Abstract
Background:
Oxidation of low density lipoproteins and their further uptake by macrophages is known to result in the formation of foam cells, which are critical in the initiation of atherosclerosis through activation of inflammatory signalling cascades. Thus, powerful dietary antioxidants are receiving attention for the reversal of such pathological states.
Materials and Methods:
Extracts of Scoparia dulcis have been used as tea and health drinks with various health promoting effects. In the present study, we examined the reactive oxygen scavenging potential as well as anti-inflammatory and anti-atherogenic efficacies, using leaf extracts obtained after successive extraction with various solvents.
Results:
A methanol extract showed potent antioxidant activity with an IC50 value of 570 μg/ml, caused hydrogen peroxide scavenging (28.9 µg/ml) and anti-inflammatory effects by improving human erythrocyte membrane stabilisation (about 86%). The methanol extract also efficiently inhibited lipid peroxidation and oxidation of low density lipoproteins, thus preventing foam cell formation in cultured RAW 264.7 cells. Furthermore, phytochemical screening of the extracts showed high accumulation of flavonoids.
Conclusions:
The foliar methanol extract of Scoparia dulcis has a strong anti-atherogenic potential and this property could be attributed maybe due to presence of flavonoids since HPLC analysis showed high concentrations of myricetin and rutin in the methanol extract.
Keywords: Anti-atherogenic, anti-inflammatory, flavonoids, foam cells, Scoparia dulcis
INTRODUCTION
Human blood vessels act as channels to deliver oxygen and nutrients to every cell in our body and remove waste and this explains why maintenance of a healthy vasculature is crucial for health, well-being and ultimately survival. Vascular endothelial cells also contribute to the formation of new blood cells through a process known as haematopoiesis. It is not surprising that abnormalities in the vasculature or vascular dysfunction are linked to diverse disorders, from cancer to heart failure and death. Better understanding of mechanism of blockage and its prevention is crucial for improving human health. Atherosclerosis is a disease of the arteries characterised by the deposition of plaques of fatty material on their inner walls and this constitutes a major risk for cardiovascular disease, which is a leading cause of death worldwide.[1] Atherosclerosis is an inflammatory process involving soluble mediators, macrophages and vascular smooth muscle cells.[1] Low density lipoproteins (LDL) play a critical role in atherogenesis, by passing through the arterial wall and accumulating locally in the intima of arterial walls where they are prone to undergo oxidation. Oxidised Low density lipoproteins (oxLDL) stimulate inflammatory signalling, trigger macrophages to accumulate cholesterol and to form foam cells of fatty streaks- the hallmark of early atherosclerotic lesions.[1] This is further followed by the development of fibrous and atheromatous plaques.[1] Furthermore, in cultured cells, it is shown that oxLDL can induce both morphological changes and DNA fragmentation, characteristic of apoptosis.[2] Oxidative processes are likely to play a key role in determining the fate of lesion formation, its progression and enhancement of the plaque stability.[3] Epidemiological evidence indicates that plants, which are rich in antioxidants and anti-inflammatory properties, are able to reduce cardiovascular pathological conditions,[4] indicating the need for more research in this area.
Scoparia dulcis L. (Scrophulariacae), also known as sweet broom weed, is an edible ethnomedicinal folklore herb used in the Indian Ayurveda to treat kidney stones.[5] Additionally, this plant has attracted global interest for its various medicinal properties. These include anti-diabetic properties, anti-hypertensive effects as well as anti-hyperlipidemic and anti-tumour effects.[6,7] Recently, Coulibaly et al.,[8] showed that the whole S. dulcis plant possessed high antioxidant and anti-inflammatory properties. Furthermore, it has been reported to lower the LDL cholesterol levels in streptozotocin-diabetic rats.[9]
In combination with the activities described above and the kidney stone dissolving property of the plant,[5] the latter property is a significant feature in carotid atherosclerosis.[10] Therefore, it could be envisioned that S. dulcis may have the potential to combat atherosclerosis although this has not been experimentally established so far. Here, we examined the foam cell preventing potential of extracts obtained from leaves of S. dulcis which has not previously been done with this plant. Since; atherosclerosis is precluded by free radical mediated oxidation and inflammation, we examined the potential of this plant to prevent oxidation and inflammation. The aim of this study was to examine the antioxidant and anti-inflammatory properties of various extracts of S. dulcis and their ability to inhibit LDL oxidation, thus preventing foam cell formation in cell line models. Furthermore, we screened the methanol extract for its phytochemical content because this could be responsible for the activity.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India), except for hydrogen peroxide (from Avantor Performance Materials India Ltd., RANKEM, Faridabad, India), potassium dihydrogen phosphate and fetal bovine serum (from HiMedia Laboratories, Mumbai, India), butyl hydroxyl anisole, DPPH, DMEM media, antibiotics (streptomycin-penicillin), thiobarbituric acid, aluminium chloride, 1,1,3,3-tetramethoxypropane, paraformaldehyde, quercetin, Oil red O stain and Folin-Ciocalteu's reagent (from Sigma-Aldrich, Bangalore, India).
