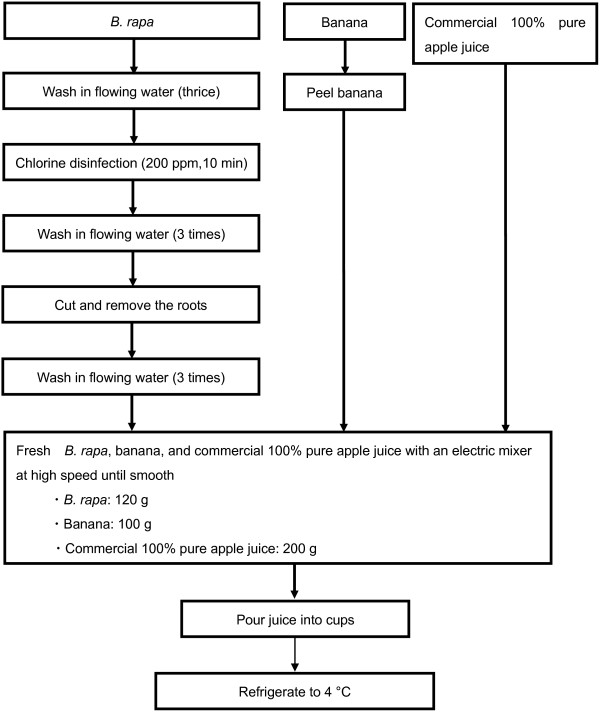
Preparation of fresh fruit and B. rapa juice mixture. The ingredients of the juice include B. rapa (120 g), banana (100 g), and 100% pure, commercial apple juice (200 g). The ingredients were blended on high speed with an electric mixer (Vitamix Model No. VM0111, VITA-MIX CORP., USA) until smooth (1 min, 37,000 rpm). Preparation of the juice mixture followed the guidelines from the “sanitary management of large scale cooking facilities manual [22].
Nutrient components of the fresh fruit and B. rapa juice mixture were analyzed by Japan Inspection Association of Food and Food Industry Environment. Nutrient components of the commercial vegetable juice were noted as per the nutritional labeling on the juice package.
Subjects
Subjects were 16 worker men with a mean age of 46.4 ± 7.1 years. The subjects volunteered after we described the study in great detail. The predominant work of the subjects was desk work. The physical activity level of the subjects were described as low. They were divided into two groups (control and intervention groups). One person had Grave’s disease in the study, who was taking medication. However, no effect on the blood marker was found, regardless of his exclusion. Other than this subject, no other subjects took medication. This study was conducted after receiving approval from the University of Shizuoka ethics committee (No. 24–13). In this study written informed consent was obtained from all the subjects.
Study design
This study was performed for 4 weeks as a single blind, randomized controlled trial from October 30th to November 27th, 2012.
Both groups consumed juice twice a day (at 10:00 a.m. and 3:00 p.m.) every weekday. The intervention group consumed freshly prepared fruit and B. rapa juice mixture (210 g). The control group consumed a commercial vegetable juice (ITOEN JUJITSU YASAI BANANA MIX, ITO EN, LTD, Tokyo, Japan) (200 g) at the same time. The fruit and B. rapa juice mixture of the intervention group and the commercial vegetable juice of the control group were of equivalent energy content. The subjects were unaware of which juice they consumed.
Anthropometric measurements and blood sampling
Subjects fasted from 20:00 of the night before body measurements and blood collection. Height, weight, body fat percentage (BFP), waist circumference, and blood pressure were measured the following morning between 08:00 and 10:00. Body weight and BFP were measured with a body fat analyzer (TBF-215; Tanita, Tokyo, Japan). Waist circumference was measured with a tape measure at the level of the navel. Blood pressure was measured with a digital automatic sphygmomanometer (HEM-5001; OMRON, Kyoto, Japan). Waist circumference and blood pressure were measured twice each, and the mean was calculated. Blood was collected between 07:00 and 10:00.
