Abstract
Background:
Gypsophila trichotoma Wend. (Caryophyllaceae) is a medicinal plant which is protected in Bulgaria by the Biodiversity Law. Previous studies have showed the presence of triterpene saponins, sterols, flavonoids, triterpens, etc.
Objective:
Gypsogenic acid, isolated from Gypsophila trichotoma roots, was evaluated for cytotoxic activity.
Materials and Methods:
The structure of the compound was elucidated by spectral methods. The cell survival fraction was determined by the MTT dye reduction assay, performed with some modifications.
Results:
Gypsogenic acid was tested in a panel of human tumor cell lines and was found to inhibit the proliferation of malignant cells. It was active against leukemic cells with lymphoid (SKW-3 and BV-173) or myeloid phenotype (HL-60, K-562, and LAMA-84), as well as against the EJ bladder carcinoma cell line. Bcr-Abl expressing myeloid cells (LAMA-84 and especially K-562) displayed lower sensitivity. HL-60/Dox cells were less sensitive to gypsogenic acid than the parent cell line, which shows that gypsogenic acid is probably a substrate of MRP-1.
Keywords: Cytotoxic activity, gypsogenic acid, Gypsophila trichotoma
INTRODUCTION
Triterpenes comprise one of the most interesting groups of natural products due to their high potential as pharmacological agents. They have been described as anti-inflammatory, antiangiogenic, antiviral, antioxidant, antibacterial, anticancer agents, as well as being immunomodulator compounds.[1] In the literature available, there are insufficient data about the pharmacological properties of gypsogenic acid. Lee et al.,[2] isolated this compound from Aceriphyllum rossii and evaluated it for in vitro cytotoxicity against the K-562 and HL-60 cell lines.[2]
Gypsophila species have been found to accumulate many saponins including both monodesmosidic and bidesmosidic triterpene saponins of gypsogenin, oleanolic acid, quillaic acid, etc.[3] Some of them have and gypsogenic acid as sapogenin.[3,4,5,6,7,8] Triterpenes can be found rarely in the genus and they occur usually as saponins. Only a few have been isolated in native form from the plants of Gypsophila.[9,10] The presence of a gypsogenic acid has been reported for G. oldhamiana.[10] In our previous investigation we isolated from G. trichotoma three triterpenes, including gypsogenic acid.[11]
Gypsophila trichotoma is a perennial herbaceous plant located in Southeastern Europe, Southwestern Asia, Kazakhstan, and Turkmenistan. The plant is found along the Black Sea coast in Bulgaria. Our previous studies of G. trichotoma resulted in the isolation of saponins, flavonoids, sterols, triterpenes, etc.[11,12,13] The hepatoprotective activity of the flavon glycoside saponarin was studied too.[14] In continuation of our investigations of G. trichotoma, in this paper we describe the cytotoxic activity of gypsogenic acid, isolated from the roots of the species, against six human tumor cell lines.
MATERIALS AND METHODS
General experimental procedure
1H Nuclear Magnetic Resonance (NMR) (400 MHz) and 13C NMR (100.6 MHz) spectra were recorded on Bruker DPX-400 using transcranial magnetic stimulation (TMS) as internal standard. All spectra were recorded in CD3OD. High-resolution electron spray ionization mass spectra (HR-ESIMS) was carried out on Agilent 6210 ESI-TOF (Agilent Technology) mass spectrometer. Thin layer chromatography (TLC) study was carried out on silica gel plates (Kieselgel 60 F254, Merck) using solvent systems n-BuOH/AcOH/H2O (4:1:1) and CHCl3/MeOH (9:1). The spots were visualized by spraying anysaldehyde/conc. H2SO4 reagent, followed by heating at 110°C. Chromatography (CC) was carried out with Diaion HP-20 (Supelco) and silica gel 60 (40-60 μm, Merck).
Plant material
The roots of G. trichotoma were collected in August 2008 at the Black Sea coast, Bulgaria. A voucher specimen (SO 103887) was deposited at the Herbarium of the Faculty of Biology, Sofia University.
Extraction and isolation
Air-dried powdered plant material of G. trichotoma (740 g) was exhaustively extracted with 80% methanol. After partial evaporation the aqueous solutions were extracted with CH2Cl2, EtOAc, and n-BuOH successively. The residue from the n-BuOH layer was separated by CC on a Diaion HP-20 column, using H2O/MeOH (100:0 → 0:100) and further purified by flash chromatography over silica gel with CH2Cl2/MeOH/H2O (18:11:1) to yield gypsogenic acid (50 mg) [Figure 1]. The structure of the compound was determined based on the spectral evidence (HRESI-MS, 1H and 13C NMR, correlation spectroscopy (COSY), heteronuclear single-quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC) experiments).[11]
Figure 1.
