Abstract
Background:
Dracaena draco L. ssp. draco is known as the “dragon's blood tree” and it is endemic from the Canary Islands and Morocco.
Objective:
Carry out phytochemical investigation of acetonic extracts of red resin obtained from the trunk of D. draco, to obtain to the isolation of the most abundant resin constituents, belonging to the class of flavonoids: flavans, along with homoisoflavans and homoisoflavanones.
Materials and Methods:
The structures of the isolated compounds were established by Nuclear Magnetic Resonance (NMR) and mass spectrometry data and comparison with literature data. The acetonic extract was evaluated for its anti-staphylococcal properties against two reference strains.
Results:
The acetonic extracts resulted inactive at the maximum tested concentration of 1000 μg/ml against free living forms of tested staphylococci, but they showed a very interesting activity in the prevention of a biofilm formation at a concentration equal to 200 μg/ml against S. aureus ATCC 25923.
Keywords: Antibiofilm activity, Dracaena draco L., dragon blood resin, flavan, homoisoflavan
INTRODUCTION
The dragon tree group consists of arborescent taxa of the genus Dracaena (Dracaenaceae, though recently given as Convallariaceae)[1] and comprises approximately 60 species which are mainly found in tropical and sub-tropical Africa. The genus also grows in Macaronesia, Arabia, Socotra, Madagascar, south-eastern Asia and northern Australia.
Dracaena draco L. is an arboreal species found in Macaronesian region and Morocco, characterised by a single or multiple trunk growing up to 12m tall, it has a slow growth, requiring about 10 years reaching 1m tall. The leaves are green, tinged with red at the base, arranged in dense rosettes at the ends of the branches. The flowers are very fragrant, form large clusters of greenish-white petals. The fruits are orange-red berries, with a single seed. When the trunk or the branches of D. draco are wounded they secrete a deep red resin so-called “Dragon's blood” [Figure 1].[2]
Figure 1.

Dragon's blood resin from Dracaena draco
The D. draco resin has been used since ancient times for artistic purposes and by traditional medicine as haemostatic, antidiarrhetic, antiulcer, antimicrobial, antiviral, wound healing, antitumor, anti-inflammatory, antioxidant, etc.[2,3,4,5,6]
D. draco morphological parts are considered as rich sources of cytostatic and cytotoxic steroidal saponins. Several studies have proved intense cytostatic activity against human acute myeloid leukaemia cells (HL-60) of the two steroidal saponins extracted from the aerial parts of D. draco.[2,6,7,8] In addition, Gonzαlez et al., reported the strong cytotoxic effect on the same cell line of the two new steroidal saponins draconins A and B isolated from D. draco bark; the mechanism of these cytotoxic compounds was established to be via activation of apoptotic process.[9] From the root of D. draco were isolated icogenin and dioscin that inhibit HL-60 cells growth by induction of apoptosis.[10] Icodeside from the D. draco leaves presents moderate cytotoxicity against both HL-60 and human epidermoid carcinoma (A-431) cells.[11]
Some phenolic compounds have been identified in D. draco resin[6,12,13,14] including flavans and methylflavans, along with flavanones, homoisoflavans, homoisoflavones, chalcones, dihydrochalcones and others (e.g. dracaenone); dracoflavylium was isolated and identified as the major red colourant in this resin.[15]
This article reports the isolation and characterization of 16 known compounds from resin samples of “dragon blood” collected by ancients speciments of D. draco L. ssp. draco growing in Palermo and the antimicrobial activity against two biofilm forming staphylococcal reference strains.
The ability to form biofilms, bacterial communities able to grow on surfaces and surrounded by an extracellular polymeric substance (EPS) matrix, is probably the most important virulence factor of staphylococci in the development of the chronic and persistent form of some infectious diseases in humans such as otitis media, osteomyelitis, endophtalmitis, urinary tract infections, acute septic arthritis, native valve endocarditis, burn or wound infections and cystic fibrosis associated infections (CF).[16]
Furthermore, staphylococcal biofilms are commonly isolated from device-related infections of medical relevance. In fact, S. aureus is an important cause of metal-biomaterial infections, while Staphylococcus epidermidis is seen more often in polymer associated infections.[17] Together, the Gram-positive pathogens S. aureus, S. epidermidis and Enterococcus faecalis represent more than 50% of the species isolated from patients with medical device-associated infections[18] and catheter-related bloodstream infections (CRBSIs) during intensive care unit (ICU) stays in four European countries (France, Germany, Italy, UK) has an estimated cost of € 163.9 million.[19]
The treatment of these kinds of infections is complicated because staphylococcal biofilms show an high degree of resistance to resistant to conventional antibiotics.[20]
Therefore; there is undoubtedly an urgent need for novel treatments, strategies and anti-staphylococcal biofilm agents. One possible strategy is the screening of novel agents (synthetic or natural) that inhibit staphylococcal biofilms through direct effects on bacterial growth and viability. This work is aimed to evaluate the anti-staphylococcal biofilm properties of D. draco resin.
MATERIALS AND METHODS
General procedures
All the ragents for extraction and isolation are analytical purity. MTT was obtained from Sigma (Co., Ltd., USA). TLC was carried out on precoated silica gel 60 F254 plates (Merck) and spots were detected under UV (254 and 366 nm). Preparative TLC was performed on precoated silica gel plates, layer thickness 1 mm (Merck). Column chromatography was carried out on silica gel 60 (230-400 mesh, Merck). 1H and 13C (BBD, DEPT 135, DEPT 90) NMR spectra were measured at 300.13 and 75.47 MHz respectively, using a Bruker AC series 300MHz spectrometer (tetramethylsilane as an internal standard): Chemical shifts are expressed in δ values (ppm). EI-MS spectra were measured on a Autospec Ultima, double focusing sector instrument, with EBE geometry (Micromass, Manchester, UK) at an ion source temperature of 200°C, an electron energy of 70 eV and a mass resolution of approximately 1000.
