Abstract
Background:
Alzheimer's disease is a neurodegenerative disease related to memory impairments and neuronal cell death. Bozhougyiqi-Tang (BZYQT), a traditional herbal medicine, has been therapeutically used for the treatment of pulmonary tuberculosis.
Objective:
The aim of this study is to evaluated the neuroprotective effect of the fermented BZYQT and compared with unfermented BZYQT in HT22 cells by MTT assay and tested the beneficial effect on memory impairments induced by scopolamine (1 mg/kg, i.p.) using the passive avoidance and Morris water maze tests.
Results:
Compared with unfermented BZYQT, the neuroprotective effect of fermented BZYQT on glutamate induced neurotoxicity in HT22 cells increased at a concentration of 100 μg/mL. Fermented BZYQT increased the step-through latency of the passive avoidance response. Furthermore, in Morris water maze test for evaluation of spatial learning and memory, escape latency time was significantly reduced by fermented BZYQT.
Conclusion:
These results suggest that the fermentation process of BZYQT led to improve neuroprotective and cognitive enhancing effect.
Keywords: Bozhougyiqi-Tang, cognitive-enhancing activity, fermentation, Morris water maze, neuroprotective effect
INTRODUCTION
Alzheimer's disease (AD), a neurodegeneration that causes disruptions in memory, cognition in the elderly population over 65 years is one form of dementia.[1,2,3] The possible cause of AD is the accumulation of amyloid plagues on the brain regions by the formation of Aβ (amyloid beta).[4,5] Cholinergic dysfunction contributed to the pathology of AD. Acetylcholine synthesized from choline and acetyl coenzyme is one of the neurotransmitter in the nerve system. Acetylcholine is involved in learning and memory performance.[6,7] AD has been linked to a hydrolysis of acetylcholine by acetylcholinesterase. Oxidative stress induced neuron cell death is associated with the pathogenesis of neurodegenerative diseases such as AD.[8,9] Glutamate, major excitatory neurotransmitter in the central nervous system has been shown to induce oxidative stress by accumulation of reactive oxygen species (ROS) and intracellular Ca2+ influx through iontropic receptors.[10,11,12,13] Cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists are used as the main drug for the treatment of AD.[14] Four cholinesterase inhibitor drug, donepezil, galantamine, rivastigmine and memantine have been currently licensed in many countries. The NMDA receptor antagonist is memantine licensed in several countries. Other treatment, such as antioxidant, Vitamin E and anti-inflammatory drugs has also been used to treatment for AD.
Traditional herbal medicine (THM) was used for the prevention and treatment of many diseases. THM included many constituents of various herbs and appeared numerous effects. Therefore, THM was appropriated for multifactorial disease, like AD.[15,16]
Bozhougyiqi-Tang (BZYQT), a THM is consisted of Panax ginseng, Astragalus membranaceus, Angelica gigas, Bupleurum falcatum, Citrus unshiu, Glycyrrhiza uralensis, Atractylodes japonica, Zingiber officinale, Zizyphus jujuba, Cimicifuga heracleifolia and used for the enhancement of digestive capacity and anti-allergy, anti-inflammatory.[17,18]
In this study, Neuroprotective activity against glutamate-induced cytotoxicity of unfermented BZYQT was evaluated in HT22 cells by MTT assay and was compared the effect of fermented BZYQT. And we investigated whether it improved scopolamine-induced memory loss in mice by a water maze test.
EXPERIMENTAL
Materials
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) was obtained from Gibco BRL. Co. Glutamate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carbboxylic acid (trolox), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and scopolamine were purchased from Sigma (U.S.A.).
Five standard compounds, hespersidin, decursin and glycyrrhizin were obtained from the Korea Food and Drug Administration and ginsenoside Rg3 is obtained from Chromadex (USA). Decursinol is purchased from Elcomscience (Korea).