Plant collection and extraction
Scoparia dulcis plants were collected from watery areas of the Thrissur district, Kerala State, India, and grown in a greenhouse under ambient conditions. The leaves were collected, washed briefly with distilled water and oven dried at 55°C (until a uniform weight was obtained) and powdered leaves (20g) were extracted sequentially with different organic solvents in the order hexane<ethyl acetate<acetone<methanol. The extraction was carried out according to the method described by Abdille et al.[11]
Antioxidant activities
The free radical scavenging activity of the extracts were measured in vitro by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay.[12] The DPPH radical scavenging activity was calculated as follows:
DPPH radical scavenging (%) =100× (A0-AS)/A0, where A0 is the absorbance of the control and AS is the absorbance of the sample. Hydrogen peroxide scavenging assay was carried out following the procedure described by Gülçin et al.[13]
Lipid peroxidation inhibition activity
The degree of lipid peroxidation was assayed by estimating the thiobarbituric acid-reactive substances (TBARS), using the method of Ohkawa et al.,[14] with minor modifications. Briefly, different concentrations of extracts (1-7mg/ml) were added to 10% rat liver homogenate. Lipid peroxidation was initiated by adding 100 μl 15 mM ferrous sulphate (FeSO4) solution to 3ml liver homogenate (final concentration was 0.5mM). After 30 min, 100 μl of this reaction mixture was added to a tube containing 1.5ml 0.67% thiobarbituric acid (TBA) in 50% acetic acid (w/v). The mixture was incubated in a boiling water bath for 30 min to complete the reaction. The absorbance of the pink coloured complex formed was measured at 535 nm using a spectrophotometer (Shimadzu UV 160 Spectrophotometer, Shimadzu, Japan). The percentage inhibition of lipid peroxidation was calculated as for the DPPH radical scavenging.
Stabilisation of human red blood cell (HRBC) membranes
The influence of the extracts on stabilisation of HRBC was assessed by the method of Bode and Oyedapo.[15] Blood from healthy human volunteers was diluted with Alsever's solution containing dextrose (2%, w/v), sodium citrate (0.8%, w/v), citric acid (0.05%, w/v) and sodium chloride (NaCl, 0.42%, w/v) in water (the ratio of blood and Alsever's solution was 1:1, v/v) and centrifuged at 1500g. The packed cells were mixed with isosaline (0.85% NaCl, w/v at pH 7.2) and centrifuged at 1500g until a clear supernatant was obtained and then a 10% (v/v) suspension with isosaline was made. The reaction mixture for each extract contained 1 mg/ml extract, 2ml hyposaline (0.36% NaCl, w/v), 1.0 ml 0.1 M phosphate buffer (pH 7.4) and 0.5 ml erythrocyte suspension to reach a total volume of 4.5 ml. The standard anti-inflammatory drug for the assay was ibuprofen.[16] The control was prepared as above without extract (or ibuprofen as control) whereas the extract control (4.5 ml) lacked the erythrocyte suspension. The reaction mixtures were incubated at 56°C for 30 min. The absorbance of the released haemoglobin was read spectrophotometrically at 560 nm (Shimadzu UV 160 Spectrophotometer, Shimadzu, Japan). The membrane stability (%) was calculated as follows:
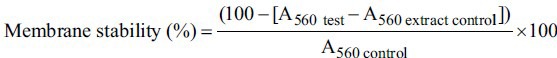
Anti-atherosclerotic effects: Protection of LDL from oxidation
Low density lipoprotein (LDL) isolation was done by the method of Orrego et al.[17] Blood from healthy human volunteers was collected in anticoagulant tubes containing 0.1% ethylene diamine tetra acetic acid (EDTA, w/v) as anticoagulant. The tubes were centrifuged at 600g for 15 min and plasma was collected. The density of plasma was then adjusted to 1.3 g/ml with potassium bromide (KBr). A volume of 1.5 ml adjusted plasma was layered under 3.5 ml of normal saline (density = 1.006 g/ml) in 5 ml ultra clear quick seal tubes to form a discontinuous NaCl/KBr density gradient. This was then centrifuged in Beckman vertical rotor Vti 65 at 369,548 g for 90 min. Collection of lipoprotein fractions was done by a peristaltic pump and the collected LDL fractions were dialysed overnight against 0.1 M phosphate buffer saline (PBS, pH 7.4) to remove KBr and EDTA.
LDL oxidation prevention capacity of extracts was assayed using the method of Orrego et al.[17] Two different concentrations of the extracts or Butyl hydroxy anisole (BHA) was incubated with LDL (100 μg protein/ml) in 50 mM PBS (pH 7.5) in a total volume of 4 ml for a period of 30 min. The positive control contained only LDL, but no extracts or BHA. Initiation of the reaction was done by the addition of 10 μM CuSO4 to all tubes. Aliquots of 0.5 ml were taken at 5 and 20 h after initiation and mixed with 0.25 ml 2.5% trichloroacetic acid (v/v) and 0.25 ml 1.0% (w/v) 2-thiobarbituric acid. The aliquots were vortexed and kept in a boiling water bath for 30 min. After 30 min, the mixtures was cooled to room temperature and the pink chromogen was extracted in n-butanol (2.0 ml) followed by spectrophotometric readings at 532 nm (Shimadzu UV 160 Spectrophotometer, Shimadzu, Japan). TBARS concentrations were calculated from a standard graph prepared using 1,1,3,3-tetramethoxypropane as standard. Results are expressed as nanomoles malondialdehyde MDA equivalents/mg of LDL protein and as per cent inhibition on MDA-TBARS formation compared to the control.