Plant material
Samples of red resin “dragon's blood” were collected from ancient specimens of Dracaena draco L. ssp. draco growing in Botanical Garden of Palermo, in July 2009.
Extraction and isolation
The dried resin (30 gm) was ground into fine powder and then extracted with acetone (100 ml) for 24h at room temperature. The extract was concentrated under reduced pressure at 40°C yielding 22 mg of dark red extract. A sample of this red acetonic extract was tested for biological activity. 10 mg of the acetonic extract was chromatographed on silica gel column and eluted with a gradient of CH2Cl2-MeOH (99:1-100 ml, 95:5-100 ml, 90:10-700 ml, 85:5-200 ml, 80:20-200 ml) yielding 13-100 ml fractions (1-13).
Fractions 3-4 (0.6 gm) were subjected to flash silica gel and eluted with gradient n-hexane-EtOAc (1:0, 9:1, 8:2, 7:3, 6:4, 1:1 each 100 ml) afforded 600 ml subfractions. Purification of combined subfractions 2-5 on several PTLC (n-hexane-EtOAc 8:2, n-hexaneCHCl3 3:1) yielded compounds 8 (45 mg), 9 (20 mg), 10 (15 mg) and 16 (10 mg).
Fractions 5-6 collected, by further CC were purified and several PTLC using n-hexane containing increasing amount of CHCl3 led to isolation of 1 (30 mg), 2 (25 mg), 3 (10 mg), 4 (5 mg), 7 (10 mg), 14 (8 mg). Similar purification of fractions 7-9 by further CC eluted with n-hexane-EtOAc gradient yielded 5 (20 mg), 6 (18 mg), 13 (15 mg) and 15 (12 mg). PTLC on fractions 10-13 using solvent CHCl3-EtOAc (9:1) led to isolation of 11 (10 mg), 12 (5 mg).
Antibacterial activity
The staphylococcal reference strains used were S. aureus ATCC 25923 and S.epidermidis RP62A well known for their ability to form biofilms. Minimum inhibitory concentrations (MICs) against planktonic strains were determined by a broth dilution micromethod as described in literature.[21]
Inhibition of biofilm formation (Safranin staining method)
The staphylococcal strains were tested for their ability to form biofilms. Briefly, bacteria were grown in Tryptic Soy Broth (TSB, Sigma) containing 2% glucose overnight at 37°C in a shaking bath and then diluted 1:200 to a suspension with optical density (OD) of about 0,040 at 570nm. Polystyrene 24-well tissue culture plates were filled with 1mL of diluted suspension and incubated for 24-h at 37°C. Then, the wells were washed 3 times with 1mL of sterile phosphate-buffered saline (PBS) and stained with 1 mL of safranin 0 1% v/v for 1 min. The excess stain was removed by placing the plates under running tap water. Plates were dried overnight in inverted position at 37°C. Safranin stained adherent bacteria in each well were re-dissolved to homogeneity in 1mL of 30% v/v glacial acetic acid, and the OD was read at 492 nm. Each assay was performed in triplicate and repeated at least twice.
The same safranin procedure was used to evaluate the activity of the tested extracts in preventing biofilm formation by adding directly sub-MIC concentrations of them to the bacterial suspension. Comparing the average optical density of the growth control wells with the sample wells the following formula was used to calculate the percentages of inhibition for each concentration of the sample:
(OD growth control- OD sample)/OD growth control × 100
Antibiofilm activity (MTT staining method)
Staphylococcal strains were grown and diluted as above mentioned. The diluted suspension was added to the wells (100 ml per well) of a polystyrene microtiter plate and incubated for 24h at 37°C. Then the wells were washed 3 times with 200 ml of sterile PBS and finally air-dried in inverted position at 37°C. Each of the wells was then filled with 100ml of Mueller-Hinton broth. With the exception of the positive (growth) control wells, the Mueller-Hinton broth was supplemented with concentrations, obtained by dilution, from 400 to 100 μgml-1 of the extract. The plates were incubated at 37°C for 24 h and then the medium was removed, the plates were air-dried in inverted position, and each well was filled with 100 ml of PBS and 5 ml of a 5 mg/ml-1 MTT (methylthiazotetrazolium) solution and incubated for 1h at 37°C. The insoluble purple formazan obtained by cleavage of MTT made by the dehydrogenase enzymes of living cells, was dissolved by a mixture of isopropyl alcohol (9 ml), Triton X-100 (Sigma) (1 ml) and HCl 37%v/v (300 μl). The OD of each well was read by a microplate reader (ELX 800, Bio-Tek instruments) at 570 nm with background subtraction at 630nm. Comparing the average optical density of the growth control wells with that of the sample wells the following formula was used to calculate the inhibition percentages for each concentration of the compound:
(OD growth control- OD sample)/OD growth control × 100
All experiments were performed at least in triplicate.
Inhibition of biofilm formation (Plate count method)
S. aureus ATCC 25923 strain was grown and diluted previously seen for safranin and MTT method. Polystyrene 24-well tissue culture plates were filled with 1 mL of diluted bacterial suspension, added with sub-MIC concentrations of extracts and incubated for 24 h at 37°C. Then, the wells were washed 3 times with 1 mL of sterile phosphate-buffered saline (PBS) and the surface of each well was scraped for 3 times. The inocula were put in test tubes with 10 ml of NaCl (0.9%v/v solution) and sonicated for 2 min. Six of 10-fold dilutions were prepared and 100 μl aliquots of each dilution were plated onto Tryptic Soy agar. Plates were then incubated at 37°C and CFU/ml were counted after 18 h. Each assay was performed in triplicate and repeated at least twice.
