Abstract
Background:
Liver diseases have become a major problem of the worldwide. More than 50% of all cases of liver failure can be attributed to drugs. Among these, acetaminophen is the most common cause.
Objective:
The aim of this study was to investigate the the hepatoprotective effects of blueberry and chitosan on tissue arginase activity, ornithine and nitric oxide levels during the acetaminophen-induced hepatotoxicity.
Materials and Methods:
Acetaminophen (250 mg/kg body weight per day), blueberry (60 mg/kg body weight per day) and, chitosan (200 mg/kg body weight per day) were administered to the rats by oral gavage during the experimental period.
Results:
Blueberry and chitosan significantly decreased liver arginase activity and ornithine levelsand and increased nitric oxide levels. Glutathione levels were remarkably increased by chitosan and blueberry treatments.
Conclusion:
The results of the present study indicate that blueberry and chitosan effectively protected against the acetaminophen-induced hepatotoxicity. The hepatoprotective effect afforded by blueberry and chitosan can be attributed to its antioxidant and anti-inflammatory activities.
Keywords: Acetaminophen hepatotoxicity, arginase, blueberry, chitosan, ornithine
INTRODUCTION
Liver diseases have become a major problem worldwide. More than 50% of all cases of liver failure can be attributed to drugs. Among these, acetaminophen (APAP) is the most common cause. APAP is a widely used and well-tolerated analgesic and antipyretic drug. Although discovered about 134 years ago and clinically used for over 100 years. While APAP produces predictable and dose-dependent hepatotoxicity, 14% of all cases of liver damage encompass unexpected and unpredictable drug reactions from a large variety of other drugs. Thus, it is now generally accepted that drugs are the single major cause of liver damage. That the liver is frequently targeted by drugs is not surprising in view of the organ's pivotal role in metabolism, vectorial transportand immune defense. Rather, what is of greater concern, is the puzzling fact that such hepatic adverse drug reactions have remained unpredictable and cannot simply be reduced to the toxicity of a drug.[1]
Hepatotoxicity of APAP is related to the initial dose and the rate of production of an electrophilic quinone metabolite, N-acetyl-p-benzoquinoneimine (NAPQI). In therapeutic doses, about 90% of ingested APAP is conjugated directly and forms sulfate and glucuronide esters before oxidation and these conjugated inactive metabolites are excreted in bile or urine. Only about 5-15% of APAP is oxidized by cytochrome P450 (CYP) enzymes to the toxic metabolite NAPQI, which is then conjugated with glutathione (GSH) and excreted in urine.[2] In APAP overdose, the conjugation pathway is saturated as GSH store is depleted, resulting in accumulation of NAPQI. When the GSH store is depleted below 30% of normal stores, free NAPQI rapidly and covalently binds to critical intracellular macromolecules and alter calcium and thiol status in liver cells leading to hepatocellular injury.[3] Hepatic enzymes, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), are routinely measured in the diagnosis of liver diseases in the clinical laboratory. These enzymes are not specific for the liver because they are widely distributed among both liver and non-hepatic tissues. In contrast, arginase, a urea cycle enzyme which hydrolyzes L-arginine to L-ornithine and urea, whereas metabolism of L-arginine by nitric oxide synthase (NOS) produces L-citrulline and nitric oxide (NO). Arginase exists abundantly in the liver, to some extent in the erythrocytes and small intestineand scarcely in the kidney and brain. Since human arginase exists almost exclusively in the liver, circulating liver-type arginase is expected to serve as a more specific marker for liver injury. The present datas supporting that arginase enzyme is an excellent marker of hepatocellular damage and also as an better indicator for prognostic diagnosis and subsequent treatment of hepatic diseases than conventional liver function tests.[4] Besides urea, arginase takes part in the biosynthesis of ornithine, a parent compound for proline, glutamate and polyamines. Therefore, being involved in so many important biochemical pathways, arginase may be a key point in the development of various pathological processes.[5] Nitric oxide is a highly reactive oxidant produced by liver parenchymal and nonparenchymal cellsand an important regulator and mediator in many physiological and pathophysiological events. The reactive nature of NO with oxygen free radicals suggests several biological pathways through which NO might either promote or reduce oxidative stress-induced cell injury. There are conflicting data in the literature to support both a protective and a cytotoxic role of NO in liver.[6,7]
