Abstract
Background:
Jaeumganghwa-tang (JEGH) is a traditional Korean herbal medicine for the treatment of chronic bronchitis, nephritis and diabetes mellitus.
Objective:
A high performance liquid chromatography-diode array detector (HPLC-DAD) method was developed for simultaneous determination of 11 major compounds such as 5- hydroxymethylfurfural, mangiferin, paeoniflorin, nodakenin, naringin, hesperidin, decursinol, berberine, glycyrrhizin, atractylenolide III and decursin, in JEGH.
Materials and Methods:
The separation was conducted on Shishedo C18 column with gradient elution of 0.1% trifluoroacetic acid–acetonitrile. Detection of wavelength was set at 205, 250, 280 and 330 nm.
Results:
The developed analysis showed a good linearity (R2 >0.9997). The range of limit of detection and limit of quantification were observed from 0.04 to 0.43 and from 0.11 to 1.30, respectively. The intra- and inter-day test relative standard deviations (RSD) were less than 3% and the accuracy was 95.98-108.44%. The recoveries were between 92.75% and 109.19% and RSD range of recoveries was measured from 0.52% to 2.78%.
Conclusion:
This HPLC-DAD method can be successfully applied for simultaneous determination of 11 major compounds in JEGH samples.
Keywords: High performance liquid chromatography-diode array detection, Jaeumganghwa-tang, liquid chromatography-mass spectrometry, simultaneous determination
INTRODUCTION
Herbal medicines have been used to treat various diseases for 1000 years.[1] Many herbal therapies have very few side-effects and expect a synergy effect by interaction of multiple bioactive compounds.[2,3,4] Recently, demand of such herbal medicines is increasing, but these herbal medicines have no standard about therapeutic effect related compounds. Therefore, the accurate quality control of compounds in herbal medicines is urgent.[2] Jaeumganghwa-tang (JEGH) is a well-known traditional Korean medicinal prescription, which contains 12 crude drugs; Angelicae gigantis Radix, Paeoniae Radix, Rehmanniae Radix Preparata, Asparagi Tuber, Atractylodis Rhizome, Aurantii Nobilis Pericarpium, Phellodendri Cortex, Liriopis Tuber, Anemarrhenae Rhizome, Glycyrrhizae Radix, Rehmanniae Radix and Rehmanniae Radix crudus. JEGH has been suggested to treat with the chronic bronchitis, nephritis and diabetes mellitus. Several studies have reported that JEGH was efficient in anti-inflammation, immune-boosting, depression of hypertension, anti-fever, pain-killing and anti-convulsion.[5,6,7,8] Furthermore, recent reports have revealed that JEGH inhibits the development of benign prostatic hyperplasia in rats and reduces the hot flush induced by tamoxifen in breast cancer patient.[9,10] In this study, a sensitive and accurate high performance liquid chromatography coupled with diode array detection (HPLC-DAD) method was developed to simultaneously determine 11 marker compounds, such as 5- hydroxymethylfurfural (HMF), mangiferin, paeoniflorin, nodakenin, naringin, hesperidin, decursinol, berberine, glycyrrhizin, atractylenolide III and decursin in JEGH. The molecular structures of these compounds are given in Figure 1. As all known, mass spectrometry (MS) is a sensitive analytical technique. Herein, the compounds were identified by liquid chromatography (LC)-MS.
Figure 1.