Anti-atherosclerotic effects: foam cell formation
LDL isolation was performed as described for lipid peroxidation activity. Oxidation of LDL was performed by the method of Greenspan et al.[18] Incubation of LDL (200 μg protein/ml) took place in 0.01 M PBS (pH 7.4), which contained 5 μM CuSO4 for 18 h at37°C. RAW 264.7 macrophage cells were procured from The National Centre for Cell Sciences (Pune, India) and grown in Dulbecco's modified Eagle's Medium supplemented with 10% (w/v) fetal bovine serum and 1% antibiotics (streptomycin-penicillin-G). Cells (3 × 105) were seeded in 12 well plates and grown in media to which oxLDL (100 μg/ml) and extracts (30 μg/ml) were added followed by incubation for 48 h. Conversion of normal RAW 264.7 cells to foam cell morphology was observed by processing the cells by Oil red O staining after 48 h, by the method of Kalayoglu and Byme.[19] Briefly, RAW 264.7 macrophages were fixed using 2% paraformaldehyde for 15 min, stained for 30 min with 1% Oil-Red O (in 60% isopropanol), washed with distilled water and observed at 60X magnification on an inverted microscope (Olympus IX 50).
Estimation of total phenolics
Total soluble phenolics of the extracts were determined by the method of Gülçin et al.[20] with Folin-Ciocalteu's reagent using gallic acid as the standard. For each extract, 1 ml aliquots (1 mg/ml) was added to test tubes and the final volume adjusted to 10 ml by the addition of distilled water. Folin-Ciocalteu's reagent (1 ml) was added to this mixture, followed by 3 ml 2% (w/v) sodium bicarbonate (NaHCO3) 3 min later. Subsequently, the mixture was shaken for 2 h at room temperature and the absorbance measured spectrophotometrically at 760 nm (Shimadzu UV 160 spectrophotometer, Shimadzu, Japan). The concentration of total phenolic compounds in the extracts was determined as μg gallic acid equivalents by using a standard gallic acid graph.
Estimation of total flavonoids
The total flavonoid content in the extracts was determined by using the colorimetric assay originally described by Basniwal et al.,[21] with slight modifications. Briefly, 0.3 ml solution of extracts (1 mg/ml) in 45% ethanol was separately mixed with 8 ml 10% aluminium chloride (w/v) in distilled water and 4 ml 0.2 M sodium acetate (CH3COONa). The mixed solution was then immediately diluted to a volume of 25 ml with deionised water, mixed thoroughly and left at room temperature for 30 min. The absorbance of the reaction mixture was measured spectrophotometrically at 350 nm (UV-2450 Spectrophotometer, Shimadzu, Japan). Total flavonoid content was calculated from a standard curve using quercetin. The results are expressed asmg quercetin equivalents/100 mg extract.
Estimation of total carotenoids
The absorbance of the different extracts was recorded spectrophotometrically at 450, 470, 645 and 661.5 nm (Shimadzu UV 160 spectrophotometer, Shimadzu, Japan). The content of chlorophyll and total carotenoids were calculated using the Lichtenthaler equation:[22]
Chlorophyll a: Chl (a) (μg/ml) = 11.24 × OD661.5 - 2.04 × OD645
Chlorophyll b: Chl (b) (μg/ml) = 20.13 × OD645 - 4.19 × OD661.5
Total chlorophyll (μg/ml) = [7.05 × OD661.5 + 18.9 × OD645]
Total carotenoids (μg/ml) = (1000 × OD470 - [1.9 × chl (a) +63.14 × chl (b)])/214
Estimation of total tannins
The tannins were quantified using the method of Price and Butler,[23] with some modifications. In short, 0.5 ml aliquots of extracts (5 μg/ml) were transferred to test tubes. To each tube, 1 ml 1% potassium ferricyanide (K3Fe(CN)6) and 1 ml 1% ferric chloride (FeCl3) were added, followed by addition of water to a final volume of 10 ml. After 5 min, the absorbance of the solutions was measured spectrophotometrically at 720 nm (Shimadzu UV 160 spectrophotometer, Shimadzu, Japan). The tannin concentrations were calculated on the basis of the optical absorbance values obtained for the standard solutions of tannic acid in the range of 5-25 μg/10 ml.
Chromatographic conditions and HPLC analysis
The HPLC flavonoid fingerprint of the methanol sequential extract (4.0 mg/ml) was performed by the method of Sonia Mesía-Vela.[24] A C-18 reverse phase column (150 mm × 4.6 mm) was used. In the HPLC programme, the mobile phases were: A = 0.1% trifluoroacetic acid (TFA) (pump A) and acetonitrile (ACN) (pump C). The gradient was 0-10 min 100% A, 10-20 min 80% A, 20-30 min 60% A and 30-40 min 40% A. Detection was at 350 nm, the flow rate 1 ml/min, the run time 40 min and sample size 20μl. Peaks were identified using flavonoid standards.