Epidemiological studies on the relationship between dietary habits and disease risk have shown that food has a direct impact on health. The capacity of some plant-derived food to reduce the risk of chronic diseases has been associated, at least in part, to the occurrence of non-nutrient secondary metabolites that have been shown to exert a wide range of biological activities. These metabolites that are present in the dietand have been associated to health benefits, include glucosinolates, sulphur containing compoundsand various groups of polyphenols. Blueberry (BB), (Wild blueberry, Vaccinium angustifolium) contains higher levels of polyphenols than most commercial fruits and vegetables, imparting very high antioxidant capacities to BB. Blueberry polyphenols are mostly flavonoid in nature, containing more than 20 different anthocyanins that contribute to their beneficial effects on liver disease, atherosclerosisand cancer. Dietary intake of BB could be beneficial in protecting against hepatotoxicity. Therefore, for these reasons wild BB could play a key role in the prevention of oxidative stress by scavenging reactive oxygen species (ROS) and free radicals.[8,9]
A number of investigators have previously demonstrated that antioxidants prevent drugs-induced hepatotoxicity, by increasing antioxidant enzyme activity. Chitosan (CS), an important polysaccharide of marine origin, is made from alkaline N-deacetylation of chitin. CS has attracted much attention as a biomedical material, owing to its anti-tumor, anti-ulcer, immuno-stimulatory, anti-bacterial and other unique biological activities. Recently, the antioxidant activity of CS has attracted the most attention.[10] However, hepatoprotective effects related to BB and CS's antioxidant activity on APAP-induced hepatic injury had not previously been demonstrated.
The present study was undertaken to investigate the antioxidative and hepatoprotective effects of BB and CS against APAP-induced liver toxicity in rats by measuring arginase enzyme activity and changes in ornithine and NO levelsand alteration of GSH levels as key indicators of hepatic detoxification.
MATERIALS AND METHODS
Chemicals
Acetaminophen and Chitosan were purchased from Sigma Chemical Co (St. Louis, MO, Germany). Blueberry (Wild Blueberry, Vaccinium angustifolium) was obtained from Life Extension., Ltd (USA).
Animals and treatments
Two month old male Spraque-Dawley rats (200-250 gm) were purchased from the Animal House, College of Medicine, Eskisehir Osmangazi University (Turkey) and maintained in a controlled environment with a 12-h light/dark cycle. Rats were supplied with standard laboratory chow and water ad libitum and left to acclimatize for one week before experiments. The experimental protocol was approved by the Local Animal Care Committee and the experimental procedures were carried out in accordance with international guidelines for the care and use of laboratory animals. Two month old male Spraque-Dawley rats (200-250 gm) were purchased from the Animal House, College of Medicine, Eskisehir Osmangazi University (Turkey) and maintained in a controlled environment with a 12-h light/dark cycle. Rats were supplied with standard laboratory chow and water ad libitum and left to acclimatize for one week before experiments. The experimental protocol was approved by the Local Animal Care Committee and the experimental procedures were carried out in accordance with international guidelines for the care and use of laboratory animals. The rats were randomly divided into seven equal groups (n = 9, each). The first group received only distilled water and served as control. Hepatotoxicity was induced in animals of the fourth, fifth, sixth and seventh groups by APAP given at a oral dose of 250 mg/kg body weight.[11] The rats of the second group received BB at a oral dose of 60mg/kg body weight without induction of APAP hepatotoxicity and served as BB control.[12] The rats of the third group received CS at a oral dose of 200 mg/kg body weight without induction of APAP hepatotoxicity and served as CS control.[10] Fifth group of animals received oral gavage of BB at a dose of 60 mg/kg body weight, 3 h after APAP administration. Sixth group of animals received oral gavage of CS at a dose of 200 mg/kg body weight, 3 h after APAP administration. Seventh group of animals received oral gavage of BB at a dose of 60 mg/kg body weight, 3 h after APAP administration and animals received oral gavage of CS at a dose of 200 mg/kg body weight half an h after BB administration. All the treatments were given daily for 14 days. Body weights and feed consumption of rats were recorded weekly. All the rats were killed on the 15th day under xylasine-ketamine anesthesia.