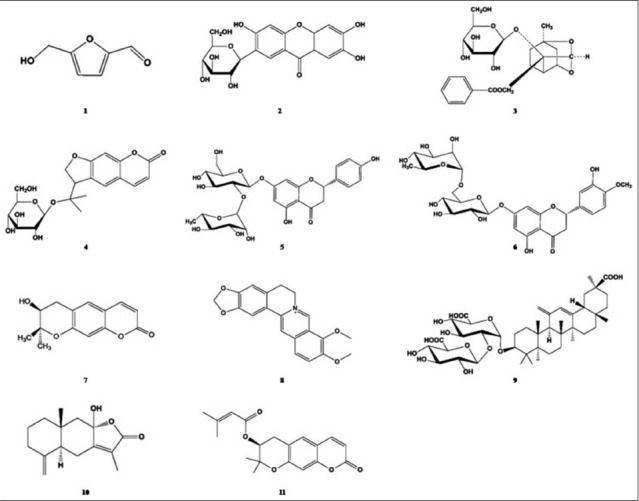
The chemical structures of 11 marker compounds of Jaeumganghwa-tang. (1) 5-Hydroxymethylfurfural, (2) mangiferin, (3) paeoniflorin, (4) nodakenin, (5) naringin, (6) hesperidin, (7) decursinol, (8) berberine, (9) glycyrrhizin, (10) atractylenolide III and (11) decursin
EXPERIMENTAL
Materials
HPLC grade water and acetonitrile was purchased from J. T. Baker. Analytical grade trifluoroacetic acid was obtained from Dae Jung. Decursin, hesperidin, naringin, berberine, glycyrrhizin and nodakenin were purchased from Korea food and drug administration. Decursinol was purchased from Elcomscience Co., Ltd. Paeoniflorin was purchased from Wako Co., Ltd. 5-HMF was purchased from Sigma Aldrich Co., Ltd. Mangiferin was purchased from Fluka Co., Ltd. Atractylenolide III was purchased from Chromadex Co., Ltd. The purities of each chemical compound were above 98%. JEGH was supplied by Korea institute of oriental medicine.
JEGH sample preparation
JEGH comprised 8 g of Paeoniae Radix, 8 g of Angelica gigantis Radix, 6 g of Asparagi Tuber, 6 g of Rehmanniae Radix Preparata, 6 g of Rehmanniae Radix crudus, 6 g of Liriopis Tuber, 6 g of Atractylodis Rhizome, 4 g of Rehmanniae Radix, 4 g of Aurantii Nobilis Pericarpium, 2 g of Anemarrhenae Rhizome, 2 g of Phellodendri Cortex and 2 g of Glycyrrhizae Radix. These herbs were deposited in water of 10 times the weight of herbs for 1 h and reflux water extracted at 115°C for 3 h. Extraction was powdered by freeze-drying method.
Preparation of standard solutions and JEGH sample
Stock standard solutions of 5-HMF (462.5 μg/ml), mangiferin (320 μg/ml), paeoniflorin (240 μg/ml), nodakenin (408.4 μg/ml), naringin (289.5 μg/ml), hesperidin (360 μg/ml), decursinol (210 μg/ml), berberine (340 μg/ml), glycyrrhizin (487.5 μg/ml), atractylenolide III (287.5 μg/ml) and decursin (560 μg/ml), were correctly weighed and dissolved in methanol. This was diluted to prepare six solutions with different concentrations for the establishment of calibration curves.
Twelve JEGH commercial samples were weighed and dissolved in methanol at 10.02 mg/ml, 12.5 mg/ml, 11.22 mg/ml, 11.86 mg/ml, 10.86 mg/ml, 10.82 mg/ml, 12.2 mg/ml, 10.66 mg/ml, 11.52 mg/ml, 12.38 mg/ml, 11.2 mg/ml and 10.92 mg/ml, respectively. All the samples were filtered through a 0.45 μm membrane filter before HPLC analysis and then all solutions were stored in the refrigerator at 4°C.
Instrumentation and conditions
The HPLC system is consisted of Dionex system with LPG 3 × 00 pump, ACC-3000 auto sampler, column oven and DAD-3000(RS) diode array ultraviolet (UV)/visible spectroscopy detector. Separation was accomplished using a Shiseido C18 column (4.6 mm I.D. × 250 mm, 5 μm pore size). Column temperature was maintained at 30°C. The mobile phase was composed of 0.1% trifluoroacetic acid aqueous solution (a) and acetonitrile (b) at a flow rate of 1.0 ml/min. The gradient flows were as follows: 5% B at 0-10 min, 5-17% B at 10-20 min, 17-30% B at 30-40 min, 30-80% B at 40-60 min. The injection volume was 20 μL. The wavelength of UV detection was set at 205 nm, 250 nm, 280 nm and 330 nm, respectively.