Preparation of BZYQT sample and fermented BZYQT
The powder of the BZYQT sample (3.0 g) was obtained from the Korea Institute of Oriental Medicine. Fermentation of BZYQT was conducted by Korea Food Research Institute (Korea). BZYQT was comprised of 3.75 g of P. ginseng, 5.63 g of A. membranaceus, 1.88 g of A. gigas, 1.13 g of B. falcatum, 1.88 g of C. unshiu, 3.75 g of G. uralensis, 3.75 g of A. japonica and 1.13 g of C. heracleifolia. The mixture of these crude drugs was extracted by heating extraction method (a water of 8-10 times the weight of herbs) for 3-4 h at 90-100°C. After successive transfer of the test organisms in MRS broth for Lactobacillus spp. at 37°C for 24 h, the activated culture was again inoculated into each broth at the same condition. It was properly diluted to obtain an initial population of 1-5 × 107 CFU/mL and served as the inoculum. The BZYQT water extract was used as the culture media for fermentation after adjusting pH to 7.0 using 1 M NaOH and autoclaved for 15 min at 121°C. After cooling, 750 mL of IS was inoculated with 7.5 mL inoculums as described above. This was incubated at 37°C for a period of 48 h. Fermented BZYQT was prepared in the form of powder by freeze-dryer.
Cell culture and MTT assay
HT22 cell was cultured as described previously. The cells were cultured in DMEM supplemented with 10% (v/v) FBS, 1% penicillin/streptomycin, NaHCO3 (2 mg/mL) and 15 mM HEPES and maintained at 37°C at humidified 5% CO2. Cell viability was evaluated by the MTT reduction assay. HT22 cells were seed into 48 well plates at a density of 6.7 × 104 cells per well. After 24 h incubation, cells were treated with 10, 100 and 1000 μg/mL of sample and trolox (>97% purity), positive control for 1 h before addition of glutamate. MTT solution (1 mg/mL) was added to each well and the incubated for 3 h. Dimethyl sulfoxide was added and the optical density was measured at 570 nm using an ELISA reader.
Animal
Male imprinting control regions mice weighing 25-30 g (age 3 weeks) were obtained from Dae Han Biolink Co. (Eumseong, Korea). The mice were housed in groups of four per cage under laboratory conditions with 12 h light/dark cycle for 1 week adaptation period. The mice were given free access commercial pellet feed and water ad libitum. All of the animal experiments procedures were conducted according to the guidelines of the Committee Animal Care and Use Committee Kangwon National University.
Morris water maze test
Spatial learning and memory was evaluated by using Morris water maze test. The water maze consisted of the large circle pool (90 cm in diameter, 40 cm in height) filled with water at 20 ± 1°C and rendered opaque by the addition of milk. The maze was divided into four equal quadrants with a white escape platform (10 cm in diameter and 26 cm high). The platform was positioned in the center of one quadrant, 1 cm below the water level. All swimming behavior of mice were monitored and analyzed by smart (version 2.5.21) video-tracking system. The mice were given two trial sessions each day for 4 consecutive days, with an inter-trial interval of 20 min. Four start locations were used. The starting point was changed on each day but the position of the platform was unchanged between trials 1 and trials 2 during test. Escape latency to find the platform evaluated during test. The maximum trial length was 120 s. If a mouse fails to find the platform within 120 s, it was guided to the platform and placed on the platform for 10s. On the day 5, the platform was removed from the maze and each mouse was subjected to a probe trial (120 s). The time that spent in the target quadrant (where the platform was hidden) was measured. 0.5% carboxymethyl cellulose (CMC; control group), fermented BZYQT sample (30, 100 and 200 mg/kg body weight) and donepezil (>97% purity, 1 mg/kg) were oral administered 120 min before the testing, respectively. After 90 min, control group was administered subcutaneously with normal saline and all the other groups were given subcutaneously with scopolamine (1 mg/kg, dissolved in normal saline) to induce amnesia. Test was performed 30 min after scopolamine treatment.
Passive avoidance test
Passive avoidance apparatus consisted of two compartments, light and dark compartments (17 × 12 × 10 cm), which equipped with an electrifiable grid floor. A guillotine door was made in the center of the partition between the two compartments. A training trial was performed on the 1st day; the mice were initially placed in the light compartment. The door between the two compartments was opened after 20 s. When mice move in the dark compartment, the guillotine door closed automatically and electric foot-shock (0.1 mA/10 g body weight) of 2 s duration was delivered through grid floor. During each trial, time taken to enter the dark compartment was a record as the training latency. Mice that waited more than 180 s to enter the dark compartment were excluded from the experiment. 0.5% CMC (control group), donepezil (positive control, 1 mg/kg) and fermented BZYQT sample (30, 100 and 200 mg/kg body weight) were orally administered for 120 min prior to start of training trial. After 90 min, amnesia was induced by scopolamine (1 mg/kg body weight) given subcutaneously. 20 h after the training, the mice replaced in the light compartment and the latency time was measured.