RESULTS
After several chromatographies on silica gel of acetone extract of dragon's blood, 16 known compounds have been identified (Scheme 1). The identification of isolated compounds has been possible by comparison of spectroscopic data obtained with those reported in literature.
Scheme 1.
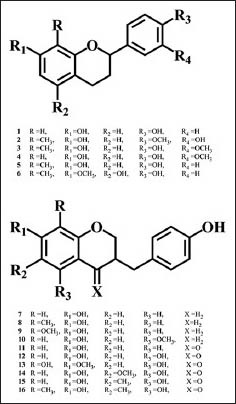
Structures of isolated compounds 1-16
Spectral data of isolated compounds
7,4′-Dihydroxyflavane (1): colorless crystal, EI-MS (70eV) m/z (rel. int): 242[M]+ (100), 136 (45), 120 (85), 107 (70), 91 (40); 1H-NMR (δ, CD3OD): 2.47 (1H, m, H-3), 2.62 (1H, m, H-3), 2.02 (1H, m, H-4), 1.98 (1H, m, H-4), 4.97 (1H, dd, J = 9.90, 2.15 Hz, H-2), 6.35 (1H, d, J = 2.5 Hz, H-8), 6.40 (1H, d, J = 8.10, 2.5 Hz, H-6), 6.90 (1H, d, J = 8.10 Hz, H-5), 6.92 (2H, d, J = 8.40 Hz, H-3′and H-5′), 7.22 (2H, d, J = 8.40 Hz, H-2′ and H-6′); 13C NMR (δ, CD3OD): 76.33 (C-2), 30.05 (C-3), 29.91 (C-4), 128.64 (C-5), 107.39 (C-6), 153.87 (C-7), 101.60 (C-8), 115.05 (C-4a), 156.90 (C-8a), 134.39 (C-1′), 127.12 (C-2′,6′), 115.88 (C-3′,5′), 155.77 (C-4′).[22]
3′,7-Dihydroxy-4′-methoxy-8-methylflavane (2): white needles, mp. 83-84°C; EI-MS (70eV) m/z (rel. int): 286[M]+ (100), 271 (6), 162 (10), 150 (60), 137 (29), 135 (18), 107 (30); 1H-NMR (δ, CDCl3): 1.97 (1H, m, H-3), 2.08 (3H, s, Me), 2.12 (1H, m, H-3), 2.69 (1H, ddd, J = 16.0, 5.0, 4.05 Hz, H-4), 2.89 (1H, ddd, J = 16.0, 10.8, 5.85 Hz, H-4), 3.90 (3H, s, OMe), 4.98 (1H, dd, J = 10.15, 2.31 Hz, H-2), 6.39 (1H, d, J = 8.19 Hz, H-6), 6.75 (1H, d, J = 8.20 Hz, H-5), 6.84 (1H, d, J = 8.3 Hz, H-5′), 6.90 (1H, dd, J = 8.3, 2.0 Hz, H-6′), 6.99 (1H, d, J = 2.0 Hz, H-2′); 13C NMR (δ, CDCl3): 77.33 (C-2), 30.10 (C-3), 24.91 (C-4), 126.64 (C-5), 107.39 (C-6), 153.87 (C-7), 111.60 (C-8), 129.10 (C-4a), 159.10 (C-8a), 134.49 (C-1′), 117.12 (C-2′,5′), 150.49 (C-3′), 152.24 (C-4′), 119.16 (C-6′).[9,14]
4′,7-Dihydroxy-3′-methoxy-8-methylflavane (3): white amorphous powder; EI-MS (70eV) m/z (rel. int): 286[M]+ (60), 271 (12), 163 (15), 162 (20), 150 (100), 137 (40), 135 (24), 107 (30); 1H-NMR (δ, CDCl3): 1.99 (1H, m, H-3), 2.18 (3H, s, Me), 2.25 (1H, m, H-3), 2.71 (1H, ddd, J = 15.8, 5.19, 3.18 Hz, H-4), 2.93 (1H, ddd, J = 15.8, 11.70, 5.82 Hz, H-4), 3.85 (3H, s, OMe), 4.97 (1H, dd, J = 10.38, 2.38 Hz, H-2), 6.36 (1H, d, J = 8.20 Hz, H-6), 6.78 (1H, d, J = 8.20 Hz, H-5), 6.90 (1H, d, J = 8.3 Hz, H-5′), 6.90 (1H, dd, J = 8.3, 2.0 Hz, H-6′), 6.96 (1H, d, J = 2.0 Hz, H-2′); 13C NMR (δ, CDCl3): 77.53 (C-2), 30.05 (C-3), 25.21 (C-4), 127.04 (C-5), 107.59 (C-6), 153.70 (C-7), 111.40 (C-8), 128.05 (C-4a), 157.90 (C-8a), 134.09 (C-1′), 112.22 (C-2′), 151.88 (C-3′), 149.24 (C-4′), 116.02 (C-5′), 129.46 (C-6′).[14]
4′,7-Dihydroxy-3′-methoxyflavane (4): yellow needles, mp. 157-159°C; EI-MS (70eV) m/z (rel. int): 272[M]+ (100), 255 (10), 241 (25), 162 (10), 150 (66), 137 (24), 107 (15); 1H-NMR (δ, (CD3)2CO): 1.89 (1H, m, H-3), 2.10 (1H, m, H-3), 2.65 (1H, ddd, J = 15.8, 4.9, 4.05 Hz, H-4), 2.82 (1H, ddd, J = 15.8, 10.10, 4.8 Hz, H-4), 3.79 (3H, s, OMe), 4.89 (1H, dd, J = 9.96, 2.37 Hz, H-2), 6.30 (1H, d, J = 2.2 Hz, H-8), 6.34 (1H, dd, J = 8.2, 2.2 Hz, H-6), 6.83 (1H, dd, J = 8.2, 2.1 Hz, H-6′), 6.84 (1H, d, J = 8.26 Hz, H-5), 6.88 (1H, d, J = 8.2 Hz, H-5′), 6.91 (1H, d, J = 2.1 Hz, H-2′); 13C NMR (δ, CDCl3): 77.44 (C-2), 30.11 (C-3), 27.02 (C-4), 128.08 (C-5), 107.99 (C-6), 156.85 (C-7), 102.40 (C-8), 114.05 (C-4a), 157.90 (C-8a), 132.24 (C-1′), 112.22 (C-2′), 149.68 (C-3′), 148.01 (C-4′), 116.02 (C-5′), 126.06 (C-6′).[14]