Validation
The method was validated in terms of linearity, precision and accuracy test according to International Conference on Harmonization guideline.[11,12,13,14,15,16]
Linearity, limit of detection and limit of quantification
The stock mixture of 5-HMF, mangiferin, paeoniflorin, nodakenin, naringin, hesperidin, decursinol, berberine, glycyrrhizin, atractylenolide III and decursin was diluted with methanol to prepare six samples with different concentrations. To get the calibration curves, standard solutions with six different concentrations were analyzed in triplicates. Calibration curves were established by plotting the peak area (y) versus concentration (x) of each analyte. The linearity was measured by the correlation coefficient (R2) values. Linearity, limit of detection (LOD) and limit of quantification (LOQ) were determined using signal-to-noise ratios of 3 and 10, respectively.
Precision and recovery
The precision of the analysis was estimated by inter- and intra-day variations. The intra-day test was analyzed by standard solutions at three different concentrations on the single day and the inter-day test was performed at same concentrations on the three different days (1, 3, 5 days). The relative standard deviation (RSD) was calculated by the standard deviation over measured amount multiply of 100. Recovery test was used to estimate the accuracy of this analysis method. The standard solutions with a certain concentration was added to JEGH sample and then analyzed at three different concentrations in 3 times. Recovery was calculated by the equation.

Analysis of JEGH sample
HPLC-DAD method was applied to JEGH samples. All samples were analyzed in triplicates. The amount of marker compounds in JEGH sample was calculated from the calibration curves of standard solutions.
LC-MS
LC-MS was used to identify each peak of marker compounds. LC-MS was performed on TSQ quantum ultra (Thermo) coupled with electro-spray ionization in positive ion mode. All samples were separated on an Atlantis dC18 column (150 × 2.0 mm I.D., 3 μm). The flow late was set at 0.2 ml/min and the injection volume was 20 μL. The ion spray voltage was set at 4000 V and the vaporizer temperature was maintained at 300°C. The sheath gas pressure and aux gas pressure was set at 35 psi, 15 psi, respectively. The capillary temperature was kept constant at 350°C. LC-MS analysis was performed under the same condition with HPLC-DAD.
RESULTS AND DISCUSSION
Optimization of HPLC-DAD condition
The 11 marker compounds belonging to different crude plants were selected for this simultaneous determination. In order to obtain the optimal analysis conditions, various columns were examined and compared (Shiseido, Phenomenex and XTerra C18 4.6 × 250 mm 5 μm pore size column). Shiseido column exhibited the best resolution and elution time. The detection wavelength was recorded at 205 nm for paeoniflorin, nodakenin, naringin, hesperidin, decursinol, atractylenolide III and decursin, 250 nm for mangiferin and glycyrrhizin, 280 nm for 5-HMF and 330 nm for berberine. The appropriate mobile phase was found to be the gradient prepared from the acetonitrile-water system. The attendance of acid in water inhibited the tailing effect of the peaks and accomplished a better separation. The marker constituents of the JEGH were detected with no effect to the other compounds. Retention times were 8.52 min for 5-HMF, 23.85 min for mangiferin, 25.85 min paeoniflorin, 35.37 min for nodakenin, 37.36 min for naringin, 39.54 min hesperidin, 43.96 min for decursinol, 45.49 min for berberine, 50.60 min for glycyrrhizin, 54.31 min for atractylenolide III and 57.68 min for decursin. Chromatogram of JEGH standard compounds mixture is shown in Figure 2.
Figure 2.
