Abstract
Background:
Ginseng root has been traditionally used for the treatment of many diseases in Korea. However, so far ginseng seed has been mostly unused and discarded. As part of our ongoing research on the ginseng seeds, the inhibitory effect of ginseng seeds on melanin production was verified to assess their potential as a skin depigmenting substance.
Materials and Methods:
The present study measured the inhibitory effect of ginseng seeds on melanin production through the tyrosinase inhibitory effect and analyzed their effects on melanin production in melan-a-cells.
Results:
Ethanol extract of ginseng seed was applied to melan-a-cells at a concentration of 100 ppm and melanin production was reduced by 35.1% without cytotoxicity. In addition, the ethanol extract of ginseng seed was shown to reduce tyrosinase activity.
Conclusion:
Because the results showed excellent melanin inhibitory activity compared with that obtained by arbutin, ethanol extracts of ginseng leaf and ginseng root at the same concentration, it can be concluded that ginseng seeds show great potential as a skin depigmenting substance.
Keywords: Melanin, panax ginseng, pigmentation, seed
INTRODUCTION
Melanin is an important factor that determines skin pigmentation of many animals including humans. Melanocytes, which produce melanin, are mainly located in the basal layer, the bottom part of the skin's epidermis and are activated by ultra violet light or inflammation to promote the biosynthesis of melanin. Biosynthesis of melanin begins with the oxidation of tyrosine by the action of tyrosinase and then proceeds through dopa, dopaquinone and dopachrome.[1,2] Melanin produced from this pathway plays a role in the protection of the skin, but overproduced melanin can cause various skin pigment problems such as melasma, freckles and black spots. Thus, many studies on the materials that inhibit melanin production have been actively conducted with a purpose of eliminating such problems.[3]
The name “ginseng” mainly refers to the Panax ginseng Meyer in Korea. Its root, after 4-6 year of cultivation, is used in both foods and medicines.[4,5] Recently, the use of its upper parts, in addition to the root, has been studied. Furthermore, hydroponically-grown ginseng leaves have been used as vegetables,[6] but so far there have been very few studies on the use of ginseng seeds.
The present study measured the inhibitory effect of ginseng seeds on melanin production via the tyrosinase inhibitory effect and analyzed their effects on melanin production in melanocytes. The inhibitory activity of ginseng root extracts and leaf fractions in melanin production has been reported,[7,8,9,10] but that of ginseng seeds has not been reported. In a previous study on the physiological activity of ginseng seeds by Kim et al., they reported the nuclear factor kappa B inhibitory effect of lupane-type triterpene which was separated from ginseng seeds,[11] while Kim and Kim reported lower DPPH scavenging activity in wild cultivated ginseng seeds compared to that in ginseng root or leaves.[12] However, other related studies have been rarely conducted.
MATERIALS AND METHODS
Samples
The seeds of Panax ginseng Meyer used in the study were purchased in Jeungpyeong-gun, Chungcheongbuk-do, Republic of Korea in March 2011, while ginseng leaves and roots were purchased in Seocheon-gun, Chungcheongnam-do, Republic of Korea in May 2011. Each sample was washed with distilled water and hot-air dried at 50°C for 36 h and then ground; the supernatant obtained by stirring the extract 3 times with ethanol was concentrated and then freeze-dried to prepare the ethanol extract.
Cell culture
Melan-a cells, melanocytes originating from mice,[13] were incubated at 37°C, 5% CO2 using RPMI 1640 culture media containing 10% fetal bovine serum, 1% penicillin-streptomycin and 200 nM phorbol-12 myristate 13-acetate. The cells were seeded in a 24-well plate at a concentration of 1 × 105 cells/well, incubated for 24 h and then each sample was treated for 3 d and incubated again for another 24 h.
