Abstract
Background:
Clove (Syzygium aromaticum) is a well-known culinary spice with strong aroma; contains a high amount of oil known as clove oil. The major phyto-constituent of the clove oil is eugenol. Clove and its oil possess various medicinal uses in indigenous medicine as an antiseptic, anti-oxidant, analgesic and neuroprotective properties. Thus, it draws much attention among researchers from pharmaceutical, food and cosmetic industries.
Objective:
The aim of the present study was to determine the anti-cholinesterase activity of the methanol extract of clove, its oil and eugenol.
Materials and Methods:
In vitro anti-cholinesterase activity of S. aromaticum was performed by a thin layer chromatography bio autography, 96 well micro titer plate and kinetic methods. Reverse phase high performance liquid chromatography (RP-HPLC) analysis was carried out to identify the biomarker compound eugenol in clove oil.
Results:
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition study revealed that eugenol possess better inhibition of the enzymes than extract and oil. Clove extract, its oil and eugenol showed better inhibition of AChE than BChE. Polyphenolic compound eugenol was detected through RP-HPLC analysis. The content of eugenol in essential oil was found to be 0.5 μg/ml. Kinetic analysis of the cholinesterase inhibition study of the extract; clove oil and eugenol have shown that they possess mixed type of inhibition for AChE and non-competitive type of inhibition for BChE.
Conclusion:
These results might be useful in explaining the effect of clove as anti-cholinesterase agent for the management of cognitive ailments like Alzheimer's disease.
Keywords: Acetylcholinesterase, Alzheimer's disease, butyrylcholinesterase, eugenol, Syzygium aromaticum
INTRODUCTION
The decrease in the brain acetylcholine (ACh) amount is the hallmark of Alzheimer's disease (AD), which is largely manifested by impairment of cognitive function leading to changes in the behavioral pattern in an older individual.[1] The mechanistic approach related to AD revealed that the minimization of acetylcholinesterase (AChE) as well as butyrylcholinesterase (BChE) activity can compensate decrease label of ACh.[2] The present cholinesterase inhibitors (ChIs) synthetic origins have been used primarily for treatment of such diseases condition. These agents are often associated with common side-effects in AD patients.[3] A safer approach to prevent the progression of such diseased condition would be use of natural anti-cholinesterases. Several reports have been published on effectiveness and some of are approved for treating cognitive disorders of AD type.[4]
Syzygium aromaticum (Myrtaceae), a tropical evergreen plant, widely used spice since time immemorial as human nutrition and flavoring agent.[5] Clove has a strong bonding with various ethnic cultures of Indian subcontinent and it has been used in rituals to medicinal practices. In Ayurveda as well as Iranian traditional medicinal formulations; clove oil is used as nervous stimulant and cognitive enhancer.[6] Phenolic compound, eugenol is reported to be the major chemical constituent present in the clove oil, which is also responsible for its biological activities. The oil also contains eugenol acetate, β-caryophyllene, chavicol, humulenes in lesser amounts.[7]
Eugenol is reported for its wide range of pharmacological properties including analgesic, anti-oxidant, anti-inflammatory, anti-allergic, anti-carcinogenic and anti-mutagenic activities.[5,8] Previous studies on eugenol with an animal model showed significance beneficial effect on nerve function. It was also reported that eugenol content of clove oil is capable of improving learning and memory process of a mice model under elevated oxidative stress condition.[6] Other experimental study shows that treatment of diabetic mice with eugenol can alter vascular and neuronal complication in brain.[9] The above mentioned publications of S. aromaticum, its oil and eugenol provided the basis of the present study to examine their anti-cholinesterase activity. Further, kinetic analysis of the anti-cholinesterase data also revealed the types of inhibition potential of the studied plant materials.
MATERIALS AND METHODS
Dried flower buds of S. aromaticum were collected from local market of Kolkata, West Bengal, India and authenticated as well. A voucher specimen (SNPS-1479) of the same has been preserved in the herbarium of the School of Natural Product Studies, Jadavpur University, Kolkata, India for further use.
Chemicals
AChE from bovine erythrocytes, BChE from equine serum, galantamine, eugenol acetylthiocholine iodide (ATCI), butyrylthiocholine iodide (BTCI), Ellman's reagent (5,5’-dithiobis-(2-nitrobenzoic acid) or DTNB were procured from Sigma (Poole, UK). Methanol and all other organic solvents (high performance liquid chromatography [HPLC] reagent grade) were purchased from Merck, India.