Statistical analysis
All experiments were carried out independently three times (n = 3) and results expressed as mean ± SEM. Statistical analyses (ANOVA) were carried out with the programmepackage SPSS, version 10.0 and means were separated by Tukey's test (P < 0.05).
RESULTS
DPPH radical scavenging activity
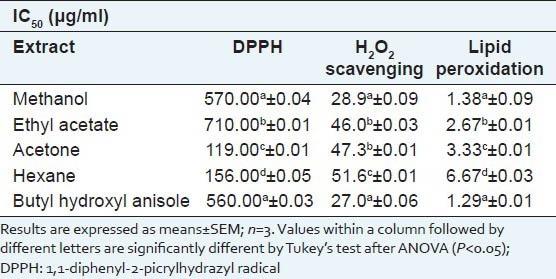
The scavenging activity of the four extracts against DPPH was concentration-dependent [Table 1]. The methanol extract showed the highest DPPH scavenging activity followed by the ethyl acetate, acetone and hexane extracts. Interestingly, the methanol extract was as effective as the positive control, BHA, in scavenging 50% of DPPH radicals present in the reaction mixture (IC50) [Table 1]. The IC50 values of ethyl acetate, acetone and hexane extracts were significantly different from each other and higher and therefore lesser activity than methanol extract.
Table 1.
Antioxidant and anti-inflammatory assays of Scoparia dulcis sequential extracts. IC50 values of different sequential extracts are compared with that of butyl hydroxyl anisole in DPPH assay, H2O2 scavenging assay and lipid peroxidation assay
H2O2 scavenging activity
H2O2 scavenging activity was evaluated by a decrease in the formation of chromogen in the Fenton reaction [Table 1]. The H2O2 scavenging activity was highest in the methanol followed by the ethyl acetate extract as indicated by their IC50 values of 28 and 46 μg/ml, respectively. The IC50 values of the different extracts were significantly different from each other except for the ethyl acetate and acetone extracts. The IC50 values of BHA and the methanol extract were not significantly different, showing that the methanol extract was as effective as BHA in scavenging H2O2.
Lipid peroxidation inhibition activity
The inhibition of lipid peroxidation by the four extracts of S. dulcis leaves on rat liver homogenate was measured by the colour intensity of the MDA-TBA complex and the ability of inhibition by different extracts was evaluated by comparing their IC50 values, the extract with the lowest IC50 value having the best inhibition capacity [Table 1]. Thus, the methanol and ethyl acetate extracts showed the highest effectiveness with IC50 values of 1.38 and 2.67 μg/ml respectively. On the other hand, the hexane and acetone extracts were not as effective in the lipid peroxidation assay as in the other antioxidant assays. The methanol extract had an IC50 value not significantly different from BHA [Table 1], thus being better than the other extracts in inhibiting lipid peroxidation. All the other solvent extracts differed significantly from BHA and the methanol extract.
Human red-blood cell membrane stabilisation
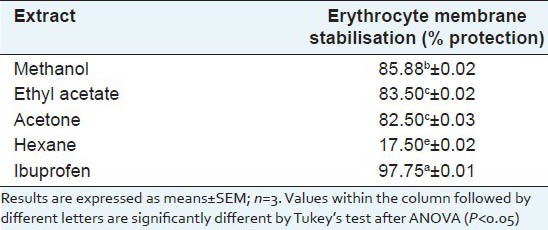
The anti-inflammatory properties of the leaf extracts of S. dulcis were tested by the HRBC membrane stabilisation method [Table 2]. The leaf extracts showed variable degrees of protection against erythrocyte cell lysis. The percentage of protection was highest in the methanol extract followed by the ethyl acetate and acetone extracts, although the level of protection afforded by the extracts was less than that of Ibuprofen. The hexane extract was able to give protection against cell lysis to a lower extent (17.50%) than the acetone extract (82.50%), which in turn had lower protection ability than the methanol extract (85.88%). The protection levels exerted by the different extracts were significantly different from each other except for the ethyl acetate (83.50%) and acetone extracts (82.50%). Thus, most leaf extracts had considerable anti-inflammatory properties and especially the methanol extract provided significant protection against erythrocyte lysis.
Table 2.