Cell viability
After eliminating the culture media, the cells were washed with phosphate buffered saline (PBS) and then 200 μL of crystal violet solution (0.1%) was added per well. The cells were incubated at room temperature for 5 min and washed with distilled water twice, after which 1 mL of EtOH was added to them. The samples were shaken at room temperature for 10 min and absorbance was measured at 590 nm.[14]
Melanin production
After eliminating the culture media, the cells were washed with PBS and then 1 mL of 1 N NaOH was added per well to dissolve the melanin. Absorbance was then measured at 400 nm.[14]
Tyrosinase activity
Forty μL of the sample dissolved in methanol and 120 μL of 8.0 mM L-dopa dissolved in 67 mM of phosphate buffer (pH 6.8) were placed in a 96-well microplate. Forty μL of mushroom tyrosinase (125 U/mL) was then added to the well. They were then incubated at 37°C for 20 min and the amount of dopachrome produced was measured at 492 nm.[15]
Intracellular tyrosinase expression
Melan-a-cells were seeded in a culture dish and incubated for 24 h and then samples at a concentration of 100 ppm were treated for 3 days. These were then incubated for another 24 h and lysis buffer (50 mM Tris-HCl, pH 8.0, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 1% NP-40, 0.02% sodium azide, 100 μg/ml PMSF, 1 μg/ml aprotinin) was added and sonicated to extract the intracellular proteins. Using 8% SDS-polyacrylamide gel, 50 μg of extracted protein was subjected to electrophoresis and then transferred to the membrane and blocked with 5% skimmed milk. The membrane was reacted with each of the primary antibody of tyrosinase and then reacted with anti-goat secondary antibody and detected using electrochemiluminescence.[16]
RESULTS AND DISCUSSION
Effects on cell viability and melanin production
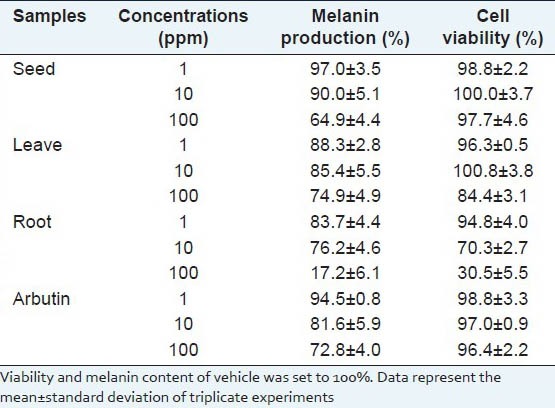
After a 3 d treatment of each extract on melan-a-cells, cell viability and melanin production were measured. The results showed that ginseng root exhibited cytotoxicity at over 10 ppm, while ginseng seeds did not exhibit significant cytotoxicity at 100 ppm but reduced melanin production by 35.1%. Ginseng leaves also reduced melanin production by 25.1% at 100 ppm, while simultaneously causing 9.2% cell death, as well as showing higher cytotoxicity and lower inhibitory activity against melanin production than those of ginseng seeds. Moreover, melanin reducing effect of ginseng seed was a better result than arbutin, a positive whitening agent,[17] which exhibited 27.2% melanin reducing effect at the 100 ppm [Table 1].
Table 1.
Effects of ginseng ethanol extracts on cell viability and melanin production in melan-a-cells
The color of melan-a cell pellet solution treated ginseng seed extract were represented at Figure 1. We reported the inhibitory effect of ethyl acetate fractions of ginseng leaf and ginseng root on melanin production.[8] However, the ethanol extract of ginseng seed showed excellent inhibitory activity in melanin production compared to the ethyl acetate fraction (27.0-28.1% at 100 ppm). Furthermore, as ethanol is a solvent that is less toxic to the human body and poses no problems in industrial use, the ethanol extract of ginseng seed is considered an excellent material for skin depigmentation compared to the extract of ginseng leaf or ginseng root.
Figure 1.

Color of melan-a cell pellet solutions treated ginseng seed extract and arbutin ①: 1 ppm ginseng seed, ②: 10 ppm ginseng seed, ③: 100 ppm ginseng seed, ④: 1 ppm arbutin ⑤: 10 ppm arbutin ⑥: 100 ppm arbutin. The ginseng seed extract and arbutin were added to melan-a-cells for 3 d and then cell pellets were dissolved in 1 mL of buffer
Effects on tyrosinase activity and intracellular tyrosinase expression
Tyrosinase is an important enzyme in promoting the oxidation of tyrosine and L-dopa in the initial steps of melanin biosynthesis. To determine the possible inhibitory effect of ginseng seed extract on tyrosinase activity, tyrosinase and its substrate L-dopa were incubated with the ethanol extract of ginseng seed and the amount of dopachrome thus produced was measured. The results show that ginseng seed 27.1% tyrosinase inhibitory effect at 100 ppm. The ginseng leaf and ginseng root all had a 23.0-24.8% tyrosinase inhibitory effect at 100 ppm [Figure 2]. The ginseng seed extract was added to melan-a-cells for 3 d and then proteins were extracted to measure the amount of expression of tyrosinase, which play an important role in melanin biosynthesis, by western immunoblotting. The ginseng seed extract did not reduce the expression of tyrosinase at 1, 10 and 100 ppm [Figure 3]. Therefore, it is considered that the inhibitory effect of ginseng seeds on melanin production may involve inhibition of tyrosianse activity except tyrosinase expression. Further studies will be needed to demonstrate the depigmenting activity by which ginseng seed inhibits melanogenesis in the animal skin.
Figure 2.