Measurement of ROS
ROS production in HT22 cells was detected using the dye 2’7’-dichlorofluorescein diacetate (DCF-DA). The cells were seeded in 48 well plates and various concentrations of fermented BZYQT sample were treated with 2 mM glutamate for 8 h. Subsequently, 10 μM DCF-DA was added to the cells. The cells were then incubated at 37°C for 30 min. The medium was removed and washed twice with phosphate buffered saline (PBS) and extracted with 1% Triton X-100 in PBS for 10 min at 37°C. ROS formation was detected at an excitation wavelength of 490 nm and emission wavelength of 525 nm.
1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay
The free radical scavenging activity was conducted by reduction of the DPPH free radical. In 96 well plate, 150 μL of different concentrations of samples added to 150 μL of DPPH methanol solution (0.4 mM). Absorbance of DPPH solution was measured using an ELISA reader at 517 nm. Scavenging activity was expressed as the % inhibition and was calculated as (1-sample absorbance/only DPPH absorbance) ×100.
Hydrogen peroxide (H2O2) scavenging assay
Different concentrations of the sample were added to the 100 μL of phosphate buffer (pH 0.5, 0.1 M) mixed with 20 μL of 10 mM H2O2 in a 96-well microplate. After incubation at 35°C for 5 min, 30 μL of 1.25 mM (2,2-azinobis (3-ethylbenzthiazolin)-6-sulfonicacid) and 30 μL of 1 unit/mL peroxide were added and again incubated at 35°C for 10 min.
High-performance liquid chromatography analysis condition
The high-performance liquid chromatography (HPLC) analysis of contents in BZYQT was performed on a Shishedo C18 column (250 × 4.6 mm i.d., 5 μm). The column temperature was set at 35°C Mobile phase system consisted of 0.1% (v/v) trifluoroacetic acid as solvent A and acetonitrile as solvent B. The gradient elution system was as follows: 15% (B) in 0-5 min, 15-35% (B) in 5-20 min, 35-50% (B) in 20-25 min, 50% (B) in 25-40 min and 50-70% (B) in 40-50 min. The flow rate was kept at 1.0 mL/min and the injection volume was 20 μL. Ultraviolet (UV) wavelengths were selected at 205 nm, 250 nm and 330 nm. According to the higher UV absorption based on literature of standard compounds, UV wavelengths of each standard compound were selected such as 205 nm for hesperidin (1), decursinol (4), ginsenoside Rg3 (8) and decursin (9), 250 nm for glycyrrhizin (5).
Statistical analysis
Each data value of Morris water maze test, MTT assay, ROS measurement, DPPH and H2O2 scavenging assay were expressed as the mean ± standard deviation. The probe trial test data in the Morris water maze test, data of MTT assay and ROS measurement were analyzed by one-way analysis of variance (ANOVA). The escape latencies of water maze test were analyzed by two-way ANOVA. If the results were significant, significant differences were determined by Tukey's post hoc test. The P < 0.05 were considered to be statistically significant.
RESULTS
The effect of BZYQT and fermented BZYQT against glutamate-induced cell death in HT22 cell was evaluated and compared. Cells treated BZYQT with 100 μg/mL was about relative protection of 4.18%, whereas cells treated fermented BZYQT with 100 μg/mL appeared to be significantly increased relative protection of 22.05% [Figure 1]. MTT assay revealed that fermentation increased the protective effects of BZYQT.
Figure 1.