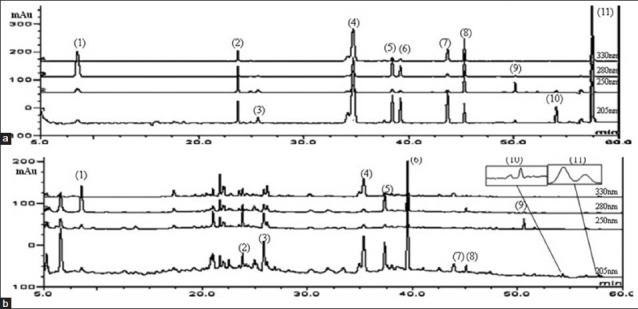
(a) Chromatograms of Jaeumganghwa-tang standard mixture and (b) sample. (1) 5-Hydroxymethylfurfural, (2) mangiferin, (3) paeoniflorin, (4) nodakenin, (5) naringin, (6) hesperidin, (7) decursinol, (8) berberine, (9) glycyrrhizin, (10) atractylenolide III and (11) decursin
Linearity
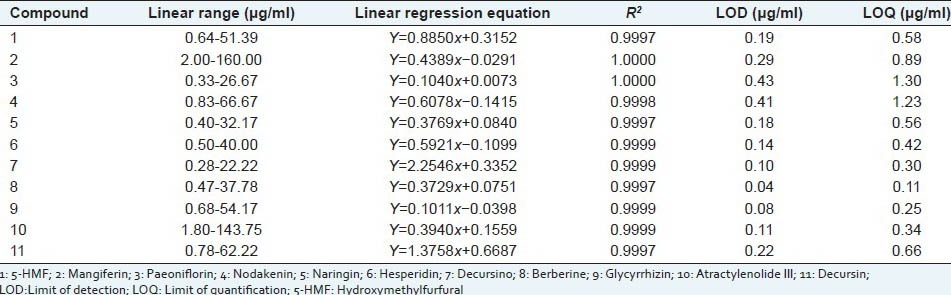
Calibration curves and each coefficient (R2) were obtained using standard solutions. The results are summarized in Table 1. All the marker compounds showed good linearity within the test range (R2 > 0.9997). The LOD and LOQ were in the ranges 0.04-0.43 and 0.11-1.30 μg/ml, respectively.
Table 1.
The regression data, LODs and LOQs for 11 marker compounds in Jaeumganghwa-tang
Precision and accuracy
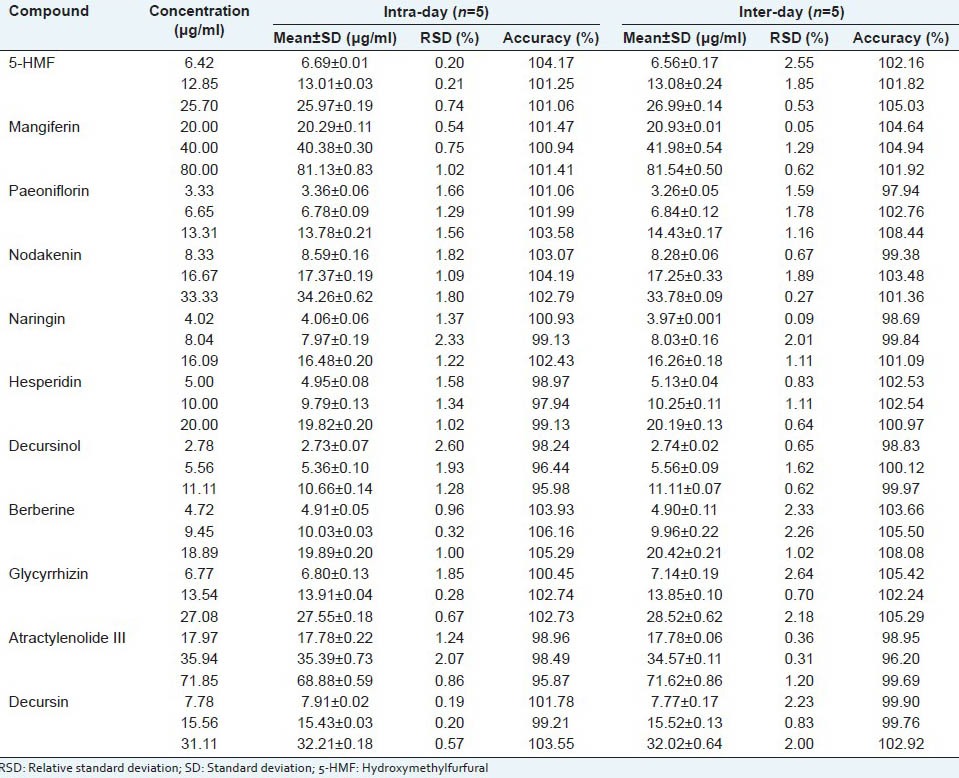
RSD value of intra- and inter-day tests was used to measure the precision for all compounds. The results of inter- and intra-day tests in measurement of 11 marker compounds are arranged in Table 2. The RSD values of intra-day and inter-day were 0.20-2.60% and 0.05-2.55%, respectively. The intra-day accuracy was in the range 95.98-106.16% and the inter-day accuracy was 96.20-108.44%.