4′,7-Dihydroxy-8-methylflavane (5): light yellow powder; EI-MS (70eV) m/z (rel. int): 256[M]+ (100), 241 (15), 137 (55), 120 (40), 107 (70), 91 (40); 1H-NMR (δ, (CD3)2CO): 1.93 (1H, m, H-3), 2.10 (3H, s, Me), 2.15 (1H, m, H-3), 2.74 (1H, ddd, J = 16.4, 5.03, 3.47 Hz, H-4), 2.93 (1H, ddd, J = 16.4, 11.40, 5.95 Hz, H-4), 4.99 (1H, dd, J = 10.25, 2.34 Hz, H-2), 6.37 (1H, d, J = 8.2 Hz, H-6), 6.77 (1H, d, J = 8.2 Hz, H-5), 6.83 (2H, d, J = 8.5, Hz, H-5′, 3′), 7.28 (2H, d, J = 8.5 Hz, H-2′, 6′); 13C NMR (δ, (CD3)2CO): 77.23 (C-2), 29.85 (C-3), 29.91 (C-4), 126.77 (C-5), 107.39 (C-6), 153.27 (C-7), 111.90 (C-8), 126.95 (C-4a), 154.80 (C-8a), 134.09 (C-1′), 117.12 (C-2′,5′), 152.68 (C-3′), 130.33 (C-2′, 6′).[12,14]
4′,5-Dihydroxy-7-methoxy-8-methylflavane (6): white amorphous powder; EI-MS (70eV) m/z (rel. int): 286[M]+ (100), 256 (41), 191 (10), 167 (72), 166 (68), 150 (25), 137 (45), 120 (65), 107 (35); 1H-NMR (δ, (CD3)2CO): 1.80 (1H, m, H-3), 1.95 (3H, s, Me), 2.25 (1H, m, H-3), 2.56 (1H, ddd, J = 16.6, 10.9, 6.01 Hz, H-4), 2.63 (1H, ddd, J = 16.6, 5.9, 3.19 Hz, H-4), 3.67 (3H, s, OMe), 4.88 (1H, dd, J = 10.23, 2.12 Hz, H-2), 6.09 (1H, s, H-6), 6.87 (2H, dd, J = 6.8, 2.0 Hz, H-5′,3′), 7.27 (2H, dd, J = 6.8, 2.0 Hz, H-2′,6′); 13C NMR (δ, CD3COCD3): 77.34 (C-2), 26.15 (C-3), 28.11 (C-4), 156.88 (C-5), 97.89 (C-6), 155.07 (C-7), 113.77 (C-8), 106.55 (C-4a), 154.90 (C-8a), 134.10 (C-1′), 127.22 (C-2′,5′), 154.33 (C-3′), 129.41 (C-2′, 6′).[14]
7-Hydroxy-3-(4-hydroxybenzyl) chromane (7): colorless needles, mp. 153-154°C; EI-MS (70eV) m/z (rel. int): 256[M]+ (90), 161 (16), 148 (68), 133 (45), 123 (61), 107 (100), 73 (20); 1H-NMR (δ, CD3OD): 2.15 (1H, m, H-3), 4.07 (1H, dd, J = 10.6, 1.25 Hz, H-2), 3.70 (1H, dd, J = 10.6, 8.65 Hz, H-2), 2.52 (1H, dd, J = 13.3, 7.45 Hz, H-4), 2.44 (1H, dd, J = 13.3, 7.40 Hz, H-4), 2.35 (1H, dd, J = 15.7, 8.90 Hz, H-9), 2.63 (1H, dd, J = 15.7, 2.0 Hz, H-9), 6.17 (1H, d, J = 3.65 Hz, H-8), 6.30 (1H, d, J = 8.3 Hz, H-5), 6.77 (1H, dd, J = 8.3, 3.65 Hz, H-6), 6.73 (2H, d, J = 8.15 Hz, H-3′, 5′), 6.90 (2H, d, J = 8.15 Hz, H-2′, 6′); 13C NMR (δ, CD3OD): 69.9 (C-2), 34.2 (C-3), 30.1 (C-4), 130.3 (C-5), 108.7 (C-6), 155.3 (C-7), 102.8 (C-8), 113.1 (C-4a), 154.8 (C-8a) 36.9 (C-9), 130.6 (C-1′), 129.9 (C-2′,6′), 115.2 (C-3′,5′), 154.5 (C-4′).[22,23]
7-Hydroxy-3-(4-hydroxybenzyl)-8-methylchromane (8), light yellow amorphous crystals; EI-MS (70eV) m/z (rel. int): 270[M]+ (80), 256 (12), 149 (31), 133 (58), 107 (100), 73 (20); 1H-NMR (δ, CDCl3): 2.20 (1H, m, H-3), 4.14 (1H, dd, J = 10.50, 1.25 Hz, H-2), 3.88 (1H, dd, J = 10.50, 8.65 Hz, H-2), 2.41 (1H, dd, J = 15.90, 5.20 Hz, H-4), 2.70 (1H, dd, J = 15.90, 8.45 Hz, H-4), 2.35 (1H, dd, J = 14.70, 8.90 Hz, H-9), 2.63 (1H, dd, J = 14.70, 1.90 Hz, H-9), 2.05 (3H, s, 8-Me), 6.60 (1H, d, J = 8.30 Hz, H-5), 6.37 (1H, d, J = 8.30 Hz, H-6), 6.79 (2H, d, J = 8.25 Hz, H-3′, 5′), 7.10 (2H, d, J = 8.25 Hz, H-2′, 6′); 13C NMR (δ, CDCl3): 71.61 (C-2), 36.04 (C-3), 31.83 (C-4), 128.03 (C-5), 108.79 (C-6), 153.32 (C-7), 112.8 (C-8), 114.1 (C-4a), 154.82 (C-8a) 37.88 (C-9), 130.96 (C-1′), 129.88 (C-2′,6′), 116.35 (C-3′,5′), 155.56 (C-4′) 9.6 (8-Me).[12]