Preparation of serum and tissue samples
The rats were anesthetized with xylasin-ketamine and sacrificed 24h after the last administration. Blood samples were collected into biochemical tubes by heart puncture. Blood samples were centrifuged for 10 min at 3500 rpm to obtain clear serum which were stored at −80◦ C. Serum samples were used for determination of aspartate aminotransferase and alanine aminotransferase activities. Livers were quickly removed, placed on ice and homogenized in 3 volumes of NaCl 0.9% and stored at −80° C. The homogenates were centrifuged at 11000rpm for 20 min at 4◦ C that and the resulting supernatant was obtained and used for determination of arginase activity and ornithine, nitric oxide, reduced glutathione (GSH) and total protein levels.
Histopathological examination of liver tissue
Samples of liver tissue from each animal were fixed in 10% formalin, dehydrated in ascending grades of alcohol and embedded in paraffin. Sections were cut at 4 μm, stained with hematoxylin and eosin (H and E) and examined under a light microscope by a pathologist unaware of the treatment protocol.
Biochemical analysis
Determination of AST and ALT activities
Hepatotoxicities were assessed by quantifying the serum activities of ALT and AST. Serum ALT and AST levels were measured using the Moduler oto-analyzer (Roche, Germany). Serum AST and ALT activities were expressed as U/L.
Determination of arginase activity
The principle of arginase activity determination was spectrophotometric measurement of urea produced by hydrolysis of L-arginine by arginase. Spectrophotometric determination was done according to thiosemicarbazide diacetlmonoxime urea (TDMU) method. For the measurement of arginase activity, 10 mM MnCl2 in 100 mM carbonate buffer (pH 9.7) was added to each tissue samples and enzyme activated by heating at 55◦ C for 20 min. The substrate, 50 mM L-arginine was then added and incubated at 37◦ C for 15 min. The reaction was terminated by the addition of an acid mix: (FeCl3 + H3PO4 + H2SO4). The urea formed was then assessed spectrophotometrically at 520 nm after the addition of colour reagent and heating at 100◦ C for 10 min. Tissue arginase activities were expressed as μmol urea mg protein−1 for 1 hour.[13,14]
Determination of ornithine levels
Ornithine was measured by a colorimetric method with ninhydrin.[15] Measurement of ornithine levels were carried out by adding 10% TCA (1:1) to the tissue samples. The mixture then was centrifuged and glacial acetic acid added. Ninhydrine reagent was also added and ornithine levels were determined by spectrophotometrically at 515 nm after the incubation at 100°C for 30 min. Tissue ornithine levels were expressed as μmol mg protein−1.
Determination of nitric oxide levels
Nitrite and nitrate were the primary oxidation products of NO subsequent to reaction with oxygen and therefore, the nitrite/nitrate concentrations in plasma were used as indicator of NO synthesis. Nitric oxide levels were determined as nitrate/nitrite levels by the Griess reaction.[16] Samples were deproteinized with Somogyi reagent. The nitrate was reduced to nitrite by copper-coated cadmium in glycine buffer (pH 9.7). Total nitrite/nitrate concentrations were calculated by using standard of sodium nitrate. Tissue NO levels were expressed as μmol mg protein−1.
Determination of reduced glutathione levels
The spectrophotometric assay method for glutathione (GSH) involved oxidation of GSH by the sulfhydryl reagent 5,5’-dithio-bis (2-nitrobenzoic acid) (DTNB) to form the yellow derivative 5’-thio-2-nitrobenzoic acid (TNB), measurable at 412 nm.[17] Tissue GSH levels were expressed as μmol mg protein−1.