Blood for plasma vitamin C analysis was collected in vacuum blood collection tubes containing sodium fluoride and dipotassium EDTA, and blood for other tests was collected in vacuum blood collection tubes containing heparin. After collection, blood collection tubes were immediately inverted and mixed, and those for other tests were left at room temperature for 30 min after collection. Collection tubes were then centrifuged for 15 min at 4°C and 3000 rpm. The plasma portion for vitamin C analysis and the serum portion for other tests were then added to microtubes and stored at -80°C until analysis. Analyses of total protein, high-density lipoprotein cholesterol (HDL-Chol), LDL-Chol, triacylglycerol, free fatty acids, and hemoglobin A1c were outsourced to SRL (Tokyo, Japan). Serum leptin was analyzed with a Human Leptin (Highly Sensitive) Assay Kit (Immuno-Biological Laboratories, Gunma, Japan). Serum adiponectin was analyzed with an Adiponectin Assay kit (Otsuka Pharmaceutical Co., Ltd, Japan). Plasma vitamin C was analyzed by the α,α’-Dipyridyl method [23,24].
Food and nutrition survey
The food and nutrition survey used was the BDHQ. The BDHQ is a 4-page fixed-portion questionnaire that asks about the consumption frequency of selected foods, but not about portion size, to estimate the dietary intake of 58 food and beverage items during the preceding month [19-21].
Statistical analysis
A normality test (Using Shapiro–Wilk test) was performed before conducting comparisons on measures before and after the intervention. When normal distribution was found, we used a paired t-test for comparisons before and after the intervention. Furthermore, a Wilcoxon signed-rank test was performed when normal distribution was not found. Data were analyzed using SPSS for Windows version 15.0 J computer software (SPSS Japan Inc, Tokyo, Japan). Values of p < 0.05 were accepted as significant. All values in the text and tables are represented as the mean ± standard deviation.
Abbreviations
B. rapa: Brassica rapa L. var. perviridis; T-Chol: Total cholesterol; LDL-Chol: Low-density lipoprotein cholesterol; BMI: Body mass index; BDHQ: Brief-type self-administered diet history questionnaire; BFP: Body fat percentage; EDTA: Ethylenediaminetetraacetate; HDL-Chol: High-density lipoprotein cholesterol; HOMA-IR: Homeostasis model assessment insulin resistance.
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
All authors managed the study. IA, HI and YS conducted blood analysis and dietary assessment. IA, HI, YS and TK undertook statistical analysis. IA, HI and TK drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
Laboratory of Nutrition Education, Department of Food and Nutritional Sciences and Environmental Health Sciences, Graduate School of Integrated Pharmaceutical and Nutritional Sciences, University of Shizuoka, Shizuoka 422–8526, Japan.
Contributor Information
Izumi Aiso, Email: s12606@u-shizuoka-ken.ac.jp.
Hiroko Inoue, Email: inoue@u-shizuoka-ken.ac.jp.
Yukiko Seiyama, Email: vj.u.klk.o0@gmail.com.
Toshiko Kuwano, Email: kuwano@u-shizuoka-ken.ac.jp.
Acknowledgments
We are grateful to subjects for participation in the study. This study was supported by the University of Shizuoka.
References
- Asia Pacific Cohort Studies Collaboration. Cholesterol, diabetes and major cardiovascular diseases in the Asia-Pacific region. Diabetologia. 2007;50:2289–2297. doi: 10.1007/s00125-007-0801-2. [DOI] [PubMed] [Google Scholar]
- Guimarães PR, Galvão AM, Batista CM, Azevedo GS, Oliveira RD, Lamounier RP, Freire N, Barros AM, Sakurai E, Oliveira JP, Vieira EC, Alvarez-Leite JI. Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz J Med Biol Res. 2000;33:1027–1036. doi: 10.1590/S0100-879X2000000900006. [DOI] [PubMed] [Google Scholar]
- Djousse L, Arnett DK, Coon H, Province MA, Moore LL, Ellison RC. Fruit and vegetable consumption and LDL cholesterol: the National Heart, Lung, and Blood Institutte Family Heart Study. Am J Clin Nutr. 2004;79:213–217. doi: 10.1093/ajcn/79.2.213. [DOI] [PubMed] [Google Scholar]
- Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Les Chik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- The Ministry of Health, Labor and Welfare. Health Japan 21. http://www.dietitian.or.jp/english/images/health_japan21.pdf.