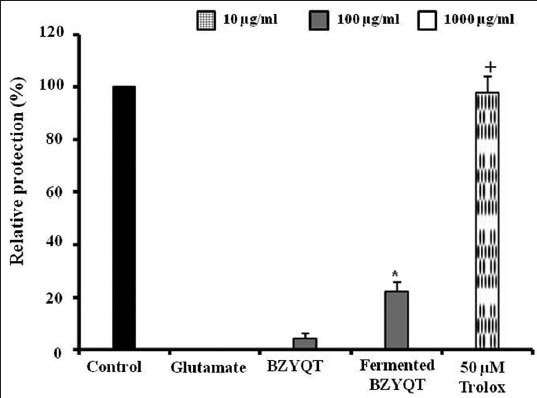
The neuroprotective effects of original Bozhougyiqi-Tang (BZYQT) and fermented BZYQT on glutamate-induced cell death in neuronal HT22 cells. Each bar represents the mean ± standard deviation of three independent experiments. +P < 0.05, ++P < 0.01 and +++P < 0.001 versus glutamate-injured cells; *P < 0.05, **P < 0.01, ***P < 0.001 versus original BZYQT (analysis of variance)
The improvement effects of fermented BZYQT on spatial learning and memory ability were assessed by Morris water maze test. The control group decreased escape latency time from day 2 to 4 in 1st and 2nd trial, respectively. In contrast, scopolamine caused a disruption of learning and memory and scopolamine - treated group increased escape latency time from day 2 to 4. Fermented BZYQT 30 mg/kg and 100 mg/kg decreased escape latency time on day 4. Fermented BZYQT (200 mg/kg) treatment group significantly decreased the escape latency time in day 3 [Figure 2a] and exhibited significantly shorter escape latency on day 3, between the 1st trial and 2nd trial. In the Morris water maze test, the effects on the Fermented BZYQT treated group were significant for treatment (F [5,144] =1.89, P < 0.05), days (F [3,144] =2.11, P < 0.01) and for the interaction between treatment and day (F [15,144] =1.42, P = 0.70). To further verify the spatial memory of mice, the probe test was conducted. Fermented BZYQT (100 mg/kg) treated mice significantly increased the time spent in the target quadrant compared to the scopolamine group [Figure 2b].
Figure 2.
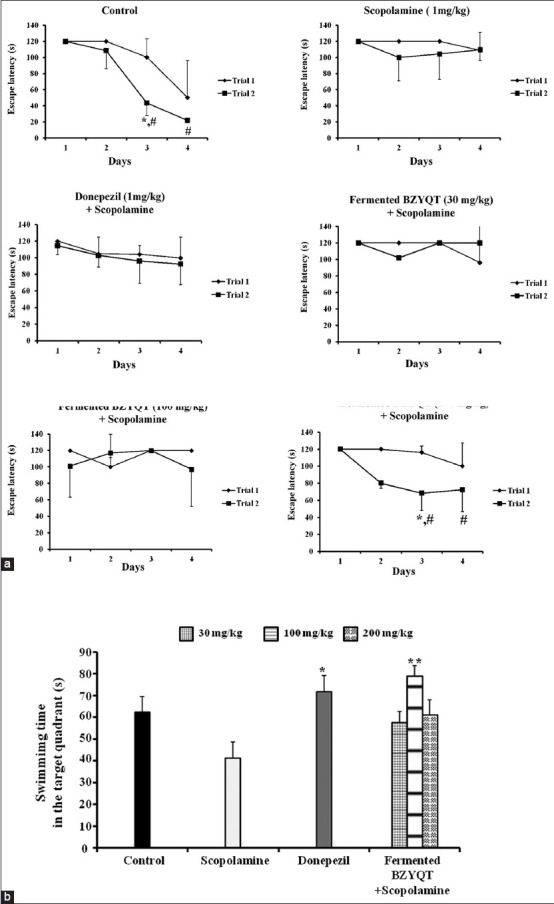
The effect of fermented Bozhougyiqi-Tang on escape latency in scopolamine injected mice in the Morris water maze test. (a) The values shown are the mean escape latency ± standard deviation (SD) (n = 7). Escape latency of the trial 2 significantly differ from the value in trial 1: *P < 0.05, **P < 0.01 and ***P < 0.001. #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with scopolamine group. (b) Mean escape latency of each group in probe trial. The time spent in the quadrant where the platform was once placed within 120 s. Data represent the mean ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 versus the control mice
Passive avoidance test have also been used to evaluate the memory enhancement of screening sample. Scopolamine-treated group (7.90 ± 6.40 s) showed lower latency compared to the control group (11.40 ± 10.90 s) [Figure 3]. The latency of fermented BZYQT (200 mg/kg) (25.60 ± 19.60 s) was significantly increased compared to the scopolamine-treated group.
Figure 3.