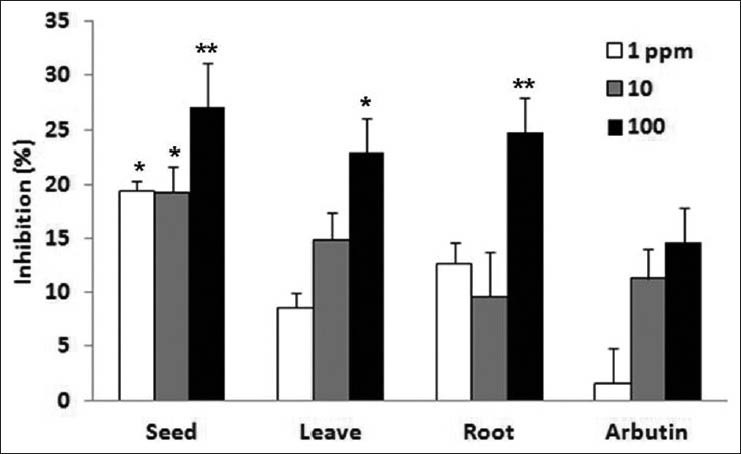
Tyrosinase inhibitory effects of ginseng extracts (Data are mean ± SD values of three experiments. *P < 0.05, **P < 0.01 compared with the control)
Figure 3.

Effect of ginseng seed extract on tyrosinase intracellular levels in melan-a-cells (Arbutin was used as positive control. Samples were treated for 3 d and then amount of expression of tyrosinase proteins were measured by western immunoblotting)
CONCLUSION
The ethanol extract of ginseng seed showed an excellent inhibitory effect on melanin production and demonstrated low cytotoxicity when applied to melanocytes, compared to the ethanol extracts of ginseng root and ginseng leaf. This inhibitory effect on melanin production was observed to be lower at concentrations below 10 ppm compared to the currently most widely used whitening substance, arbutin, but higher at concentrations over 100 ppm. From the above results, it is considered that ginseng seeds show great potential for use as a skin depigmenting substance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pawelek JM. After dopachrome? Pigment Cell Res. 1991;4:53–62. doi: 10.1111/j.1600-0749.1991.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 2.del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–8. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 3.Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, et al. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20:921–34. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 4.Ha DC, Ryu GH. Chemical components of red, white and extruded root ginseng. J Korean Soc Food Sci Nutr. 2005;34:247–54. [Google Scholar]
- 5.Kim DH. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SY, Cho CW, Lee Y, Kim SS, Lee SH, Kim KT. Comparison of ginsenoside and phenolic ingredient contents in hydroponically-cultivated ginseng leaves, fruits, and roots. J Ginseng Res. 2012;36:425–9. doi: 10.5142/jgr.2012.36.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im SJ, Kim KN, Yun YG, Lee JC, Mun YJ, Kim JH, et al. Effect of radix ginseng and radix trichosanthis on the melanogenesis. Biol Pharm Bull. 2003;26:849–53. doi: 10.1248/bpb.26.849. [DOI] [PubMed] [Google Scholar]
- 8.Hwang EY, Choi SY. Quantitative analysis of phenolic compounds in different parts of panax ginseng C. A. Meyer and its inhibitory effect on melanin biosynthesis. Korean J Med Crop Sci. 2006;14:148–52. [Google Scholar]
- 9.Hwang EY, Kong YH, Lee YC, Kim YC, Yoo KM, Jo YO, et al. Comparison of phenolic compounds contents between white and red ginseng and their inhibitory effect on melanin biosynthesis. J Ginseng Res. 2006;30:82–7. [Google Scholar]
- 10.Song M, Mun JH, Ko HC, Kim BS, Kim MB. Korean red ginseng powder in the treatment of melasma: An uncontrolled observational study. J Ginseng Res. 2011;35:170–5. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JA, Son JH, Yang SY, Song SB, Song GY, Kim YH. A new lupane-type triterpene from the seeds of Panax ginseng with its inhibition of NF-κB. Arch Pharm Res. 2012;35:647–51. doi: 10.1007/s12272-012-0408-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim JK. Antioxidant activity and functional component analysis of Korean mountain ginseng's different sections. J Korean Soc Food Sci Nutr. 2006;35:1315–21. [Google Scholar]
- 13.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–8. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 14.Choi SY, Hwang JS, Kim S, Kim SY. Synthesis, discovery and mechanism of 2,6-dimethoxy-N-(4-methoxyphenyl) benzamide as potent depigmenting agent in the skin. Biochem Biophys Res Commun. 2006;349:39–49. doi: 10.1016/j.bbrc.2006.07.206. [DOI] [PubMed] [Google Scholar]
- 15.Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, et al. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun. 1998;243:801–3. doi: 10.1006/bbrc.1998.8169. [DOI] [PubMed] [Google Scholar]
- 16.Choi SY. Inhibitory effects of geranic acid derivatives on melanin biosynthesis. J Cosmet Sci. 2012;63:351–8. [PubMed] [Google Scholar]
- 17.Lim YJ, Lee EH, Kang TH, Ha SK, Oh MS, Kim SM, et al. Inhibitory effects of arbutin on melanin biosynthesis of alpha-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch Pharm Res. 2009;32:367–73. doi: 10.1007/s12272-009-1309-8. [DOI] [PubMed] [Google Scholar]