- Shenoy SF, Kazaks AG, Holt RR, Chen HJ, Winters BL, Khoo CS, Poston WS, Haddock CK, Reeves RS, Foreyt JP, Gershwin ME, Keen CL. The use of a commercial vegetable juice as a practical means to increase vegetable intake. Nutr J. 2010;9:38. doi: 10.1186/1475-2891-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SF, Poston WS, Reeves RS, Kazaks AG, Holt RR, Keen CL, Chen HJ, Haddock CK, Winters BL, Khoo CS, Foreyt JP. Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: a randomized controlled trial. Nutr J. 2010;9:8. doi: 10.1186/1475-2891-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Nieman DC, Gillitt ND, Shanely RA, Cialdella-Kam L, Henson DA, Sha W. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: a metabolomics-based approach. Int J Sport Nutr Exerc Metab. 2013;23:150–160. doi: 10.1123/ijsnem.23.2.150. [DOI] [PubMed] [Google Scholar]
- Potter AS, Foroudi S, Stamatikos A, Patil BS, Deyhim F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr J. 2011;10:96. doi: 10.1186/1475-2891-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Reddy KS, Radhakrishnan J, Kumar DA, Zehra A, Agawane SB, Madhusudana K. Influence of antioxidant rich fresh vegetable juices on starch induced postprandial hyperglycemia in rats. Food Funct. 2011;2:521–528. doi: 10.1039/c1fo10093a. [DOI] [PubMed] [Google Scholar]
- Suido H, Tanaka T, Tabei T, Takeuchi A, Okita M, Kishimoto T, Kasayama S, Higashino K. A mixed green vegetable and fruit beverage decreased the serum level of low-density lipoprotein cholesterol in hypercholesterolemic patients. J Agric Food Chem. 2002;50:3346–3350. doi: 10.1021/jf0116698. [DOI] [PubMed] [Google Scholar]
- Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Liu G, Sun H, Kitajima S, Morimoto M, Watanabe T, Yamada N, Fan J. Overexpression of lipoprotein lipase in transgenic Watanabe heritable hyperlipidemic rabbits improves hyperlipidemia and obesity. J Biol Chem. 2004;279:7521–7529. doi: 10.1074/jbc.M311514200. [DOI] [PubMed] [Google Scholar]
- Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 2001;276:40071–40079. doi: 10.1074/jbc.M105456200. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kawasaka T, Nakamura M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br J Nutr. 1997;78:737–750. doi: 10.1079/BJN19970191. [DOI] [PubMed] [Google Scholar]
- Fuhrman B, Elis A, Aviram M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. 1997;233:658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- Moreno FS, Rossiello MR, Manjeshwar S, Nath R, Rao PM, Rajalakshmi S, Sarma DS. Effect of beta-carotene on the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat liver. Cancer Lett. 1995;96:201–208. doi: 10.1016/0304-3835(95)03933-N. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Ushio F, Amano K, Morihara M, Todoriki O, Uehara Y, Toyooka E. Serum biomarker-based validation of a self-administered diet history questionnaire for Japanese subjects. J Nutr Sci Vitaminol. 2000;46:285–296. doi: 10.3177/jnsv.46.285. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, Fukui M, Date C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14:1200–1211. doi: 10.1017/S1368980011000504. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, Fukui M, Date C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22:151–159. doi: 10.2188/jea.JE20110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare. Sanitary management of large scale cooking facilities manual (in Japanese) http://www.mhlw.go.jp/topics/syokuchu/01.html.
- Murata A. Plasma vitamin C concentrations of Japanese (in Japanese) Vitamins. 2006;80:513–515. [Google Scholar]
- Murata A, Ishimatsu H, Uchi Y, Kang YC, Kato F. Determination of vitamin C by the α, α’-dipyridyl method (in Japanese) Bull Fac Agr Saga Univ. 1986;61:9–19. [Google Scholar]