Preparation of plant extract
Fresh flower buds of S. aromaticum were shade dried and pulverized to coarse power. The plant extract was prepared by cold maceration. A hydroalcholic mixture was added with 25 g powder with occasional shaking for 72 h to obtain effective extraction.[10] The extract was collected after filtration in a beaker. The above procedure was repeated thrice for exhaustive extraction. All the extracts were pooled together and distilled under reduced pressure using rotary vacuum evaporator to collect the final semisolid extract which dried in a lyophilizer to remove residual moisture, yield was found to be 8% w/w.
Isolation of clove oil
Clove oil was isolated by Clevenger apparatus adapting the reported method.[11] 10 g of powdered clove was soaked in water and boiled at 100°C for 5 h to collect the buff colored oil on water. The collected oil was dried with anhydrous sodium sulfate to remove moisture and reserved in a refrigerator at 4°C until further study, yield of the oil was found to be 6% v/w.
Standardization of the extract through HPLC
Reverse-phase HPLC (RP-HPLC) analysis of clove oil and standard eugenol were performed on HPLC Shimadzu Prominence, Kyoto, Japan. Clove oil (10 mg/ml) and standard eugenol [Figure 1] (1 mg/ml) were dissolved in methanol for the chromatography analysis. Isocratic mobile phase (methanol:water: 75:25) condition was maintained throughout the process. The samples were eluted from the column at room temperature with flow rate of 1 ml/min which was detected at 254 nm. LC solution software was used for the study of chromatogram. Identification and quantification of eugenol was verified by peak areas obtained in HPLC analysis.
Figure 1.
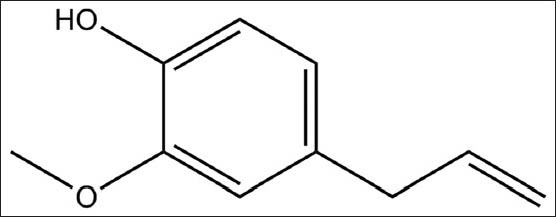
Chemical structure of eugenol
ChIs activity
Bio-autography method
Thin layer chromatography (TLC) analysis of AChE and BChE inhibition was carried out according to the method.[12] 2.5 mm silica gel TLC plate, F254 (Merck, Darmstadt, Germany) was spotted with methanol extract (10 mg/ml) and it was developed with an optimized mobile phase methanol:water (75:25). The plate was dried at room temperature. Freshly prepared 1 mM DTNB or Ellman's reagent[13] and 1 mM ATCI solution in phosphate buffer was sprayed over the plate for AChE inhibiton and 1 mM BTCI solution instead of ATCI was used for studying BChE inhibition. The plates were dried for 25 s and then 3 U/ml of AChE/BChE enzyme solutions were sprayed over the respective plates. The plates were kept aside for 5 min to develop colorless spots over a yellow background as AChE/BChE inhibition zones. The plates were immediately photographed, as the white spots gets disappear fast. To confirm the true inhibition, another TLC plate was developed in a similar manner without spotting the extract, called as false positive. Appearance of white spots at the similar place on the TLC plate with extract considered as false inhibition.
96 well micro titer plate assay
The inhibition activity of AChE/BChE was determined by modified method[13] by using Bio Rad 96-well microplate reader (680 XR, USA).[14] Extract, oil, eugenol and galantamine as a positive control were dissolved in methanol for the study. In each well of separate 96-well micro titer plate, 125 μL of 3 mM, DTNB (Ellman's reagent), 25 μL of 15 mM ATCI/BTCI in phosphate buffer, 50 μL of phosphate buffer (pH: 7.2) and extract/oil/eugenol at various constrations (5-100 μg/ml) and galantamine (1-20 μg/ml) were added. The plates was measured at 405 nm at every 13 s for 65 s. AChE/BChE (25 μL of 0.22 U/ml) in phosphate buffer was added to all the wells and reading measured at 405 nm at every 13 s for 104 s.[14] This procedure was repeated for 6 times. IC50 value was calculated by plotting the inhibition percentages against the logarithm of the concentration.
Kinetic study
Method applied for the kinetic study of enzyme inhibition was similar to the 96-well microplate assay with the main difference being the fixed inhibitor concentration and varied substrate (ATCI/BTCI) concentration (0.75-14 mM). The kinetic parameter Km (Michaelis-Menten constant), Vmax (maximal velocity) and dissociation constant/inhibition constant (Ki) was calculated over through the Lineweaver-Burk and their secondary replots. The experiment was performed in triplicate.[13]
Statistical analysis
All the results of the data were expressed as mean ± standard error of the mean. The linear regression parameters were used to determine the IC50 value of enzyme activity. Kinetic parameters (Km, Vmax and Ki) were obtained by using non-linear regression analysis. Comparison of enzyme kinetic data was performed by using one-way analysis of variance followed by Newman-keuls comparison test. P < 0.05 was considered to be significant. Graphs were plotted using Graph Pad Prism 5.01.