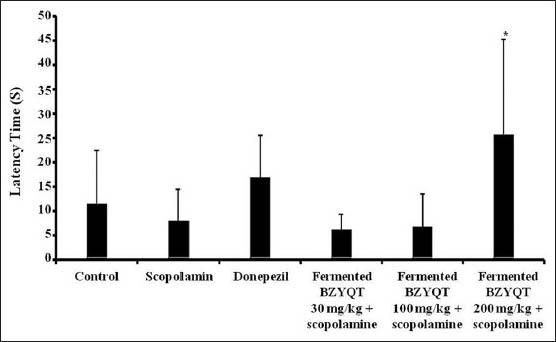
The passive avoidance test of mice treated with control group (0.5% carboxymethyl cellulose 10 mL/kg, p.o.), scopolamine (1 mg/kg, s.c.), donepezil (1 mg/kg, p.o.), scopolamine + fermented Bozhougyiqi-Tang (BZYQT) (30 mL/kg, p.o.), scopolamine + fermented BZYQT (100 mL/kg, p.o.) and scopolamine + fermented BZYQT (200 mL/kg, p.o.), respectively. The values are expressed as mean ± standard deviation. *P < 0.05, **P < 0.01 and ***P < 0.001 versus the scopolamine-treated group
To evaluate the effect of fermented BZYQT (1, 10 and 100 μg/mL) on glutamate induced oxidative stress, we determined the levels of ROS production by dye 2′7′-DCF-DA in HT22 cells. As shown in Figure 4, fermented BZYQT at the concentration of 100 μg/mL significantly inhibited intracellular accumulation of ROS in HT22 cells exposed to glutamate.
Figure 4.
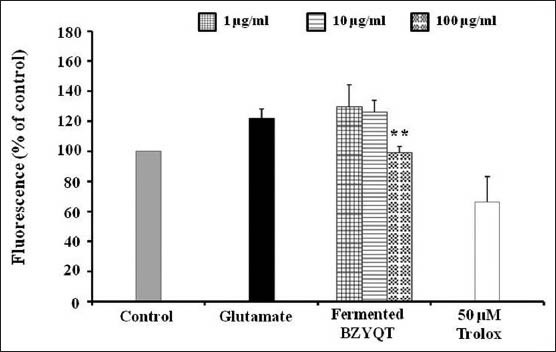
Inhibition of reactive oxygen species (ROS) generation. HT22 cells were treated with 1, 10 and 100 ìg/mL of fermented Bozhougyiqi-Tang and then exposed to 2 mM glutamate for 8 h increased ROS production. Trolox (10 ìM) was used as positive control. Each bar represents the mean ± standard deviation of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus glutamate-injured cells (analysis of variance)
The antioxidant activity of the fermented BZYQT was determined by radical scavenging activity assay. DPPH radical scavenging effect of fermented BZYQT inhibited DPPH formation by 50% at a concentration of 3183.72 μg/mL (IC50). H2O2 scavenging assay showed that fermented BZYQT was very active with IC50 value of 1594.81 μg/mL.
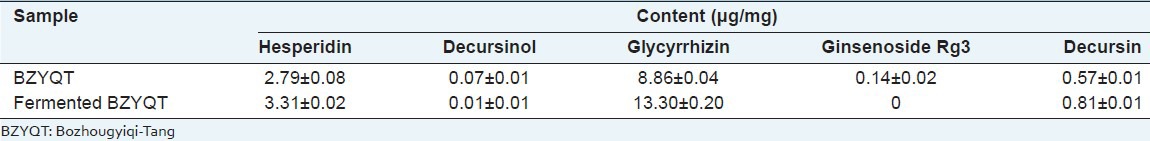
To determine the change of compounds in BZYQT by fermentation, the simultaneous determination of original BZYQT and fermented BZYQT was applied by HPLC coupled with diode array detector (DAD). Hesperidin (1), decursinol (2), glycyrrhizin (3), ginsenoside Rg3 (4) and decursin (5) in BZYQT identified by comparing both the UV spectrum and retention times of reference compounds [Figure 5]. The contents of five compounds in fermented BZYQT varied compared with original BZYQT. The contents of hesperidin, glycyrrhizin and decursin are increased. The content of decursinol was decreased and ginsenoside Rg3 is not detected [Table 1].
Figure 5.