7-Hydroxy-3-(4-hydroxybenzyl)-8-methoxylchromane (9): yellow oil; EI-MS (70eV) (rel. int): 286[M]+ (85), 256 (15), 148 (18), 133 (45), 120 (61), 107 (100), 73 (32); 1H-NMR (δ, CDCl3): 2.17 (1H, m, H-3), 4.18 (1H, dd, J = 10.50, 1.25 Hz, H-2), 3.77 (1H, dd, J = 10.50, 8.65 Hz, H-2), 2.67 (1H, dd, J = 15.60, 5.1 Hz, H-4), 2.41 (1H, dd, J = 15.30, 8.90 Hz, H-4), 2.35 (1H, dd, J = 15.70, 8.90 Hz, H-9), 2.63 (1H, dd, J = 15.70, 2.0 Hz, H-9), 3.74 (3H, s, 8-OMe), 6.56 (1H, d, J = 8.30 Hz, H-5), 6.37 (1H, d, J = 8.30 Hz, H-6), 6.79 (2H, d, J = 8.25 Hz, H-3′, 5′), 7.06 (2H, d, J = 8.25 Hz, H-2′, 6′); 13C NMR (δ, CDCl3): 71.4 (C-2), 36.2 (C-3), 32.1 (C-4), 125.6 (C-5), 109.5 (C-6), 150.4 (C-7), 137.3 (C-8), 115.6 (C-4a), 149.8 (C-8a) 38.6 (C-9), 132.1 (C-1′), 130.9 (C-2′,6′), 117.2 (C-3′,5′), 157.5 (C-4′), 61.6 (8-OMe).[23]
7-Hydroxy-3-(4-hydroxybenzyl)-5-methoxychromane (10): Colorless needles; mp 127-129°C; EI-MS (70eV) m/z (rel. int): 286[M]+ (92), 161 (25), 148 (61), 133 (20), 123 (40), 107 (100), 73 (12); 1H-NMR (δ, CD3OD): 2.05 (1H, m, H-3), 3.98 (1H, dd, J = 8.10, 10.40 Hz; H-2), 3.60 (1H, dd, J = 1.6, 10.4 Hz; H-2), 3.67 (3H, s, 5-OMe), 2.57 (1H, dd, J = 5.0,14.4 Hz, H-4), 2.07 (1H, dd, J = 8.45,14.43 Hz, H-4), 6.00 (1H, d, J = 2.10 Hz, H-6), 5.85 (1H, d, J = 2.10 Hz, H-8), 2.35 (1H, dd, J = 15.70, 8.90 Hz, H-9), 2.63 (1H, dd, J = 15.70, 2.0 Hz, H-9), 6.96 (2H, d, J = 8.25 Hz, H-3′, 5′), 6.76 (2H, d, J = 8.25 Hz, H-2′, 6′); 13C NMR (δ, CD3OD): 70.20 (C-2), 34.72 (C-3), 25.55 (C-4), 158.63 (C-5), 92.81 (C-6), 157.24 (C-7), 95.95 (C-8), 37.82 (C-9), 102.65 (C-4a), 155.76 (C-8a), 134.14 (C-1′), 130.45 (C-2′,6′), 155.84 (C-4′), 115.64 (C-3′,5′), 65.2 (5-OMe).[24]
7-Hydroxy-3-(4-hydroxybenzyl)-chroman-4-one (11): white needles, mp 153-155°C; EI-MS (70eV) m/z (rel. int): 270 [M]+ (90), 253 (15), 133 (30), 107 (100), 73 (60). 1H-NMR (δ, CDCl3): 2.61 (1H, m, H-3), 4.15 (1H, dd, J = 7.5, 11.5 Hz, H-2), 3.97 (1H, dd, J = 7.7, 11.5 Hz, H-2), 6.52 (1H, dd, J = 8.7, 2.4 Hz, H-6), 7.80 (1H, d, J = 8.7 Hz, H-5), 6.38 (1H, d, J = 2.4 Hz, H-8), 3.17 (1H, dd, J = 13.5, 4.2 Hz, H-9), 2.65 (1H, dd, J = 13.5, 10.8 Hz, H-9), 6.78 (2H, d, J = 8.4 Hz, H-3′,5′), 7.09 (2H, d, J = 8.4 Hz, H-2′,6′). 13C NMR (δ, CDCl3): 69.5 (C-2), 47.6 (C-3), 193 (C-4), 110.4 (C-4a), 128.8 (C-5), 114.5 (C-6), 167.9 (C-7), 103.8 (C-8), 163.6 (C-8a), 32.8 (C-9), 130.2 (C-1′), 130.1 (C-2′,6′), 158.3 (C-4′), 114.1 (C-3′,5′).[12,22,25]
5,7-Dihydroxy-3-(4-hydroxy-benzyl)-chroman-4-one (12): white powder; EI-MS (70eV) m/z (rel. int): 286[M]+ (80), 253 (17), 133 (28), 107 (100), 73 (70); 1H-NMR (δ, CDCl3): 2.80 (1H, m, H-3), 4.07 (1H, dd, J = 4.2, 11.4 Hz, H-2), 4.15 (1H, dd, J = 11.4, 7.5 Hz, H-2), 6.20 (1H, d, J = 2.4 Hz, H-6), 5.88 (1H, d, J = 2.4 Hz, H-8), 3.15 (1H, dd, J = 17.5, 4.2 Hz, H-9), 2.60 (1H, dd, J = 17.5, 10.4 Hz, H-9), 6.78 (2H, d, J = 8.4 Hz, H-3′,5′), 7.25 (2H, d, J = 8.4 Hz, H-2′,6′); 13C NMR (δ, CDCl3): 68.86 (C-2), 45.62 (C-3), 197.65 (C-4), 163.34 (C-5), 96.17 (C-6), 166.96 (C-7), 95.21 (C-8), 30.88 (C-9), 100.95(C-4a), 162.68 (C-8a), 129.18 (C-1′), 130.10 (C-2′,6′), 155.81 (C-4′), 115.66 (C-3′,5′).[25]