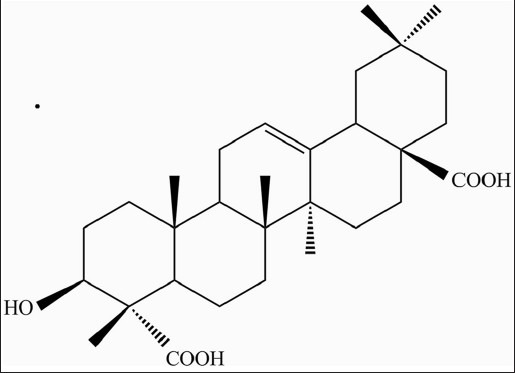
Chemical structure of gypsogenic acid
Cell lines and culture conditions
The following human cell lines were used: EJ (urinary bladder carcinoma), SKW-3 (T-cell leukemia), BV-173, K-562 and LAMA-84 (chronic myeloid leukemia), and HL-60 (acute myeloid leukemia) and its resistant variant HL-60/Dox which is characterized by the expression of the multi-drug resistance-associated protein MRP-1. All leukemic cell lines were obtained from DSMZ (Braunschweig, Germany) and EJ cells were obtained from the American Type Culture Collection (Rockville, MD, USA). All cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 2 mM L-glutamine (all from Lonza, Belgium) under standard conditions (37°C in an incubator with humidified atmosphere containing 5% CO2). The cell cultures were supplemented with fresh medium two or three times per week to maintain them in log phase. HL-60/Dox cells were maintained in medium containing 0.2 μM doxorubicin in order to sustain their multidrug-resistance (MDR) phenotype. One week prior to cytotoxicity determination however, they were kept in drug-free medium in order to avoid synergistic interaction between doxorubicin and the tested compound.
MTT assay for cell survival and proliferation
Exponentially growing cells were seeded into 96-well microplates (100 μl/well at a density of 2 × 105 cells/ml for leukemic cells or 5 × 104 for EJ cells) and exposed to various concentrations of gypsogenic acid for 72 h. The cell survival fraction was determined by the MTT (3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazoliumbromide) dye reduction assay,[15] performed with some modifications.[16] Briefly, after incubation with the test compound, MTT solution (10 mg/ml in PBS) was added (10 μl/well). Plates were further incubated for 3 h at 37ºC and the formazan crystals formed were dissolved by addition of 110 μl solvent (5% formic acid in 2-propanol) per well and mixing. Absorption was measured by an automated microtiter plate spectrophotometer (Labexim LMR-1, Lengau, Austria) at 550 nm. For each concentration at least four wells were used. Complete medium (100 μl), MTT solution (10 μl) and 5% formic acid in 2-propanol (110 μl) were used as blank solution.
Statistics and evaluation of cytotoxic effects
Cell survival fractions were calculated as percentages of respective untreated controls, taken for 100%. Using the GraphPad Prism 5.01 program (GraphPad Software, San Diego, California, USA), concentration-effect curves was fitted, which were then used to interpolate respective IC50 values and their 95% confidence intervals (CI).
RESULTS AND DISCUSSION
Fractionation of methanolic extract, obtained from the roots of G. trichotoma, by a combination of CC over Diaion HP-20 and silica gel resulted in the isolation of gypsogenic acid.[11]
Gypsogenic acid has previously been shown to possess antibacterial and trypanocidal activity. It inhibited the growth of six cariogenic gram-positive bacterial strains[17] and was active against blood trypomastigote forms of Trypanosoma cruzi.[18] Gypsogenic acid was also found to have antihepatotoxic activity in a CCl4-based model of liver injury.[19] In the present study, gypsogenic acid was evaluated for cytotoxic activity against a panel of human tumor cell lines and concentration-dependent cytotoxic effects were observed [Figures 2 and 3]. BV-173 cells were most sensitive (IC50 = 41.4 μM; 95% CI: 38.6-44.3 μM), HL-60 cells ranked second (IC50 = 61.1 μM; 95% CI: 57.7-64.7 μM), and SKW-3 third (IC50 = 81.5 μM; 95% CI: 79.1-84.0 μM). Gypsogenic acid had similar efficacy against HL-60/Dox, LAMA-84, and EJ cells with IC50 values ranging between 100 and 125 μM. K-562 cells were the least sensitive to gypsogenic acid (IC50 = 227.6 μM; 95% CI: 212.6-243.7 μM). Our data for the K-562 cell line are in accordance with those obtained by Lee et al.,[2] who studied gypsogenic acid isolated from A. rossii, while HL-60 cells were more sensitive in our experiment.