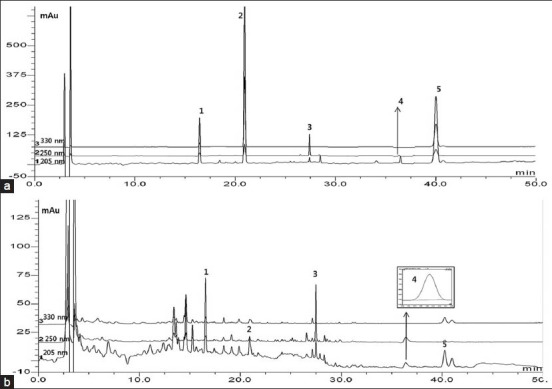
High-performance liquid chromatography-diode array detector chromatograms of five standard compounds (a) and BZYQT preparation sample (b) at 205, 250 and 330 nm. Hesperidin (1), decursinol (2), glycyrrhizin (3), ginsenoside Rg3 (4) and decursin (5)
Table 1.
Contents (μg/mg) of the five compounds in the original BZYQT sample and fermented BZYQT
DISCUSSION
To increase the activity of BZYQT, it was fermented. We analyzed the neuroprotective effect of fermented BZYQT and compared with the original BZYQT. Fermented BZYQT significant neuroprotective effect against glutamate-induced neurotoxicity in HT22 cells at a concentration of 100 μg/mL and effect of fermented BZYQT was higher than the original BZYQT. Based on in vitro test, we used water maze and passive avoidance test. Water maze test and passive avoidance test were used widely to investigate the spatial memory and learning and memory retrieval, respectively.[19,20,21] In water maze test, we observed that scopolamine-treated group exposed to fermented BZYQT showed improving spatial impairment in mice. Similar results were found in the passive avoidance test. It had reported that the passive avoidance test is related to long-term or reference memory. Step-through latencies increased significantly by treatment with fermented BZYQT on scopolamine-induced amnesic mice. Cell death due to oxidative stress induces the impairment of memory.
The NMDA type of glutamate receptor highly concentrated in the cortex and hippocampus has been implicated in memory and in AD.[22,23] Scopolamine is very widely used in screening of memory enhancing activity. Scopolamine, muscarinic cholinergic antagonist blocks cholinergic neurotransmission and impairs cognitive functions including learning and memory.[24] Several studies reported that the NMDA receptors interact with cholinergic system and these mechanisms can influence hippocampal learning and memory.[25] Thus, we estimated that fermented BZYQT prevented impaired spatial learning and long-term memory by blocking non-selective muscarinic acetylcholine antagonist and NMDA receptors. Further, we investigated the anti-oxidative activity and measured ROS concentration to elucidate the mechanism of neuroprotection of fermented BZYQT. Fermented BZYYQT is shown anti-oxidative activity in DPPH and H2O2 scavenging activity and protected neuron cells through reducing ROS level.
In results of HPLC-DAD analysis, five compounds, hesperidin, glycyrrhizin, decursinol, ginsenoside Rg3 and decursin in BZYQT were identified. The contents of hesperidin, glycyrrhizin and decursin were increased through fermentation. Hesperidin, a flavone glycoside in C. unshiu have been reported that protected primary cultured rat cortical cells against Aβ25-35-indused neuronal damage and cell death due the activation of the PI3 and mitogen-activated protein kinesis pathways.[26] Decursin in A. gigas is known to protective primary cultured rat cortical cells against glutamate-induced oxidative stress with decursinol and to have anti-amnestic activity in vivo through inhibition of acetylcholinesterase activity.[27,28] Glycyrrhizin in G. uralensis have been found to protect cell apoptosis caused by ischemic stress in PC12 cells via PI3K/Akt signaling and mitochondrial Bcl-2 family modulation.[29]
Herbal medicines, such as BZYQT have various activity compounds of herbs. Combination of compounds appeared synergistic effects and synergy enhanced bioactivity of herbal medicines. Enhanced neuroprotective effect of fermented BZYQT could be due to an interaction of hesperidin, glycyrrhizin and decursin.
In summary, the results of in vitro and in vivo test demonstrated that the fermented BZYQT improved learning and memory in scopolamine-induced memory deficit and has neuroprotective effect against glutamate induced oxidative stress in HT22 cells. We suggested that fermented BZYQT may have therapeutic potential for the treatment of AD or memory impaired disorders and further study of mechanism of fermented BZYQT will required.