Protein measurement
Protein was assayed by the method of Bradford et al., with serum bovine albumin as standard.[18]
Statistical analysis
All data analyses were performed by using SPSS Statistics 20 and SigmaStat 3.5. The descriptive statistisics were demonstrated with n (sample size), mean and standard deviation for continuous variables and, n (sample size), median and 25th and 75th percentiles for categorical variables. Contionuous normally distributed measurements were compared across the groups by using One Way ANOVA with Tukey Method, Student Newman Keuls Method multiple comparisons. Score variables that don’t show normal distribution were compared across the groups by using Kruskal-Wallis with Tukey Method, Student Newman Keuls Method multiple comparison tests. Spearman correlations analysis was applied to determine correlations among non-normally distributed variables. P value less than 0.05 (P < 0.05) was accepted significant.
RESULTS
Aspartate aminotransferase and alanine aminotransferase activities
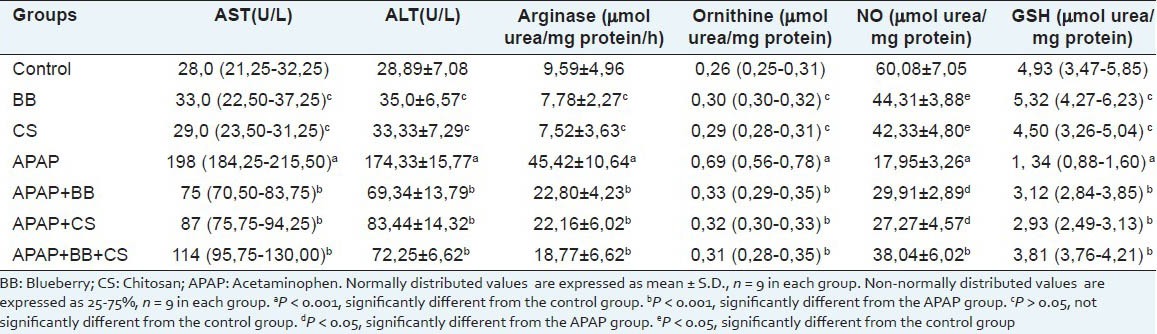
There were statistically significant (P < 0.001) increases in the serum activities of AST and ALT in the group treated with APAP as compared with the control and groups treated with BBand CS [Table 1]. Equally, the activities of these enzymes were statistically (P < 0.001) decreased in the groups treated with APAP + BB, APAP + CS and APAP + BB + CS only when compared with the APAP group. There was no statistically significant difference in the activities of AST and ALT in the groups of BB and CS when compared with the control (P > 0.05). Treatment with the BB and CS offered a protective effect against increase in the activities of AST and ALT induced by APAP.
Table 1.
Effects of blueberry and chitosan treatments on the biochemical parameters in rats exposed to acetaminophen hepatotoxicity
Arginase activitiy
Hepatic arginase activity was increased significantly (P < 0.001) in the group treated with APAP as compared with the control and groups treated with BBand CS [Table 1]. There was no statistically significant difference in the arginase activity in the groups of BB and CS when compared with the control (P > 0.05). However; treatment with BB, CSand BB + CS combination, offered protection against APAP-induced arginase activity. The arginase activity was statistically (P < 0.001) decreased in the groups treated with APAP + BB, APAP + CSand APAP + BB + CS only when compared with the APAP group.
Ornithine levels
The tissue ornithine level was increased in rats treated with APAP alone when compared with the control and groups treated with BBand CS (P < 0.001) and however; there was no statistically significant difference in the ornithine level in the groups of BB and CS when compared with the control (P > 0.05). The ornithine level was statistically (P < 0.001) decreased in the groups treated with APAP + BB, APAP + CS and APAP + BB + CS only when compared with the APAP group. Treatment with BB, CS and BB + CS combination ameliorated the induction caused by APAP in ornithine level.
Nitric oxide levels
Treatment with APAP caused a significant (P < 0.001) decrease in NO level as compared with the control. There was a statistically significant (P < 0.05) increase in the NO level in the groups treated with APAP + BB, APAP + CS compared with the APAP group. However, there was a highly statistically significant (P < 0.001) increase in the NO level in the group treated with APAP + BB + CS. On the other hand, treatment with BB + CS combination was more effective than the groups treated with APAP + BB, APAP + CS.