5,8-dihydroxy-7-methoxy-3-(4-hydroxy-benzyl)-chroman-4-one (13): white powder, mp 172-174°C; EI-MS (70eV) m/z (rel. int): 316[M]+ (45), 253 (7), 133 (8), 107 (100), 73 (30); 1H-NMR (δ, CDCl3): 3.02 (1H, m, H-3), 4.12 (1H, dd, J = 4.2, 11.4 Hz, H-2), 4.20 (1H, dd, J = 11.4, 7.5 Hz, H-2), 6.19 (1H, s, H-6), 3.82 (3H, s, 7-OMe), 3.0 (1H, dd, J = 15.5, 4.2 Hz, H-9), 2.65 (1H, dd, J = 15.5, 10.4 Hz, H-9), 6.72 (2H, d, J = 8.4; Hz, H-3′,5′), 7.05 (2H, d, J = 8.4 Hz, H-2′,6′). 13C NMR (δ, CDCl3): 68.81 (C-2), 45.94 (C-3), 196.44 (C-4), 154.32(C-5), 92.50 (C-6), 152.22(C-7), 130.15 (C-8), 31.08 (C-9), 102.01 (C-4a), 157.12 (C-8a), 127.98 (C-1′), 129.80 (C-2′,6′), 155.77 (C-4′), 115.16 (C-3′,5′), 56.02 (7-OMe).[26]
5,7-dihydroxy-6-methoxy-3-(4-hydroxy-benzyl)-chroman-4-one (14): yellow needles, mp 198-200°C; EI-MS (70eV) m/z (rel. int): 316[M]+ (100), 301 (35), 283 (15), 265 (10), 210 (92), 107 (80); 1H-NMR (δ, (CD3) 2CO): 2.94 (1H, m, H-3), 4.16 (1H, dd, J = 8.1, 11.6 Hz, H-2), 4.30 (1H, dd, J = 11.6, 4.5 Hz, H-2), 5.97 (1H, s, H-8), 3.15 (1H, dd, J = 13.6, 10 Hz, H-9), 2.65 (1H, dd, J = 13.6, 4.5 Hz, H-9), 3.77 (3H, s, 6-OMe), 7.15 (2H, d, J = 8.4 Hz, H-2′,6′), 6.84 (2H, d, J = 8.4 Hz, H-3′,5′); 13C NMR (δ, CD3OD): 70.42 (C-2), 47.62 (C-3), 199.91 (C-4), 156.77 (C-5), 129.63 (C-6), 159.34 (C-7), 95.13 (C-8), 102.94 (C-4a), 160.05 (C-8a), 32.66 (C-9), 130.62 (C-1′), 131.04 (C-2′,6′), 157.25 (C-4′), 116.17 (C-3′,5′), 60.85 (6-OMe).[26,27]
5,7-Dihydroxy-6-methyl-3-(4-hydroxy-benzyl)-chromane-4-one (15): white needles, mp 167-169°C; EIMS (70eV) m/z (rel. int): 300[M]+ (100), 193 (50), 179 (30), 166 (25), 166 (61), 107 (80); 1H-NMR (δ, CD3OD): 2.76 (1H, m, H-3), 4.07 (1H, dd, J = 11.5, 4.0, H-2), 4.22 (1H, dd, J = 11.5, 7.0, H-2), 5.95 (1H, s, H-8), 2.65 (1H, dd, J = 10.4, 13.6 Hz, H-9), 3,09 (1H, dd, J = 4.0, 13.6 Hz, H-9), 1.95 (3H, s, 6-Me), 6.74 (2H, d, J = 8.4 Hz, H-5′,3′), 7.05 (2H, d, J = 8.4 Hz, H-2′,6′). 13C NMR (δ, CD3OD): 70.02 (C-2), 48.55 (C-3), 199.85 (C-4), 102.88 (C-4a), 162.98 (C-5), 104.78 (C-6), 165.81 (C-7), 95.1 (C-8), 160.71 (C-8a), 33.47 (C-9), 130.47 (C-1′), 131.72 (C-2′,6′), 157.27 (C-4′), 116.46 (C-3′,5′), 7.32 (6-Me).[12,28]
5,7-dihydroxy-6,8-dimethyl-3-(4-hydroxy-benzyl)- chromane-4-one (16): colorless needles mp 102-104°C; EI-MS (70eV) m/z (rel. int): 314[M]+ (100), 208 (72), 179 (30), 152 (25), 133 (11), 107 (80); 1H-NMR (δ, CD3OD): 2.74 (1H, m, H-3), 4.07 (1H, dd, J = 11.5, 4.1, H-2), 4.22 (1H, dd, J = 11.5, 7.0, H-2), 2.63 (1H, dd, J = 10.4, 13.6 Hz, H-9), 3,07 (1H, dd, J = 4.2, 13.6 Hz, H-9), 1.97 (3H, s, 6-Me), 1.98 (3H, s, 8-Me), 6.74 (2H, d, J = 8.4 Hz, H-5′,3′), 7.05 (2H, d, J = 8.4 Hz, H-2′,6′). 13C NMR (δ, CD3OD): 70.02 (C-2), 48.15 (C-3), 199.85 (C-4), 102.58 (C-4a), 160.98 (C-5), 104.78 (C-6), 164.81 (C-7), 103.81 (C-8), 160.71 (C-8a), 33.47 (C-9), 130.47 (C-1′), 131.72 (C-2′,6′), 157.27 (C-4′), 116.46 (C-3′,5′), 7.92 (6-Me), 7.50 (8-Me).[28,29]
DISCUSSION
The resin resulted inactive against free living (planktonic) staphylococcal strains S. aureus ATCC 25923 and S. epidermidis RP62A at the maximum tested concentration of 1000 μg/mL.