Figure 2.
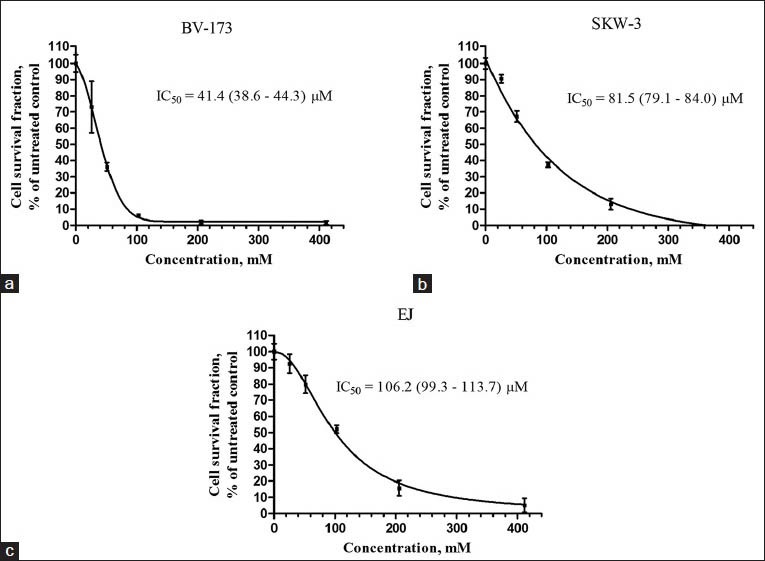
Survival of (a) BV-173, (b) SKW-3, and (c) EJ, tumor cells after exposure to gypsogenic acid for 72 h. Cell survival fractions were measured using the MTT dye reduction assay and are given as percentages of the respective untreated controls. Bars denote standard deviation
Figure 3.
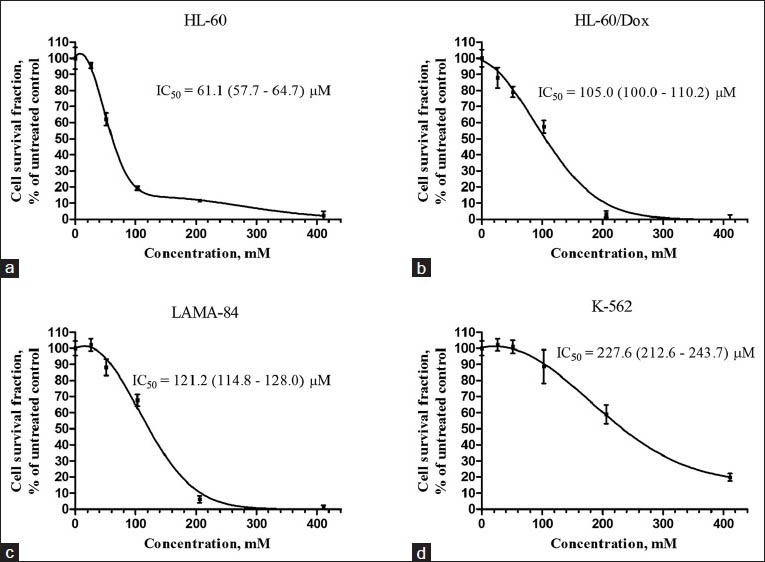
Survival of (a) HL-60, (b) HL-60/Dox, (c) LAMA-84, and (d) K-562, tumor cells after exposure to gypsogenic acid for 72 h. Cell survival fractions were measured using the MTT dye reduction assay and are given as percentages of the respective untreated controls. Bars denote standard deviation
CONCLUSION
In summary, gypsogenic acid isolated from G. trichotoma exhibited moderate cytotoxic activity against leukemic cells with lymphoid (SKW-3 and BV-173) or myeloid phenotype (HL-60, K-562, and LAMA-84), as well as against the EJ bladder carcinoma cell line. Bcr-Abl expressing myeloid cells (LAMA-84 and especially K-562) displayed lower sensitivity. HL-60/Dox cells were less sensitive to gypsogenic acid than the parent cell line, which shows that the compound is probably a substrate of MRP-1.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–60. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- 2.Lee I, Yoo JK, Na M, Min BS, Lee J, Yun BS, et al. Cytotoxicity of triterpenes isolated from Aceriphyllum rossii. Chem Pharm Bull (Tokyo) 2007;55:1376–8. doi: 10.1248/cpb.55.1376. [DOI] [PubMed] [Google Scholar]
- 3.Yotova M, Krasteva I, Nikolov S. Triterpenoid saponins from genus Gypsophila L. (Caryophyllaceae) In: Koh R, Tay I, editors. Saponins: Properties, Applications and Health Benefits. United States: Nova Publishers; 2012. pp. 99–122. [Google Scholar]
- 4.Böttger S, Melzig MF. Triterpenoid saponins of the Caryophyllaceae and Illecebraceae family. Phytochem Lett. 2011;4:59–68. [Google Scholar]
- 5.Elbandy M, Miyamoto T, Lacaille-Dubois MA. New triterpenoidal saponins from Gypsophila repens. Helv Chim Acta. 2007;90:260–70. [Google Scholar]
- 6.Elgamal MH, Soliman HS, Karawya MS, Duddeck H. Isolation of two triterpene saponins from Gypsophila capillaris (Forssk.) Nat Prod Lett. 1994;4:217–22. doi: 10.1016/0031-9422(94)00900-e. [DOI] [PubMed] [Google Scholar]
- 7.Elgamal MH, Soliman HS, Karawya MS, Mikhova B, Duddeck H. Isolation of triterpene saponins from Gypsophila capillaris. Phytochemistry. 1995;38:1481–5. doi: 10.1016/0031-9422(94)00900-e. [DOI] [PubMed] [Google Scholar]