Glutathione levels
Treatment with APAP caused a significant (P < 0.001) decrease in hepatic GSH level as compared with the control and groups treated with BBand CS (P < 0.001) [Table 1]. However, there was no statistically significant difference in the GSH level in the groups of BB and CS when compared with the control (P > 0.05). The GSH level was statistically (P < 0.001) increased in the groups treated with APAP + BB, APAP + CS and APAP + BB + CS only when compared with the APAP group. Treatment with BB, CSand BB + CS combination ameliorated the inhibition caused by APAP in GSH level.
Histopathology analysis
Control, BB and CS groups showing portal triad, normal arrangement of hepatocytes with nuclei [Figure 1a–c]. APAP overdose caused marked liver damage in the form of intense cellular degeneration, sinusoidal dilatation and cellular inflammation of hepatocytes with vascular congestion [Figures 1d and 2a]. When BB [Figure 2b] was given to rat after to the APAP treatment, APAP + BB group showing mild cellular inflammation and the area of liver damage was reduced to compare with APAP group. However, CS was given to rat after to the APAP treatment, APAP + CS group showing mild sinusoidal dilatation and the area of liver damage was reduced to compare with APAP group [Figure 2c]. BB and CS combination treatment cause a significant changes in the liver histologyand showing portal triad, normal arrangement of hepatocytes with nucleiand showing normal hepatic architecture. BB + CS combination treatments markedly attenuated the APAP-induced liver tissue injury and restored the same histopathological picture observed in the control group [Figure 2d].
Figure 1.
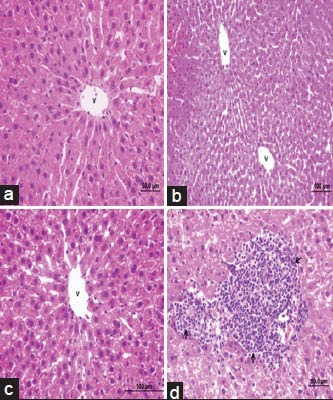
Photomicrographs of rat liver (H and E, ×100, ×50) from: (a) control group showing normal hepatic architecture; (b) Blueberry group showing normal hepatic architecture; (c) Chitosan group showing normal hepatic architecture; (d) Acetaminophen group showing intense cellular degenaration, cellular inflammation of hepatocytes
Figure 2.
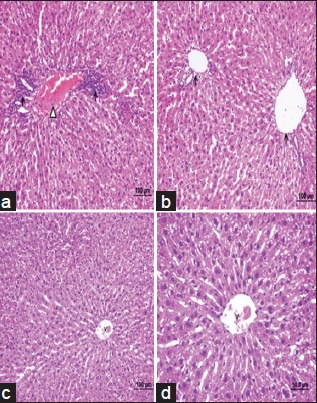
Photomicrographs of rat liver (H and E, ×100, ×50) from: (a) Acetaminophen group showing intense cellular degenaration, sinusoidal dilatation and cellular inflammation of hepatocytes with vascular congestion. (b) acetamiophen plus blueberry group showing mild cellular inflammation and the area of liver damage was reduced to compare with acetaminophen group; (c) acetamiophen plus chitosan group showing mild sinusoidal dilatation and the area of liver damage was reduced to compare with acetaminophen group; (d) Acetaminophen plus blueberry plus chitosan group showing portal triad, normal arrangement of hepatocytes with nuclei, and showing normal hepatic architecture
DISCUSSION
The liver is an organ that plays a major role in metabolizing endogenous and exogenous materials. Acute and chronic liver diseases, where in sufficient detoxification of harmful substances is not carried out, lead to pathological health problems.[30] APAP is one among to produce acute and chronic liver damage if over doses have been consumed. It is mainly metabolized in the liver to glucuronide and sulphate conjugates that are subsequently excreted. The hepatotoxicity of APAP has been attributed to the formation of a highly reactive metabolite NAPQI by the hepatic cytochrome P-450 which in turn disrupts the structure and function of lipid and protein macromolecules in the membrane.[19] The laboratory features of hepatotoxicity induced by APAP resemble other kinds of acute inflammatory liver disease with prominent increase of AST and ALT levels. In the present study, the serum levels of hepatic enzymes AST and ALT were increased and reflected the hepatocellular damage in the APAP-induced hepatotoxicity animal model. ALT and AST catalyze the reductive transfer of an amino group from alanine or aspartate, respectively, to alpha-ketoglutarate to yield glutamate and pyruvate or oxaloacetate, respectively. Damaged hepatocytes release their contents including ALT and AST into the extracellular space. The released enzymes ultimately enter into circulation and thereby increase the serum levels of ALT and AST compared to control subjects.[20] The BB and CS treatments could lower the AST and ALT levels in these APAP-intoxicated groups. The ability of BB and CS to prevent the increase in the activities of these enzymes is the primary evidence of their hepatoprotective activity.
