Abstract
Objective:
To investigate possible effects of aqueous taxus baccata extract on adenosine deaminase (ADA) activity in cancerous and noncancerous human tissues and to clarify mechanism(s) of its anticancer potential.
Materials and Methods:
Cancerous and noncancerous human gastric and colon tissues were used in the study. The extracts were prepared in distilled water. Before and after treatment with the extracts, ADA activities in the tissue homogenates were measured.
Results:
ADA activity was found to be higher in gastric tissue compared with colon tissue, but no differences were found between ADA activities of cancerous and noncancerous tissues for both as well. In the plant extract studies, it was found that taxus extract significantly inhibited ADA activity both in cancerous and noncancerous gastric and colon tissues.
Conclusion:
Our results suggest that aqueous extract from taxus baccata inhibits ADA activities in both gastric and colon tissues significantly. It is suggested that in addition to other proposed mechanisms, accumulated adenosine due to the inhibition of ADA enzyme might also play part in the anticancer properties of taxus species.
Keywords: Adenosine deaminase, cancer, taxol, taxus baccata
INTRODUCTION
Cancer is one of the leading health problems in the world. Side effects of chemotherapy and radiation therapy have been known obviously. Since the therapies at the moment do not result in successful results for some types of cancers, scientists have started to investigate natural remedies for the treatment of cancer. In this regard, plant-derived compounds were evaluated first, because of the findings that used some plants in a rural population to treat certain types of cancers, which yielded positive results. In fact, plants have a long history in the treatment of cancer[1] and studies related to anticancer agents from plant sources started in the 1950s with the discovery of some alkaloids like vinca major and cytotoxic podophyllotoxins.[2,3]
The taxanes are a group of plant-derived chemotherapeutic agents.[4] Paclitaxel (taxol®) isolated from the Pacific Yew, Taxus brevifolia Nutt. (a species of the genus Taxus, Taxaceae) is the best known agent among them. Taxus baccata (European yew) is another important source of paclitaxel. A recent review focused on the production of paclitaxel and related taxanes in Taxus baccata using cell suspension culture technology.[5] Various parts of Taxus brevifolia and other Taxus species are used for the treatment of some cancerous and noncancerous conditions.[1] Paclitaxel, which is used in the treatment of several cancers,[6,7] is found in the leaves of various Taxus species, and their semi-synthetic conversions such as docetaxel (Taxotere®), have provided natural sources of plant-derived drugs.[8]
D-Adenosine deaminase (ADA) is an enzyme (EC 3.5.4.4) involved in purine metabolism. It is needed for the breakdown of adenosine and for the turnover of nucleic acids in tissues. It is present virtually in all mammalian cells and its primary function in humans is the development and maintenance of the immune system.[9] However, the full physiological role of ADA is not yet completely understood.[10] ADA association has also been observed with epithelial cell differentiation, neurotransmission, and gestation maintenance.[11] It has also been proposed that ADA, in addition to adenosine breakdown, stimulates release of excitatory amino acids and is necessary for the coupling of A1 adenosine receptors and heterotrimeric G proteins.[10]
Although some molecular mechanisms are supposed for the action of taxus species in the cancer process, it seems quite possible that there might be some other mechanisms unknown yet. Therefore, further studies are needed. As to the subject, we evaluated to investigate possible effects of aqueous taxus baccata extract on ADA activity in cancerous and noncancerous human gastric and colon tissues in order to make contribution to the attempts to clarify its anticancer potential.
MATERIALS AND METHODS
The study protocol was approved by the Ethical Committee of Clinical Research in Ankara University Faculty of Medicine (Decision No: 32-690, Date: June 13, 2011). Nine cancerous colon tissues and nine noncancerous adjacent colon tissues were obtained from patients with colon cancer by surgical operation. Sixteen cancer and 16 non cancer gastric tissues were similarly obtained from patients with gastric cancer. Tissues were first cleaned by saline solution and the stored at -80°C until the analysis time. In the analysis process, first they were homogenized in saline solution (20%, w/v). After homogenization, homogenates were centrifuged at 5000 rpm for 30 min to remove debris and to obtain clear supernatant fraction. Then, the analyses were performed in this fraction.[12]
The extracts were prepared by soaking plant (taxus baccata needles) into the distilled water at the concentration of 10% (w/v) and waiting for 24 hours at room temperature by rotating continuously. After the debris was removed, plant supernatants were centrifuged at 10,000 rpm for 20 min and upper clear part was taken and used in the assays as the plant extract.