Table 2.
Intra-and inter-day precisions of the assay
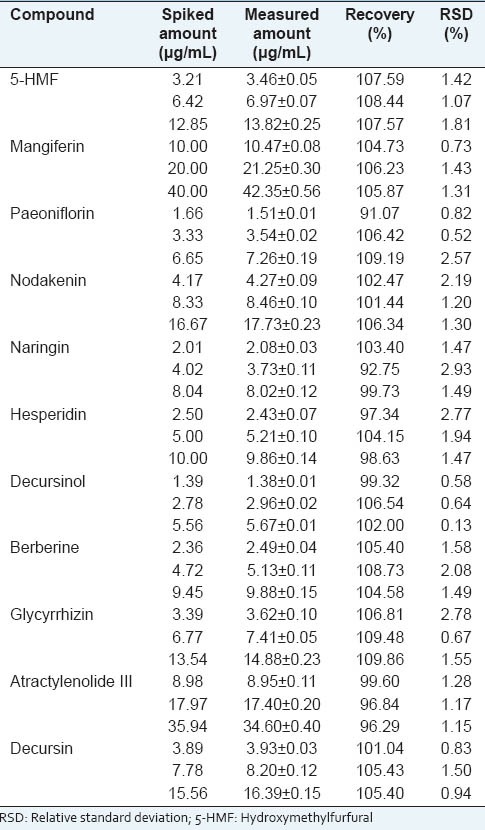
The recovery of 11 marker compounds was evaluated by standard addition method. It was found that the overall recoveries were in the range of 92.75-109.19% and the RSD values were measured from 0.52% to 2.78% [Table 3]. These results demonstrate that this analysis method has suitable precision and accuracy for simultaneous determination of JEGH.
Table 3.
Recoveries of the 11 marker compounds in Jaeumganghwa-tang
Application
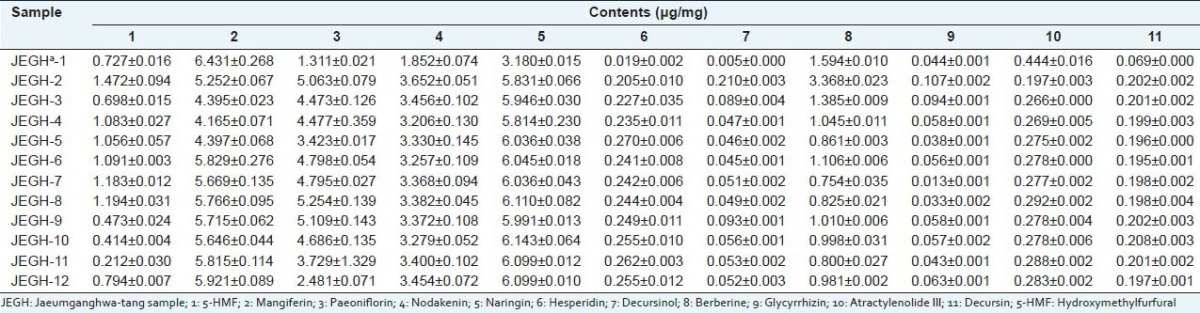
The developed HPLC-DAD method was applied to the determination of 11 marker compounds in 12 commercial JEGH samples. Each peak was confirmed by the distinction of retention time and UV spectrum with those of each standard compound. All 11 marker compounds were detectable in 12 samples as shown in Table 4. Paeoniflorin and hesperidin has the largest amount in JEGH. The amount of 5-HMF, naringin, berberine, glycyrrhizin and decursin was the highest in JEGH-2. Similarly, the quantity of paeoniflorin and mangiferin was higher in JEGH-1 than in the other samples. The contents of marker compounds varied among each sample. For example, the contents of hesperidin varied from 3.18 μg/g to 6.14 μg/g, which was almost doubled. This variation might due to different regions of cultivation and manufacturing process.