Erythrocyte membrane stabilisation of Scoparia dulcis sequential extracts compared with that of the standard anti-inflammatory drug, ibuprofen
Inhibition of LDL oxidation
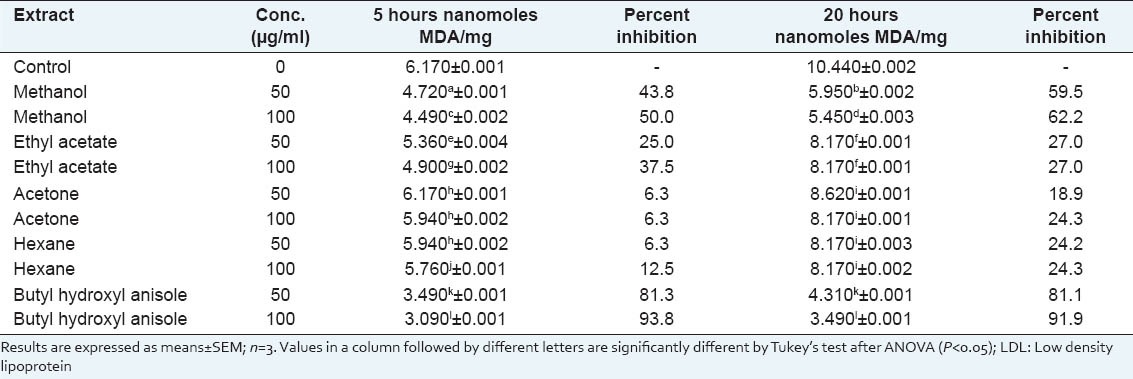
CuSO4-induced LDL oxidation was noted as a time dependent increase in TBARS over a period of 20 hours (h)[Table 3]. The recording time points (5 and 20 h) were selected according to the level of protection provided by the plant extracts. As the concentration of different extracts were increased at 5 h, the percentage inhibition of LDL oxidation also increased. However, exceptions were the acetone and hexane extracts which did not show significant concentration dependent changes. Of the remaining two extracts, the methanol extract showed the highest protective effect. However, at 5h, the percentage inhibition was significantly lower compared to that of BHA. Interestingly, it was seen that whereas the protective effect of BHA stabilised at 20h, the LDL oxidation inhibition percentage of all extracts increased significantly except for the ethyl acetate extract (100 μg/ml) for which the protection percentage decreased significantly at 20 h. The significant increase was both concentration and time dependent for the methanol extract (100 μg/ml showing the highest inhibition) as seen in Table 3. Thus, the methanol extract of S. dulcis leaves was effective in inhibiting LDL oxidation and there was a sustained inhibition with increasing time and increasing concentrations.
Table 3.
Prevention of LDL oxidation by different concentrations of Scoparia dulcis sequential leaf extracts compared to that of butyl hydroxyl anisole
Inhibition of foam cell formation from macrophages induced by oxidised LDL
It is known that incubation of macrophage cells with oxLDL causes the cells to take up LDL in an unregulated manner and this results in formation of foam cells. The effect of the four sequential extracts of S. dulcis on conversion of oxLDL-treated macrophages (RAW 264.7) to foam cells was determined and it was seen that after the addition of the methanol extract at a concentration of 30 μg/ml, oxLDL-treated cells showed a significant decrease or no formation of foam cells at all [Figure 1]. Ethyl acetate, acetone and hexane extracts did prevent the formation of foam cells.
Figure 1.
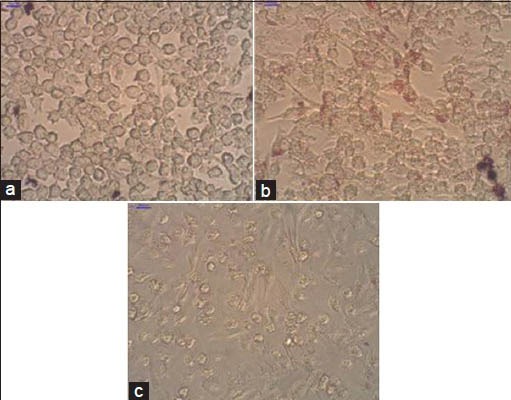
Conversion of cultured mice RAW 264.7 cells to foam cells after treatment with extracts of S. dulcis. (a) Control cells; (b) Cells treated with oxidised LDL resulting in foam cells (stained with Oil red O). The formation of foam cells is seen by the Oil red O stain taken up by cells as shown by the arrows. (c) Cells treated with oxidised LDL and 30 ìg/ml methanol extract. Note absence of foam cells as evident from the absence of stained cells
Phytochemical screening of leaf extracts
The yield and various phytochemical constituents of the different solvent extracts of S. dulcis leaves are shown in Table 4. The amount of extractable components from the dried leaves, expressed as percentage yield, varied significantly from 16.06% (methanol extract) to 1.50% (acetone extract). The amount of total phenolics in terms of gallic acid equivalents varied significantly from 0.10 mg/100 mg in the hexane extract to 1.03 mg/100 mg in ethyl acetate extract. The amounts of total flavonoids varied significantly at the level of P < 0.05 in the different extracts and ranged from 2.50 (hexane extract) to 8.69 (methanol extract) mg quercetin/100 mg extract.
Table 4.
Content of phytochemical in Scoparia dulcis sequential leaf extracts
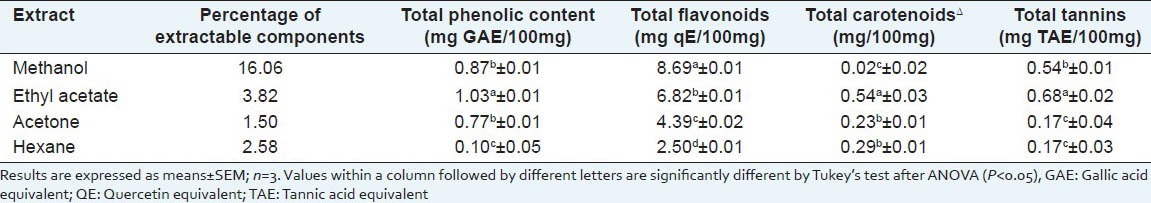
The ethyl acetate extract contained significantly higher amounts of carotenoids (0.54 mg/100 mg) and tannins (0.68 mg TAE/100 mg) than the other extracts, whereas the lowest levels of these phytochemicals were 0.02 mg/100 mg (methanol extract) and 0.17 mg/100 mg (acetone and hexane extracts), respectively.