Protein concentrations of the tissues were measured by the Lowry method[13] and ADA activities were measured by the method described by Guisti before.[14]
Statistical analysis
Statistical evaluations were made by Wilcoxon test and P values lower than 0.05 were evaluated as significant
RESULTS
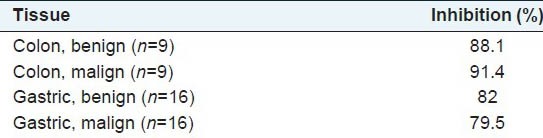
Results are shown in Table 1. As seen from Table 1, the taxus extract significantly inhibits ADA activity both in cancerous and noncancerous gastric and colon tissues. Inhibition percents were higher in colon tissues than gastric tissues [Table 2]. Furthermore, we observed that ADA activity was higher in the gastric tissue compared with the colon tissue. However, we found no differences between ADA activities of cancerous and noncancerous tissues for both tissues studied.
Table 1.
Mean±SD values for ADA activities (IU/mg protein) in the groups with and without extract
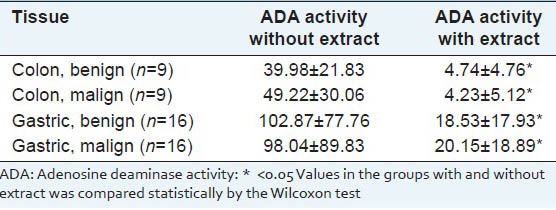
Table 2.
Inhibition percents observed owing to treatment with the extract in the groups
DISCUSSION
Plants are the important sources of effective drugs for the treatment of some types of cancers and they also provide important sources for the development of novel agents with anticancer potential.[15,16]
Among these plants, taxus baccata has long been under interest in this regard. This plant has some components with alkaloid structure. Although its alkaloids share common structural similarities with some others, they have a unique taxane ring. These alkaloids interrupt mitosis by promoting and stabilizing microtubule formation. Taxol targets microtubules produced by dimeric proteins. Microtubules are keys for cell division, and tubulins polymerize to form microtubules in the presence of microtubule associated protein (MAP) and GTP. It seems possible that taxol stabilizes microtubules. As a result of this, microtubules become nonfunctional, which interferes cell dividing and blocks cell cycle. Due to abnormal clusters formed in this way, nonfunctional microtubules get distributed.[17,18,19]
Additionally, taxol prevents spread of metastatic cancer cells by inhibiting cell migration. Paclitaxel principally acts at the G-2/M-phase junction. However, docetaxel acts mainly in S-phase of mitosis. These compounds are not toxic to nondividing cells because they do not require mitotic spindle. Therefore, they act only on proliferating cells.[18] Additionally, as the microtubule system is essential for the release of various cytokines, modulation of cytokine release by this drug may play a major role in its antitumor activity.[20]
From a scientific perspective of view, use of ADA inhibitors has helped much in understanding the mechanism of action of adenosine metabolites and analogs. ADA inhibitors have also led to the understanding of the regulatory processes associated with immunodeficiency characterized by a lack of ADA, and of maturation of the immune response.[21] One of them, pentostatin (Nipent) is a nucleoside analog having potential to inhibit ADA enzyme. Inhibition of ADA blocks the deamination reactions in the purine salvage pathway, result of which is the inhibition of ribonucleotide reductase. As a result, this process depletes the nucleotide pool and limits DNA synthesis.[22]
Looking at our results, it seems that ADA activity is higher in gastric tissue compared with colon tissue, and there are no differences between ADA activities of cancerous and noncancerous cancer tissues in both tissues. Our results, however, suggest that components of aqueous extract from taxus baccata significantly inhibits ADA activities in both gastric and colon tissues. Inhibition percents in ADA activity are almost at the same degree in the cancerous (malign) and noncancerous adjacent tissues (benign).