Table 4.
Contents of 11 marker compounds in commercial Jaeumganghwa-tang samples
LC-MS
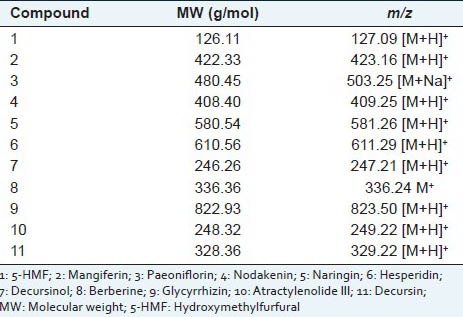
LC-MS is a sensitive and accurate method for the simultaneous determination. LC-MS method was applied to identify the maker compounds of JEGH in this study. In positive ion mode, most of the m/z data were [M + H]+. As shown in Figure 3, 5-HMF molecular weight (MW - 126.11 g/mol), mangiferin (MW - 422.33 g/mol), nodakenin (MW - 408.40 g/mol), naringin (MW - 580.54 g/mol), hesperidin (MW - 610.56 g/mol), decursinol (MW - 246.26 g/mol), glycyrrhizin (MW - 822.93 g/mol), atractylenolide III (MW - 248.32 g/mol) and decursin (MW - 328.36 g/mol) gave protonated adducts at m/z 127.09, 423.16, 409.25, 581.26, 611.29, 247.21, 823.50, 249.22 and 329.22, respectively. [M + Na]+ signal of paeoniflorin was observed at m/z 503.25 and M + signal of berberine was shown at m/z 336.24. These results are described in Table 5.
Figure 3.
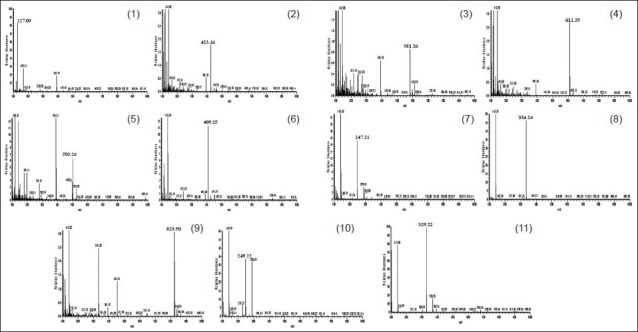
Liquid chromatography-mass spectrometry spectra of maker compound in Jaeumganghwa-tang. (1) 5-Hydroxymethylfurfural, (2) mangiferin, (3) paeoniflorin, (4) nodakenin, (5) naringin, (6) hesperidin, (7) decursinol, (8) berberine, (9) glycyrrhizin, (10) atractylenolide III and (11) decursin
Table 5.
Mass data of marker compounds
CONCLUSION
In this study, we developed an HPLC-DAD method for simultaneous determination of 11 marker compounds in JEGH and validated linearity, precision and accuracy for this method. The 11 marker compounds were properly separated with no apparent interference from other compound and were successfully applied to 12 commercial samples. Compared with the previous study using seven marker compounds, this newly established method was more accurate in quantitative analysis of compounds in JEGH.[17] Furthermore, an analytical method involving both HPLC-DAD and LC-MS might be available for more sensitive quality analysis. In conclusion, the results showed that this analysis method was sensitive and reliable and it could be used to improve the quality control of JEGH.
ACKNOWLEDGEMENT
The work was supported by a grant (K12050) from the Korea Institute of Oriental Medicine.
Footnotes
Source of Support: The work was supported by a grant (K12050) from the Korea Institute of Oriental Medicine
Conflict of Interest: None declared.