- 8.Nie W, Luo JG, Kong LY. New triterpenoid saponins from the roots of Gypsophila pacifica Kom. Carbohydr Res. 2010;345:68–73. doi: 10.1016/j.carres.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Borges Del Castillo J, Zeitoun B, Arriaga F, Vazquez P. Steryl glucosides of Gypsophila struthium. Fitoterapia. 1986;57:61–4. [Google Scholar]
- 10.Luo JG, Liu J, Kong LY. New pentacyclic triterpenes from Gypsophila oldhamiana and their biological evaluation as glycogen phosphorylase inhibitors. Chem Biodivers. 2008;5:751–7. doi: 10.1002/cbdv.200890071. [DOI] [PubMed] [Google Scholar]
- 11.Yotova M, Krasteva I, Jenett-Siems K, Zdraveva P, Nikolov S. Triterpenoids in Gypsophila trichotoma Wend. Phytochem Lett. 2012;5:752–5. [Google Scholar]
- 12.Krasteva IN, Popov IS, Balabanova VI, Nikolov SD, Pencheva IP. Phytochemical study of Gypsophila trichotoma Wend. (Caryophyllaceae) Quim Nova. 2008;31:1125–6. [Google Scholar]
- 13.Krasteva I, Jenett-Siems K, Kaloga M, Nikolov S. 3-O-Sulfo-triterpenoid saponins from Gypsophila trichotoma Wend. Z Naturforsch B. 2009;64:319–22. [Google Scholar]
- 14.Vitcheva V, Simeonova R, Krasteva I, Yotova M, Nikolov S, Mitcheva M. Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. On cocaine-induced oxidative stress in rats. Redox Rep. 2011;16:56–60. doi: 10.1179/174329211X12989133691530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Yosifov DY, Todorov PT, Zaharieva MM, Georgiev KD, Pilicheva BA, Konstantinov SM, et al. Erucylphospho-N, N,N-trimethylpropylammonium (erufosine) is a potential antimyeloma drug devoid of myelotoxicity. Cancer Chemother Pharmacol. 2011;67:13–25. doi: 10.1007/s00280-010-1273-5. [DOI] [PubMed] [Google Scholar]
- 17.Scalon Cunha LC, Andrade de Silva ML, Cardoso Furtado NA, Vinhólis AH, Gomes Martins CH, da Silva Filho AA, et al. Antibacterial activity of triterpene acids and semi-synthetic derivatives against oral pathogens. Z Naturforsch C. 2007;62:668–72. doi: 10.1515/znc-2007-9-1007. [DOI] [PubMed] [Google Scholar]
- 18.Cunha WR, Martins C, da Silva Ferreira D, Crotti AE, Lopes NP, Albuquerque S. In vitro trypanocidal activity of triterpenes from miconia species. Planta Med. 2003;69:470–2. doi: 10.1055/s-2003-39719. [DOI] [PubMed] [Google Scholar]
- 19.Hikino H, Ohsawa T, Kiso Y, Oshima Y. Analgesic and antihepatotoxic actions of dianosides, triterpenoid saponins of Dianthus superbus var. longicalycinus herbs. Planta Med. 1984;50:353–5. doi: 10.1055/s-2007-969730. [DOI] [PubMed] [Google Scholar]