HPLC profiling for flavonoids in the methanol extract
The HPLC flavonoid fingerprint of the Scoparia dulcis methanol extract is shown in Figure 2. The largest peak, eluted at retention time 23.97 min, was identified to be rutin from standards run using the same programme. Another peak eluted at 24.53 min was identified to be myricetin from the standards. The smaller peaks were not identified.
Figure 2.
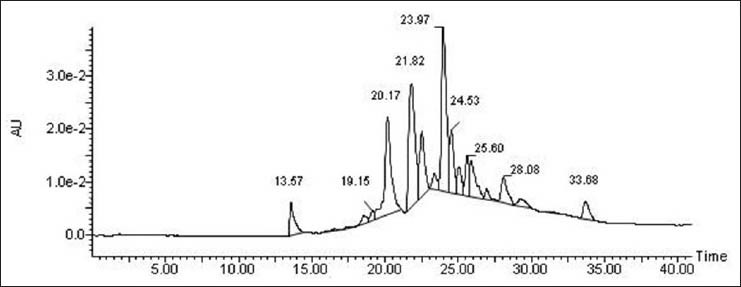
HPLC chromatogram HPLC chromatogram showing flavonoids in the methanol extract from Scoparia dulcis leaves. using C-18 column
DISCUSSION
Plant-based antioxidants can elicit increased synthesis of endogenous antioxidant defences or themselves act directly as antioxidants.[25] The choice of the method used for antioxidant analysis has often been questioned. Therefore, we used an array of different methods for assessing antioxidant activity to analyse the bio-efficacies of different extracts. Scoparia dulcis is an edible plant (also used to prepare health drinks in some areas) that has been used extensively as a medicinal herb since ancient times in India. In the present study, sequential leaf extracts of Scoparia dulcis were studied to assess their capacities to scavenge reactive oxygen molecules. Furthermore the capacity of the extracts to inhibit the formation of foam cells through inhibition of LDL oxidation was examined, as these factors contribute significantly to the initiation of atherosclerosis. It was found that only the methanol extract had the ability to prevent foam cell formation at a concentration of 30μg/ml and furthermore, this extract also showed the highest antioxidant activity which is comparable to that of the standard drug BHA. Previously, S. dulcis has been found to be able to reduce the cholesterol level in blood serum of rats.[9] However this is the first report on the foam cell prevention ability of Scoparia dulcis leaf extracts. Thus, our findings that the antioxidant and anti-inflammatory capacity of S. dulcis leaves is at the same level as that of BHA, coupled with the fact that the plant is edible whereas BHA is carcinogenic,[26] suggest a future possible use of this plant for pharmaceutical purposes or as a neutraceutical through its use in beverages such as tea and juices to help in the prevention of atherosclerosis. However, further research is required for the fulfilment of this purpose.
In vitro incubation of macrophages in the methanol extract of S. dulcis inhibited the formation of foam cells at a concentration as low as 30 μg/ml [Figure 1]. The oxidative modification hypothesis of atherosclerosis suggests that circulating LDL particles are modified by oxidation and such modified LDL molecules are then taken up by macrophages located inside the arterial wall, resulting in building up cholesterol plaques.[1] Such cholesterol-dependent macrophage formation is known to be the first step in initiating atherosclerotic plaques. Hence, it is important to consume natural dietary antioxidative nutrients, capable of reducing plasma cholesterol and suppressing oxidation of LDL so that their accumulation in macrophages is prevented since this will eventually reduce the development of atherosclerosis.[1]
Since the S. dulcis methanol extract showed high antioxidant and anti-inflammatory potential, as illustrated by the ability to prevent foam cell formation, it could potentially reduce the development of aortic atherosclerotic lesions. The inhibition of foam cell formation could possibly be due to inhibition of oxidation of LDL by the antioxidant potential of the plant extract as has been established in other studies. Thus, Esterbauer et al.,[27] reported that aldehyde products from lipid hydroperoxide breakdown processes were responsible for the modification of the LDL apoprotein, thereby inhibiting lipid peroxidation and preventing the formation of foam cells, which in turn prevented the initiation of the atherosclerotic lesions. Furthermore, Hishikawa et al.,[28] suggested that the antioxidant activities of many polyphenols are responsible for their protection against atherosclerosis. In addition, a direct relation between the polyphenol content and antioxidant activity was seen in Carthamus tinctorious.[29]
Azizova et al.,[30] showed that erythrocyte membranes during atherosclerotic conditions are prone to lysis in the presence of oxidised LDL. We found that the methanol extract caused efficient inhibition of lipid peroxidation, which was further supported by its potential to stabilise erythrocyte membranes.