REFERENCES
- 1.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Shen P, Cheng Y. Identification and determination of the major constituents in traditional Chinese medicine Si-Wu-Tang by HPLC coupled with DAD and ESI-MS. J Pharm Biomed Anal. 2004;34:705–13. doi: 10.1016/s0731-7085(03)00650-2. [DOI] [PubMed] [Google Scholar]
- 3.Jiang WY. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol Sci. 2005;26:558–63. doi: 10.1016/j.tips.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Yi LZ, Liang YZ. Traditional Chinese medicine and separation science. J Sep Sci. 2008;31:2113–37. doi: 10.1002/jssc.200800134. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Kim HJ, Kim WS, Park HJ, Moon G, Kim DW, et al. Inhibitory effect of Jaeumganghwa-tang in allergic inflammatory reaction. Korean J Orient Int Med. 2004;25:174–82. [Google Scholar]
- 6.Beak KM, Jo HK, Yoo HR, Kim YS, Seo IC. The effect of Kamijaeumganghwatang (KJI) on hypertension. Korean J Orient Int Med. 2006;27:1–15. [Google Scholar]
- 7.Jung DY, Ha HK, Lee HY, Lee JA, Lee JK, Huang DS, et al. Stimulation of the immune response by Yin-Tonifying formula. J Korean Orient Med. 2010;31:112–23. [Google Scholar]
- 8.Ghee HK. Experimental studies on the efficacy of Gamijaumganghwatang. J Korean Orient Med. 1987;8:9–19. [Google Scholar]
- 9.Shin IS, Lee MY, Lim HS, Seo CS, Ha HK, Shin HK. Jaeumganghwa-tang, a traditional herbal formula inhibits the development of benign prostatic hyperplasia in rats. J Korean Soc Appl Biol Chem. 2010;55:205–12. [Google Scholar]
- 10.Zheng HM, Lee YW, Yoo HS, Cho CK. Case study of a breast cancer patient accompanying with hot flush by tamoxifen whose condition was improves by Jayeumganghwa-tang. Korean J Orient Int Med. 2010;31:395–400. [Google Scholar]
- 11.Wei H, Sun L, Tai Z, Gao S, Xu W, Chen W. A simple and sensitive HPLC method for the simultaneous determination of eight bioactive components and fingerprint analysis of Schisandra sphenanthera. Anal Chim Acta. 2010;662:97–104. doi: 10.1016/j.aca.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Weon JB, Yang HJ, Ma JY, Ma CJ. Simultaneous determination of six active components in traditional herbal medicine ‘Oyaksungisan’ by HPLC-DAD. J Nat Med. 2012;66:510–5. doi: 10.1007/s11418-011-0617-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZJ, Wo SK, Wang L, Lau CB, Lee VH, Chow MS, et al. Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;50:232–44. doi: 10.1016/j.jpba.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Liang X, Zhang X, Dai W, Lv Y, Yan S, Zhang W. A combined HPLC-PDA and HPLC-MS method for quantitative and qualitative analysis of 10 major constituents in the traditional Chinese medicine Zuo Gui Wan. J Pharm Biomed Anal. 2009;49:931–6. doi: 10.1016/j.jpba.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lay HL, Chen CC, Huang SC, Cha TM, Wu TS, Lin IH. Simultaneous analysis of nine components in patch preparations of Ru-Yi-Jin-Huang-San by high-performance liquid chromatography. J Nat Med. 2010;64:194–202. doi: 10.1007/s11418-009-0384-y. [DOI] [PubMed] [Google Scholar]
- 16.ICH. Guidance for Industry, Q2 (R1) Validation of Analytical Procedures: Test and Methodology, Methodology. 2005:6–12. [Google Scholar]
- 17.Seo CS, Kim JH, Shin HK. Simultaneous determination of seven constituents in herbal prescription Jaeumganghwa-tang using HPLC-PDA. Afr J Tradit Complement Altern Med. 2013;10:113–23. [PMC free article] [PubMed] [Google Scholar]