In this study, APAP-induced hepatotoxicity was evidenced by biochemical measurements and histopathological changes that coincided with the observations of other investigators. Current evidence suggests that intracellular GSH plays an essential role in detoxification of APAP and prevention of APAP-induced toxicity in the liver. GSH, a thiol antioxidant, is known to act as protector in various cytotoxic conditions. Studies have reported reduced GSH pool during cytotoxicity and oxidative stress situations. APAP hepatotoxicity is initiated by the formation of a reactive metabolite, which depletes GSH and binds to cellular proteins, especially in the mitochondriaand leading to hepatocellular injury.[21,22] In this study, high-dose APAP treatment significantly induced toxicity, as indicated by elevated AST and ALT levels and decreased GSH contents in the liver of rats. Meanwhile, the increases in AST and ALT levels, accompanied by a significant reduction in GSH level, implicated liver damage, whereas the histopathological data of the tissues confirmed APAP-induced organ damage with histopathological evidences. However, BB and CS ameliorated hepatotoxicityand as assessed by both biochemical and histopathological analysis. Our results demonstrated that treatments of rats with BB and CS protected, against hepatic damages induced by toxic dose of APAP, as assessed by biochemical measurements and histopathological examination. At the same time, the depletion of GSH induced by APAP in the rat liver were inhibited in by treatments of rats with BB, CS and BB combination.
Hepatic enzymes in serum, AST and ALT, are routinely measured in serum for the diagnosis of hepatic disease; however, these enzymes are not liver specific because they are widely distributed in nonhepatic tissues. In contrast, urea cycle enzyme, i.e. liver-type arginase exist almost exclusively in the liver and may serve as more specific marker of liver. It has been reported that some of the urea cycle enzymes leak rapidly from hepatocytes when liver cells are damaged. Although there are several “hepatic marker” enzymes, including the urea cycle enzymes, it is not known which one of them is the most suitable enzyme for early detection of hepatocellular injury.[23] In the present study, we examined the clinical importance of arginase enzyme activity and ornithine levels in hepatotoxicity induced by APAP. To confirm the most suitable enzyme for this purpose, it is important to verify changes in the activity of arginase enzyme after liver damage. Our results confirm the significant increase the activity of arginase enzyme and ornithine levels in the liver tissue of APAP group administered rats as compared to that of control group. This was paralleled by significant decrease in the levels of non-enzymatic GSH free radical scavengers. Some studies indicate the importance role of arginase enzyme activity in experimentally APAP-induced hepatic injury.[23,24]
Arginase enzyme, catabolizes the hydrolysis of L-arginine to produce L-ornithine and urea. With any liver damage, arginase is released into the bloodstream and acts to deplete arginine while increasing ornithine production. The diseases to the liver caused by APAP has become an important problem of the world. Large amounts of arginase, sufficient to result in marked arginine depletion, are released from hepatocytes following hepatic damage. Since the L-arginine/NOS pathway has been recognized to play critical roles during acute and chronic liver diseases, the purpose of this study was to determine if arginase blockade could effectively attenuate liver damage induced by APAP. Recent studies have been suggested that increased arginase activity may decrease NO production by limiting the availability of intracellular arginine. Moreover, increased arginase activity has been shown to decrease NO production in macrophages by competitive inhibition through decreasing the available substrate, arginine.[14,25] The studies in recent years suggest that NO may act as an antioxidant and may interact with superoxide anion and other radicals to produce less toxic species. In contrast, other evidence suggests that NO may interact with reactive oxygen intermediates to form more toxic species.