In order to determine whether there was a correlation between the phytochemical content and antioxidant and anti-inflammatory activity in S. dulcis, a phytochemical screening was carried out and it was found that the methanol extract had the highest amount of flavonoids followed by the ethyl acetate extract. Since the methanol extract showed high levels of antioxidant and anti-inflammatory activity, and also because this extract contained high amount of flavonoids, it is probable that the flavonoids present in the extract could be responsible for the positive activity. Earlier, Pereira et al.,[31] correlated high antioxidant activity of plant extracts to high polyphenolic content. Flavonoids are a class of strong anti-lipoperoxidant agents, and they have also been found to strongly scavenge reactive oxygen species known to cause oxidative stress.[32] Reactive oxygen species are involved in tissue injury through initiation of lipid peroxidation, leading to LDL oxidation. Flavonoids were also shown to localise within activated macrophages in human atherosclerotic lesions and prevent the uptake of oxidised LDL through the down-regulation of scavenger receptors,[33] thus indicating their role in prevention of atherosclerosis.
In vitro and in vivo experiments with flavonoids from other sources have demonstrated their strong dietary antioxidant potential, ability to inhibit LDL oxidation, platelet aggregation and suppression of enzymes involved in lipid and lipoprotein metabolism.[34,35] In addition, flavonoids, via suppression of LDL oxidation, may also induce endothelium-dependent vasorelaxation and may increase reverse cholesterol transport.[34] The reason why flavonoids inhibit LDL oxidation could be their chelation of copper ions, as has been demonstrated in the case of quercetin.[36] Grassi et al.,[37] reported that flavonoids possess protective effects against the initiation and progression of atherosclerosis. These results also suggest that the activity of S. dulcis could be due to its flavonoid content, such as rutin, myricetin or quercetin. However the role of other classes of flavonoids such as flavanones, dihydroflavonols and polymethoxylated flavonoids cannot be ruled out and needs further investigation. Furthermore, HPLC analyses of the methanol extract [Figure 2] showed that there was a high accumulation of rutin and myricetin and these compounds have been known to play antioxidant roles and possess LDL oxidation prevention capacity.[38,39,40] Collectively, our results indicate that S. dulcis has high antioxidant and anti-inflammatory properties as shown using a cell line model and that this plant could also be a good source of anti-atherosclerotic compounds due to its sustained protection from oxidative modification of LDL and prevention of foam cell formation. Moreover, S. dulcis is a cost-effective medicinal plant because of its fast growth rate, low need for fertilisation and care as well as its abundant availability. Our work shows that this plant could potentially be a medicinally important food plant with a wide range of applications. However, further analyses of the biological activity of individual flavonoids need to be carried out for developing therapeutic and neutraceutical formulations.
CONCLUSION
This study demonstrated that the methanol extract of S. dulcis leaves had the highest capacity in terms of scavenging free and peroxyl radicals. The extract also showed strong anti-inflammatory properties since it caused the highest inhibition of lipid peroxidation which is the main cause of numerous diseases including cardiovascular and neurodegenerative disorders. The extract showed prevention of the initial stages of atherosclerosis, LDL oxidation and foam cell formation, thus indicating its strong protective effect against inflammation. Since the methanol extract was found to be rich in flavonoids, the observed biological effect could be due to the presence of flavonoids here. However, more work needs to be done on characterisation and purification of the flavonoids which could be considered in food formulations for the production of inexpensive nutritional food items with health-benefitting properties.
ACKNOWLEDMENTS
We thank the Council of Scientific and Industrial Research for providing the funds needed for this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem. 1998;273:9681–7. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- 3.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 4.Corral-Aguayo RD, Yahia EM, Carrillo-Lopez A, Gonzalez-Aguilar G. Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J Agric Food Chem. 2008;56:10498–504. doi: 10.1021/jf801983r. [DOI] [PubMed] [Google Scholar]
- 5.Farook NA, Rajesh S, Jamuna M. Inhibition of mineralization of urinary stone forming minerals by medicinal plants. Eur J Chem. 2009;6:938–42. [Google Scholar]
- 6.Satyanarayana K. Chemical examination of Scoparia dulcis (Linn):Part-I. J Indian Chem Soc. 1969;46:765–6. [Google Scholar]
- 7.Freire SM, Emim JA, Lapa AJ, Souccar C, Torres LM. Analgesic and antiinflammatory properties of Scoparia dulcis L. extracts and glutinol in rodents. Phytother Res. 1993;7:408–14. [Google Scholar]
- 8.Coulibaly AY, Kiendrebeogo M, Kehoe PG, Sombie PA, Lamien CE, Millogo JF, et al. Antioxidant and anti-inflammatory effects of Scoparia dulcis L. J Med Food. 2011;14:1576–82. doi: 10.1089/jmf.2010.0191. [DOI] [PubMed] [Google Scholar]
- 9.Pari L, Latha M. Antihyperlipidemic effect of Scoparia dulcis (Sweet broomweed) in streptozotocin diabetic rats. J Med Food. 2006;9:102–s7. doi: 10.1089/jmf.2006.9.102. [DOI] [PubMed] [Google Scholar]