Biofilm formation was estimated for the two strains used in this study by staining adherent cells on polystirene surfaces with safranin and reading the optical densities. We confirmed that the tested reference strains formed dense biofilms[30] and we evaluated the ability to prevent biofim formation of D. draco resin by using the sub-MIC concentrations of 400, 200 and 100 μg/mL. The resin resulted ineffective in inhibiting the biofilm formation of S. epidermidis RP62A when evaluated with safranin staining method, but showed interesting inhibition percentages respectively of 74, 55.9 and 31.8 against S. aureus ATCC 25923 at above mentioned concentrations. The effectiveness of resin in preventing biofilm formation of S. aureus ATCC 25923 biofilm was also evaluated in terms of log reductions based on viable plate count. The number of CFU/ mL for detached bacterial cells grown on plastic surface was assessed after scraping and sonicating and is reported in Figure 2. At 400 μg/mL, the resin had a very strong activity against S. aureus ATCC 25923 (4.0-log reduction) and a good activity (3.0-log reduction) was observed at 200 μg/mL. A weaker inhibition of biofilm formation was determined at 100 μg/mL (1.5-log reduction). From the experimental data obtained with both methods, we can observe that D. draco resin showed interesting anti-adhesion property against tested S. aureus strain, such property could be useful to prevent biofilm formation on natural surfaces like the host's tissues or on the surfaces of medical devices.
Figure 2.
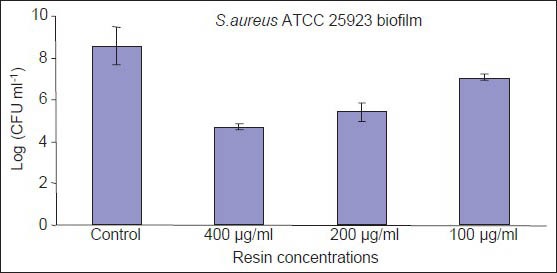
Numbers (CFU/ml) of S. aureus ATCC 25923 in untreated biofilm and in the presence of Dracaena draco resin. Data reported as means ± SD
Finally, the anti-biofilm activity of resin against a pre-formed biofilm of S. aureus ATCC25923 was evaluated by using MTT method. The MTT used to stain live and adherent bacteria is a respiratory indicator and reveals bactericidal activity by antimicrobials.[31] The resin resulted active with inhibition percentages respectively of 98 and 96.5% at the concentrations of 400 and 200 μg/mL, but showed a weak activity (less than 20%) at 100 μg/mL. It can be speculated that D. draco resin at the highest concentrations efficiently penetrates in the staphylococcal biofilm killing micro-organisms.
Of course, to better understand the utility of D. draco's resin as unusual source of antibiofilm agents, new experimental work is needed and we are planning to evaluate and compare the antibiofilm properties of isolated novel molecules against a wide range of biofilm forming micro-organisms of clinical and veterinary interest.
CONCLUSIONS
The phytochemical investigation of the resin of Dracaena draco L. spp. draco has led to the isolation and the structural characterization of 16 known compounds.
D. draco resin significantly affects S. aureus ATCC 25923 biofilm formation at sub-MIC concentrations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pearson JJ, Prendergast HD. Daemonorops Dracaena and Other Dragon's Blood. Econ Bot. 2001;55:474–7. [Google Scholar]
- 2.Gupta D, Bleakley B, Gupta RK. Dragon's blood: Botany, chemistry and therapeutic uses. J Ethnopharmacol. 2008;115:361–80. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Milburn M. Dragon's Blood in East and West Africa, Arabia and the Canary Islands. Africa. 1984;39:486–93. [Google Scholar]
- 4.Ballabio R. Plantes médicinales endémiques de l’île de Madère. Phytothérapie. 2004;2:41–6. [Google Scholar]
- 5.Silva BM, Santos RP, Mendes LS, Guedes de Pinho P, Valentão P, Andrade PB, et al. Dracaena draco L. fruit: Phytochemical and antioxidant activity assessment. Food Res Int. 2011;44:2182–9. [Google Scholar]
- 6.Camarda L, Di Stefano V, Pitonzo R. Ch. 11. New York, NY, USA: Nova Science Publishers Inc; 2011. In Resin Composites: Properties, Production and Applications; pp. 353–74. [Google Scholar]
- 7.Darias V, Bravo L, Rabanal R, Sanchez Mateo C, Gonzalez Luis RM, Hernandez Perez AM. New contribution to the ethnopharmacological study of the Canary Islands. J Ethnopharmacol. 1989;25:77–92. doi: 10.1016/0378-8741(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 8.Mimaki Y, Kuroda M, Ide A, Kameyama A, Yokosuka A, Sashida Y. Steroidal saponins from the aerial parts of Dracaena draco and their cytostatic activity on HL-60 cells. Phytochemistry. 1999;50:805–13. doi: 10.1016/s0031-9422(98)00614-1. [DOI] [PubMed] [Google Scholar]
- 9.González AG, Hernandez JC, Leon F, Padrón JI, Estévez F, Quintana J, et al. Steroidal saponins from the bark of Dracaena draco and their cytotoxic activities. J Nat Prod. 2003;66:793–8. doi: 10.1021/np020517j. [DOI] [PubMed] [Google Scholar]