Endogenous NO plays a hepatoprocective role in drug hepatotoxicity. This effect of NO could be attributed to its ability to interact with superoxide anion and other radicals to produce less toxic species.[26] In the present study, we found that NO levels were lower in APAP group than in healthy controls. However, it is still controversial whether this decrease is beneficial or damaging? Our results indicate that arginase is capable of diminishing NO production. This regulatory mechanism may be particularly by reducing L-arginine availability to NOS. The studies of NO production in activated macrophages has suggested that both the NO synthesis pathway and the arginase pathway rely on the extracellular supply of L-arginine and that 90% of L-arginine is consumed by arginase, while 10% of the L-arginine routes to the NO synthesis pathway. This view is supported by our data showing that arginase has a higher activity in APAP group and NO levels decreased. The changes in arginase activity may subsequently alter L-arginine availability for NOS and thus influence NO production. Therefore, we speculated that arginase might play an important role in the modulation of NO production.[25]
Epidemiological data and in vitro and in vivo studies demonstrate the protective effects of naturally occurring antioxidants against several degenerative diseases. High consumption of fruit and vegetables probably plays an important role in the prevention of illness. Dietary intake of nutraceutics, such as vitamins, flavonoids and anthocyanins, which are widely found in fruits, could be beneficial in protecting against APAP-induced hepatotoxicity. Tissue inflammation plays a critical role in liver pathology via induction of cellular injury. Blueberry also reduced the inflammatory process by reducing leukocyte infiltration in the liver tissues.[27,28] To our knowledge, the present study is the first to directly demonstrate the effects of BB and CS on APAP-induced hepatotoxicity. We found that the activity of arginase and ornithine levels in the liver were significantly lower in all treatment groupsand the NO levels in BB with and without CS groups showed a tendency to increase in comparison with the APAP group. Intake of BB, alone and with CS, ameliorates the APAP-induced liver injury.
Chitosan has profound applications in the fields of pharmaceuticals and biomedicines since it is having antibacterial, haemostatic, fungistatic, antitumoral and anticholesteremic properties. The main advantage of CS is that it is non-toxic. The studies indicated the antioxidant role and free radical scavenging properties of chitosan against experimentally induced hepatic injury.[29] In the present study, administration of CS decreased the activity of arginase and ornithine level to near normal status by its cytoprotectivity and may be also due to the inhibitory effect on arginase enzyme. Therefore, we found that the NO levels in the liver were increased significantly in CS with and without BB groups. The major and novel findings of this investigation are: 1) arginase enzyme activity are increased after APAP-induced hepatotoxicityand result in rapid circulating NO depletion; 2) NO induction with BB and CS is associated with significantly decreased arginase activityand 3) reduced arginase activity and ornithine levels ameliorate liver histology and biomarkers of hepatocellular injury.
In summary, activity of arginase enzyme blockade is associated with decreased hepatocellular injury. Thus, strategies aimed at arginase blockade following liver may be important in a variety of clinical settings. We showed that the BB and CS protective effects on APAP-induced hepatotoxicity. They reduce the hepatocytes injury, the inflammationand improve the barrier functions and antioxidant activity. These effects could have worked in harmony to produce the general protective effect that we have demonstrated. Thus BB and CS can be classified as an hepatoprotective agents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Boelsterli UA. Diclofenac-induced liver injury: A paradigm of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2003;192:307–22. doi: 10.1016/s0041-008x(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 2.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: New vistas of an old drug. CNS Drug Rev. 2006;12:250–75. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manimaran A, Sarkar SN, Sankar P. Toxicodynamics of subacute co-exposure to groundwater contaminant arsenic and analgesic-antipyretic drug acetaminophen in rats. Ecotoxicol Environ Saf. 2010;73:94–100. doi: 10.1016/j.ecoenv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Ikemoto M, Tsunekawa S, Awane M, Fukuda Y, Murayama H, Igarashi M, et al. A useful ELISA system for human liver-type arginaseand its utility in diagnosis of liver diseases. Clin Biochem. 2001;34:455–61. doi: 10.1016/s0009-9120(01)00254-5. [DOI] [PubMed] [Google Scholar]