ACKNOWLEDGEMENTS
This research was supported by a grant (K11050) from the Korea Institute of Oriental Medicine. Fermented BZYQT provided from Korea Food Research Institute (KFRI, Korea).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986;19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 2.Crapper DR, DeBoni U. Brain aging and Alzheimer's disease. Can Psychiatr Assoc J. 1978;23:229–33. doi: 10.1177/070674377802300406. [DOI] [PubMed] [Google Scholar]
- 3.Citron M. Alzheimer's disease: Treatments in discovery and development. Nat Neurosci. 2002;5(Suppl):1055–7. doi: 10.1038/nn940. [DOI] [PubMed] [Google Scholar]
- 4.Portelius E, Zetterberg H, Andreasson U, Brinkmalm G, Andreasen N, Wallin A, et al. An Alzheimer's disease-specific beta-amyloid fragment signature in cerebrospinal fluid. Neurosci Lett. 2006;409:215–9. doi: 10.1016/j.neulet.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–27. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 6.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 7.Kása P, Rakonczay Z, Gulya K. The cholinergic system in Alzheimer's disease. Prog Neurobiol. 1997;52:511–35. doi: 10.1016/s0301-0082(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 9.Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 11.Rössler OG, Bauer I, Chung HY, Thiel G. Glutamate-induced cell death of immortalized murine hippocampal neurons: Neuroprotective activity of heme oxygenase-1, heat shock protein 70, and sodium selenite. Neurosci Lett. 2004;362:253–7. doi: 10.1016/j.neulet.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Boje KM, Arora PK. Microglia as a source and target of cytokines. Brain Res. 1992;587:250–6. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology. 1996;35:963–8. doi: 10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 14.Mehta M, Adem A, Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer's disease. Int J Alzheimers Dis 2012. 2012 doi: 10.1155/2012/728983. 728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang WY. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol Sci. 2005;26:558–63. doi: 10.1016/j.tips.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Lu AP, Jia HW, Xiao C, Lu QP. Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol. 2004;10:1854–6. doi: 10.3748/wjg.v10.i13.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SY, Lee KY, Park MJ, Kim YC, Markelonis GJ, Oh TH, et al. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Mem. 2003;79:11–8. doi: 10.1016/s1074-7427(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 18.Seo M, Lee K, Park J, Hong S. The current trend of research about Bojungikki-Tang. Korean J Orient Med. 2010;16:83–90. [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–46. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Altman HJ, Stone WS, Ogren SO. Evidence for a possible functional interaction between serotonergic and cholinergic mechanisms in memory retrieval. Behav Neural Biol. 1987;48:49–62. doi: 10.1016/s0163-1047(87)90574-7. [DOI] [PubMed] [Google Scholar]
- 22.Cotman CW, Monaghan DT, Ottersen OP, Storm-Mathisen J. Anatomical organization of excitatory amino receptors and their pathways. Trends Neurosci. 1987;10:273–80. [Google Scholar]
- 23.Collingridge GL, Bliss TV. NMDA receptors-Their role in long term potentiation. Trends Neurosci. 1987;10:288–93. [Google Scholar]
- 24.Khakpai F, Nasehi M, Haeri-Rohani A, Eidi A, Zarrindast MR. Scopolamine induced memory impairment;possible involvement of NMDA receptor mechanisms of dorsal hippocampus and/or septum. Behav Brain Res. 2012;231:1–10. doi: 10.1016/j.bbr.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Aigner TG. Pharmacology of memory: Cholinergic-glutamatergic interactions. Curr Opin Neurobiol. 1995;5:155–60. doi: 10.1016/0959-4388(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 26.Huang SM, Tsai SY, Lin JA, Wu CH, Yen GC. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol Nutr Food Res. 2012;56:601–9. doi: 10.1002/mnfr.201100682. [DOI] [PubMed] [Google Scholar]
- 27.Kang DS, Kam CW, Park DI. The experimental study on the anti-allergic effect of Boiungikgi-Tang. J Life Sci. 2003;13:73–82. [Google Scholar]
- 28.Kang SY, Kim YC. Decursinol and decursin protect primary cultured rat cortical cells from glutamate-induced neurotoxicity. J Pharm Pharmacol. 2007;59:863–70. doi: 10.1211/jpp.59.6.0013. [DOI] [PubMed] [Google Scholar]
- 29.Kao TC, Shyu MH, Yen GC. Neuroprotective effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid in PC12 cells via modulation of the PI3K/Akt pathway. J Agric Food Chem. 2009;57:754–61. doi: 10.1021/jf802864k. [DOI] [PubMed] [Google Scholar]