- 10.Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, et al. Kidney stones and subclinical atherosclerosis in young adults: The CARDIA study. J Urol. 2011;185:920–5. doi: 10.1016/j.juro.2010.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdille MH, Singh RP, Jayaprakasha GK, Jena BS. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005;90:891–6. [Google Scholar]
- 12.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol. 1995;28:25–30. [Google Scholar]
- 13.Gülçin I, Köksal E, Elmastas M, Aboul-Enein HY. Determination of in vitro antioxidant and radical scavenging activity of Verbascum oreophilum C. Koch var. joannis (Fam. Scrophulariaceae) Res J Biol Sci. 2007;2:372–82. [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Bode SO, Oyedapo OO. Biological activities and phytoconstituents of the lower plant Platycerium angolense, Welwex Hook. J Med Plants Res. 2011;5:1321–9. [Google Scholar]
- 16.Oyedapo OO, Akinpelu BA, Akinwunmi KF, Adeyinka MO, Sipeolu FO. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int J Plant Physiol Biochem. 2010;2:46–51. [Google Scholar]
- 17.Orrego R, Leiva E, Cheel J. Inhibitory effect of three C-glycosylflavonoids from Cymbopogon citratus (lemongrass) on human Low Density Lipoprotein oxidation. Molecules. 2009;14:3906–13. doi: 10.3390/molecules14103906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan P, Yu H, Mao F, Gutman RL. Cholesterol deposition in macrophages: Foam cell formation mediated by cholesterol-enriched oxidized low density lipoprotein. J Lipid Res. 1997;38:101–9. [PubMed] [Google Scholar]
- 19.Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–9. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 20.Gülçin I, Oktay M, Küfrevioglu ÖÌ, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol. 2002;79:325–9. doi: 10.1016/s0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 21.Basniwal PK, Suthar M, Rathore GS, Gupta R, Kumar V, Pareek A, et al. In-vitro antioxidant activity of hot aqueous extract of Helicteres isora Linn. fruits. Nat Prod Rad. 2009;8:483–7. [Google Scholar]
- 22.Lichtenthaler HK. Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–82. [Google Scholar]
- 23.Price ML, Butler LG. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J Agric Food Chem. 1977;25:1268–73. [Google Scholar]
- 24.Mesia-Vela S, Bielavsky M, Torres LM, Freire SM, Lima-Landman MT, Souccar C, et al. In vivo inhibition of gastric acid secretion by the aqueous extract of Scoparia dulcis L. in rodents. J Ethnopharmacol. 2007;111:403–8. doi: 10.1016/j.jep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 26.Ito N, Fukushima S, Haqlwara A, Shibata M, Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst. 1983;70:343–52. [PubMed] [Google Scholar]
- 27.Esterbauer H, Wäg G, Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993;49:566–76. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- 28.Hishikawa K, Nakaki T, Fujita T. Oral flavonoid supplementation attenuates atherosclerosis development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:442–6. doi: 10.1161/01.ATV.0000148404.24271.fc. [DOI] [PubMed] [Google Scholar]
- 29.Paramesha M, Ramesh CK, Krishna V, Kumar YS, Parvathi KM. Hepatoprotective and in vitro antioxidant effect of Carthamus tinctorious L, var Annigeri-2-, an oil-yielding crop, against CCl4-induced liver injury in rats. Pharmacogn Mag. 2011;7:289–97. doi: 10.4103/0973-1296.90406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azizova OA, Piryazev AP, Nikitina NA, Savchenkova AP, Lopukhin YM. Effect of oxidized LDL on hemolytic resistance of erythrocyte. Bull Exp Biol Med. 2002;134:137–8. doi: 10.1023/a:1021175912810. [DOI] [PubMed] [Google Scholar]
- 31.Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, et al. Walnut (Juglans regia L.). leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol. 2007;45:2287–95. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Bok SH, Jeong TS, Lee CH, Choi MS, Park YB. Switzerland: ISB 2001 Congress XVIII, Zürich, International Society of Biomechanics; 2011. Antiatherogenic or hypolipidemic effects of rutin and buckwheat extracts in animal models; pp. 620–2. [Google Scholar]
- 33.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries. Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–34. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 34.Reed J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42(Suppl):S301–16. doi: 10.1080/10408390209351919. [DOI] [PubMed] [Google Scholar]
- 35.Liu CM, Ma JQ, Sun YZ. Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol. 2011;49:3119–27. doi: 10.1016/j.fct.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu[2]+ions: Implications for their antioxidant properties. Biochem J. 1998;330:1173–8. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi D, Desideri G, Ferri C. Flavonoids: Antioxidants against atherosclerosis. Nutrients. 2010;2:889–902. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yildirim HK, Akçay YD, Güvenç U, Sözmen EY. Protection capacity against low-density lipoprotein oxidation and antioxidant potential of some organic and non-organic wines. Int J Food Sci Nutr. 2004;55:351–62. doi: 10.1080/09637480412331319781. [DOI] [PubMed] [Google Scholar]
- 39.Chopra M, Fitzsimons PE, Strain JJ, Thurnham DI, Howard AN. Nonalcoholic red wine extract and quercetin inhibit LDL oxidation without affecting plasma antioxidant vitamin and carotenoid concentrations. Clin Chem. 2000;46:1162–70. [PubMed] [Google Scholar]
- 40.Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, et al. Antioxidant properties of quercetin. Adv Exp Med Biol. 2011;701:283–9. doi: 10.1007/978-1-4419-7756-4_38. [DOI] [PubMed] [Google Scholar]