- 5.Chrzanowska A, Gajewska B, Barańczyk-Kuźma A. Arginase isoenzymes in human cirrhotic liver. Acta Biochim Pol. 2009;56:465–9. [PubMed] [Google Scholar]
- 6.Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;234:124–34. doi: 10.1016/j.tox.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Sesti S, Martino G, Mazzulla S, Chimenti R. Effect of bradykinin on nitric oxide production, urea synthesis and viability of rat hepatocyte cultures. BMC Physiol. 2005;5:2. doi: 10.1186/1472-6793-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: Facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy KS, Boswall IA, Scanlan AP, Gottschall-Pass KT, Sweeney MI. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr Res. 2009;29:130–8. doi: 10.1016/j.nutres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Jeon TI, Hwang SG, Park NG, Jung YR, Shin SI, Choi SD, et al. Antioxidative effect of chitosan on chronic carbon tetrachloride induced hepatic injury in rats. Toxicology. 2003;187:67–73. doi: 10.1016/s0300-483x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 11.Olaleye MT, Rocha BT. Acetaminophen-induced liver damage in mice: Effects of some medicinal plants on the oxidative defense system. Exp Toxicol Pathol. 2008;59:319–27. doi: 10.1016/j.etp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, et al. Effect of a polyphenol-rich wild blueberry extracton cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198:352–8. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Geyer JW, Dabich D. Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem. 1971;39:412–7. doi: 10.1016/0003-2697(71)90431-3. [DOI] [PubMed] [Google Scholar]
- 14.Erbas H, Aydogdu N, Kaymak K. Effects of N-acetylcysteine on arginase, ornithine and nitric oxide in renal ischemia-reperfusion injury. Pharmacol Res. 2004;50:523–7. doi: 10.1016/j.phrs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Chinard FP. Photometric estimation of proline and ornithine. J Biol Chem. 1952;199:91–5. [PubMed] [Google Scholar]
- 16.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3. [PubMed] [Google Scholar]
- 17.George S, Jyothi M, Mathew B, Shashidhar S. Changes in glutathione, glutathione-linked enzymes and hexose mono-phosphate shunt enzymes in senile cataract. Indian J Physiol Pharmacol. 2003;47:191–6. [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Smilin B, Aseervatham G, Shamna R, Sangeetha B, Sasikumar JM. In vivo antioxidant activity of bark extract of Bixa orellana L. against acetaminophen–induced oxidative stress. Asian Pac J Trop Biomed. 2012;1691:700–5. [Google Scholar]
- 20.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Manimaran A, Sarkar SN, Sankar P. Toxicodynamics of subacute co-exposure to groundwater contaminant arsenic and analgesic–antipyretic drug acetaminophen in rats. Ecotoxicol Environ Saf. 2010;73:94–100. doi: 10.1016/j.ecoenv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Atakisi O, Erdogan HM, Atakisi E, Citil M, Kanici A, Merhan O, et al. Effects of reduced glutathione on nitric oxide level, total antioxidant and oxidant capacity and adenosine deaminase activity. Eur Rev Med Pharmacol Sci. 2010;14:19–23. [PubMed] [Google Scholar]
- 23.Ikemoto M, Tsunekawa S, Toda Y, Totani M. Liver-type arginase is a highly sensitive marker for hepatocellular damage in rats. Clin Chem. 2001;47:946–8. [PubMed] [Google Scholar]
- 24.Sajedianfard J, Saeb M, Edalatpisheh MR. Therapeutic effect of cimetidine on acetaminophen-induced hepatotoxicity in rabbits. Comp Clin Pathol. 2009;18:325–8. [Google Scholar]
- 25.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol. 1998;274:342–8. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 26.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulationand liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998;56:773–9. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 27.Sharifudin SA, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MA, Arulselvan P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm Biol. 2013;51:279–88. doi: 10.3109/13880209.2012.720993. [DOI] [PubMed] [Google Scholar]
- 28.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–56. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Santhosh S, Sini TK, Anandan R, Mathew PT. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur J Pharmacol. 2007;572:69–73. doi: 10.1016/j.ejphar.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Li W, Kim YH, Lee YW. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp Toxicol Pathol. 2013;65:73–80. doi: 10.1016/j.etp.2011.06.003. [DOI] [PubMed] [Google Scholar]