CONCLUSION
It seems quite possible that in addition to other proposed mechanisms, accumulated adenosine due to the inhibition of ADA enzyme might also play an important function in the anticancer properties of taxus species.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Himes RH. Interactions of the catharanthus (Vinca) alkaloids with tubulin and microtubules. Pharmacol Ther. 1991;51:257–67. doi: 10.1016/0163-7258(91)90081-v. [DOI] [PubMed] [Google Scholar]
- 3.Noble RL. The discovery of the vinca alkaloids - chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344–51. [PubMed] [Google Scholar]
- 4.Crown J, O’Leary M. The taxanes: An update. Lancet. 2000;355:1176–8. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- 5.Malik S, Cusidó RM, Mirjalili MH, Moyano E, Palazón J, Bonfill M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011;46:23–34. [Google Scholar]
- 6.Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother. 2002;3:755–66. doi: 10.1517/14656566.3.6.755. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 8.Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM, Cragg GM. Taxus spp. needles contain amounts of taxol comparable to the bark of Taxus brevifolia: Analysis and isolation. J Nat Prod. 1990;53:1249–55. doi: 10.1021/np50071a017. [DOI] [PubMed] [Google Scholar]
- 9.Kingston D. Taxol and its analogs. In: Cragg G, Kingston DG, Newman DJ, editors. Anticancer Agents from Natural Products. Boca Raton, FL: Brunner-Routledge Psychology Press, Taylor and Francis Group; 2005. pp. 89–122. [Google Scholar]
- 10.Wilson DK, Rudolph FB, Quiocho FA. Atomic structure of adenosine deaminase complexed with a transition-state analog: Understanding catalysis and immunodeficiency mutations. Science. 1991;252:1278–84. doi: 10.1126/science.1925539. [DOI] [PubMed] [Google Scholar]
- 11.Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, et al. Adenosine deaminase: Functional implications and different classes of inhibitors. Med Res Rev. 2001;21:105–28. doi: 10.1002/1098-1128(200103)21:2<105::aid-med1002>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Durak İ, Biri H, Ergüder İB, Devrim E, Şenocak Ç, Avcı A. Effects of garlic and black grape extracts on the activity of adenosine deaminase from cancerous and noncancerous human urinary bladder tissues. Med Chem Res. 2007;16:259–65. [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Guisti G. Enzyme activities. In: Bergmeyer H, editor. Methods of Enzymatic Analysis. Weinhem, Bergest: Verlag Chemia; 1974. pp. 1092–8. [Google Scholar]
- 15.Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9:581–91. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 17.Vohora SB, Kumar I, Shah SA, Khan MS. Effect of bioflavonoids of Taxus baccata on the central nervous system. Indian J Med Res. 1980;71:815–20. [PubMed] [Google Scholar]
- 18.Manish L, Mangesh B, Sabale V. Taxus - the panacea for cancer treatment. Anc Sci Life. 2005;24:152–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, et al. Taxanes: A new class of antitumor agents. Cancer Invest. 1995;13:381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 20.Smith RE, Thornton DE, Allen J. A phase II trial of paclitaxel in squamous cell carcinoma of the head and neck with correlative laboratory studies. Semin Oncol. 1995;22(Suppl 6):41–6. [PubMed] [Google Scholar]
- 21.Glazer RI. Adenosine deaminase inhibitors: Their role in chemotherapy and immunosuppression. Cancer Chemother Pharmacol. 1980;4:227–35. doi: 10.1007/BF00255266. [DOI] [PubMed] [Google Scholar]
- 22.Brown JB, Lee G, Grimm GR, Barrett TA. Therapeutic benefit of pentostatin in severe IL-10-/- colitis. Inflamm Bowel Disc. 2008;14:880–7. doi: 10.1002/ibd.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]