- 10.Hernández JC, Léon F, Quintana J, Estévez F, Bermejo J. Icogenin, a new cytotoxic steroidal saponin isolated from Dracaena draco. Bioorg Med Chem. 2004;12:4423–9. doi: 10.1016/j.bmc.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Hernández JC, Léon F, Estévez F, Quintana J, Bermejo J. A homo-isoflavonoid and a cytotoxic saponin from Dracaena draco. Chem Biodivers. 2006;36:2–67. doi: 10.1002/cbdv.200690008. [DOI] [PubMed] [Google Scholar]
- 12.Camarda L, Merlini L, Nasini G. Dragon's blood from Dracaena draco structure of novel homoisoflavonoid. Heterocycles. 1983;20:39–43. [Google Scholar]
- 13.Gonzáles AG, León F, Sanchez-Pinto L, Padrón JI, Bermejo J. Phenolic Compounds of Dragon's blood from Dracaena draco. J Nat Prod. 2000;63:1297–9. doi: 10.1021/np000085h. [DOI] [PubMed] [Google Scholar]
- 14.González AG, León F, Hernández JC, Padrón JI, Sanchez-Pinto L, Bermejo Barrera J. Flavans of Dragon×s blood from Dracaena draco a nd Dracaena tamaranae. Biochem Syst Ecol. 2004;32:179–84. [Google Scholar]
- 15.Melo MJ, Sousa M, Parola AJ, Melo JS, Catarino F, Marcalo J, et al. Identification of 7,4-Dihydroxy-5-methoxyflavylium in “Dragon's Blood” :To be or not to be an anthocyanin. Chemistry. 2007;13:1417–22. doi: 10.1002/chem.200600837. [DOI] [PubMed] [Google Scholar]
- 16.Schillaci D. Staphylococcal Biofilms: Challenges in the discovery of novel anti-infective agents. J Microbial Biochem Technol. 2011;3:4–6. [Google Scholar]
- 17.Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 18.Donelli G, De Paoli P, Fadda G, Marone P, Nicoletti G, Varaldo PE. A multicenter study on central venous catheter-associated infections in Italy. J Chemother. 2001;13:251–62. doi: 10.1179/joc.2001.13.Supplement-2.251. [DOI] [PubMed] [Google Scholar]
- 19.Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter-related bloodstream infection in intensive care units of four European countries: Literature- and registry-basedestimates. J Hosp Infect. 2009;72:97–103. doi: 10.1016/j.jhin.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Høib N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–32. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Schillaci D, Petruso S, Sciortino V. 3,4,5,3′,5′-Pentabromo-2 (2′-hydroxybenzoyl) pyrrole: A potential lead compound as anti-Gram-positive and anti-biofilm agent. Int J Antimicrob Agents. 2005;25:338–40. doi: 10.1016/j.ijantimicag.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Zheng QA, Zhang YJ, Yang CR. A new meta-homoisoflavane from the fresh stems of Dracaena cochinchinensis. J Asian Nat Prod Res. 2006;8:571–7. doi: 10.1080/1028602042000204126. [DOI] [PubMed] [Google Scholar]
- 23.Masaoud M, Ripperger H, Porzel A, Adam G. Flavonoids of dragon's blood from Dracaena cinnabari. Phytochemistry. 1995;38:745–9. [Google Scholar]
- 24.Zheng QA, Li HZ, Zhang YJ, Yang CR. Flavonoids from the Resin of Dracaena cochinchinensis. Helv Chim Acta. 2004;87:1167–71. [Google Scholar]
- 25.Morales-Serna JA, Estrada-Reyes R, Marquez C, Cárdenas J, Salmón M. Homoisoflavanones from Agave tequilana Weber. Molecules. 2010;15:3295–301. doi: 10.3390/molecules15053295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adinolfi M, Barone G, Belardini M, Lanzetta R, Laonigro G, Parrilli M. 3-Benzyl-4-chromanones from Muscari comosum. Phytochemistry. 1984;21:2091–3. [Google Scholar]
- 27.Silayo A, Ngadjui BT, Abegaz BM. Homoisoflavonoids and stilbenes from the bulbs of Scilla nervosa subsp. rigidifolia. Phytochemistry. 1999;52:947–55. [Google Scholar]
- 28.Nguyen AT, Fontaine J, Malonne H, Duez P. Homoisoflavanones from Disporopsis aspera. Phytochemistry. 2006;67:2159–63. doi: 10.1016/j.phytochem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Huang PL, Gan KH, Wu RR, Lin CN. Benzoquinones, a homoisoflavanone and other constitutents from Polygonatum alte-lobatum. Phytochemistry. 1997;44:1369–73. doi: 10.1016/s0031-9422(96)00652-8. [DOI] [PubMed] [Google Scholar]
- 30.Schillaci D, Petruso S, Raimondi MV, Cusimano MG, Cascioferro S, Scalisi M, et al. Pyrrolomycins as potential anti-staphylococcal biofilms agents. Biofouling. 2010;26:433–8. doi: 10.1080/08927011003718673. [DOI] [PubMed] [Google Scholar]
- 31.Kuzma L, Rozalski M, Walencka E, Rozalska B, Wysokinska H. Antimicrobial activity of diterpenoid from hairy roots of Salvia sclarea L. Salvipisone as apotential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine. 2007;14:31–5. doi: 10.1016/j.phymed.2005.10.008. [DOI] [PubMed] [Google Scholar]