RESULTS
Standardization of the extract through HPLC
In the present study, eugenol content in S. aromaticum oil was accessed through RP-HPLC analysis. The retention time of eugenol in the extract [Figure 2a] was found at 13.633 min which was similar to the retention time of standard eugenol [Figure 2b]. The calibration curve of eugenol has shown linear relationship between peak area and standard eugenol concentration with a correlation coefficient, r2 = 0.9958. The amount of eugenol in oil was found to be 0.5 μg/ml.
Figure 2.
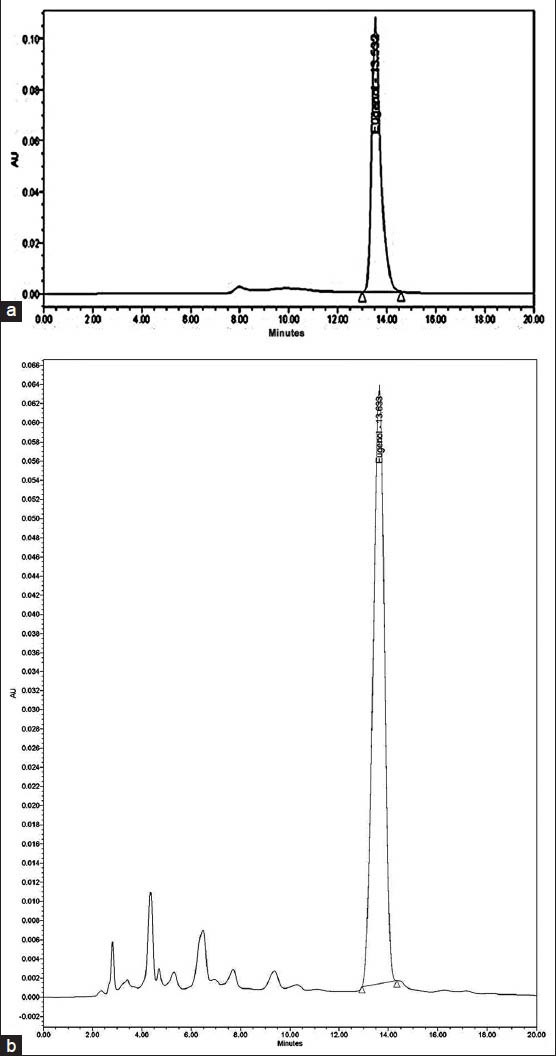
High performance liquid chromatography Chromatogram of (a) clove oil and (b) standard eugenol
ChIs activity
Bio-autography method
AChE/BChE inhibitory activity of the standardized methanol extracts (10 mg/ml) of S. aromaticum was evaluated by using TLC bioautographic assay. Biochemical detection of plant extract was screened through an optimized mobile phase. As a result, variable white spot with yellow staining was observed in a TLC plate [Figure 3a–c]. The white area of TLC plate was corresponds to the enzyme inhibition of both AChE and BChE. It was confirmed that the plant extract has potential ChIs activity.
Figure 3.

Thin layer chromatography bio-autography plate showing (a) false inhibition activity (b) acetylcholinesterase inhibition activity (c) butyrylcholinesterase inhibition activity; S = Standard galantamine solution, T = Test solution of methanol extract, (→) indicates white spot on TLC plate which corresponds the positive (plate b and c) or negative (plate a) result of the chemical reaction
96 well micro titer plate assay
AChE and BChE inhibitory activities of the test and standard compounds have been shown in Table 1. The result shown that the test sample along with standard compound have significant both AChE and BChE inhibitory activity. Essential oil has showed high AChE inhibition (IC50: 49.73 ± 1.33), compared to low AChE inhibition label of methanol extract (IC50: 61.5 ± 1.88) and standard compound eugenol (IC50: 49.73 ± 1.33) and reference compound galanthamine have possessed the highest AChE inhibition activity with an IC50 value of 10.14 ± 0.71. With respect to BChE inhibition, bio marker compound eugenol has showed potent inhibition activity with IC50 value of 63.51 ± 1.88. In contrast, methanol extract (IC50 : 103.53 ± 1.47) and essential oil (IC50 : 88.14 ± 1.74) has exhibited weak inhibition activity than reference compound galantamine (IC50 : 21.15 ± 0.18). Dose response curve of the extract, oil, eugenol and galantamine has been shown in Figures 4 and 5.
Table 1.
Data of IC50 values
Figure 4.
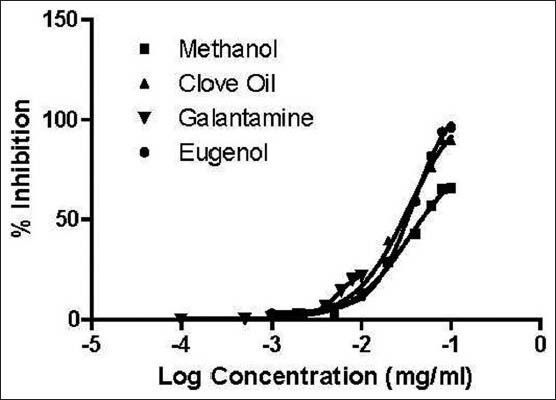
Dose response curve of methanol extract, clove oil, galantamine and eugenol against acetylcholinesterase
Figure 5.
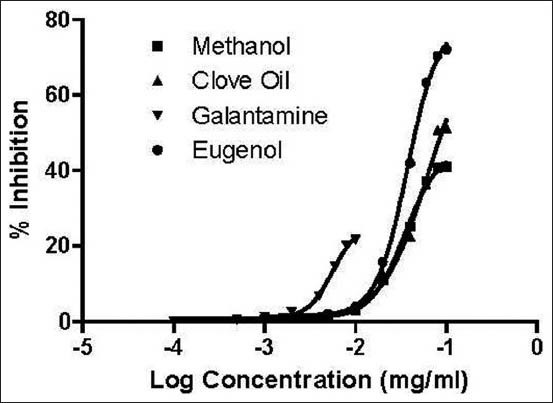
Dose response curve of methanol extract, clove oil, galantamine and eugenol against butyrylcholinesterase
Kinetic study
Enzyme kinetics of AChE inhibitory activities of methanol extract of S. aromaticum extract, essential oil, eugenol and galantamine is shown in Table 2 and Figure 6a–c. Vmax, Km and Ki values were determined through Lineweaver-Burk plot. Vmax value (54.09 ± 1.38, 32.72 ± 1.26, 64.06 ± 3.0) of S. aromaticum methanol extract, essential oil and eugenol were decrease with the control group (92.76 ± 3.44), whereas the Km value of above constituents were an increase with comparison of the same control group (40.99 ± 2.98). However, Vmax value of reference compound galantamine (92.76 ± 3.44) was showing any significant change in respect of the control group. Moreover, Eugenol and galatamine was shown significant (P < 0.05) kinetic value, when it compared with the control group. In the case of BChE, the results of all test compounds were shown in Table 2 and Figure 7a–c. Vmax value of S. aromaticum methanol extract, essential oil and eugenol (61.54 ± 2.83, 68.44 ± 1.29, 58.62 ± 2.83) were significantly increase by comparing the control group (68.18 ± 3.8), while Km value of these test compounds were remain unchanged. Further, the reference compound galantamiine was shown an increase in Vmax value (48.59 ± 0.9) with a decrease in Km value (25.57 ± 2.1) by comparing Vmax value (68.18 ± 3.8) and Km value (24.11 ± 2.7) of the control group. Data result also indicated that galantamine have significant (P < 0.05) value than the control group. In case of AChE activity, Ki value of galantamine exhibit 1.761 ± 0.2, which was less in value of remaining test chemical compounds. Further, Ki value (2.545 ± 0.49) of galantamine for BChE inhibition was also decreases with other test compounds.
Table 2.
Summary of kinetic data
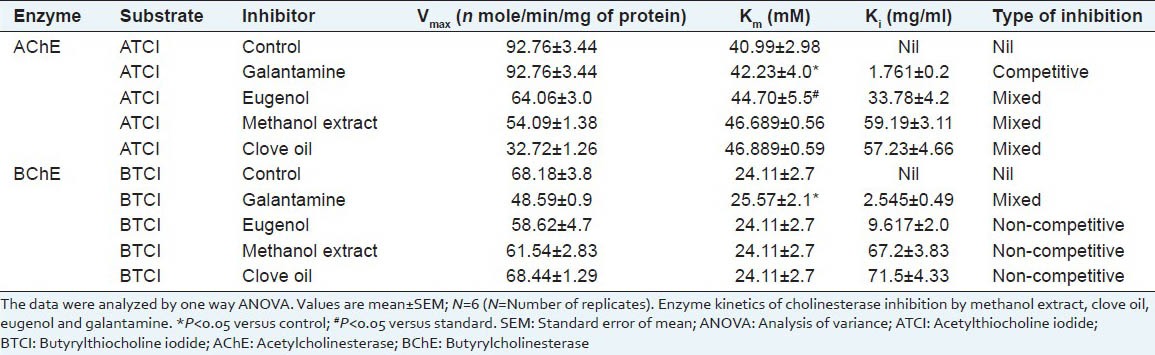
Figure 6.
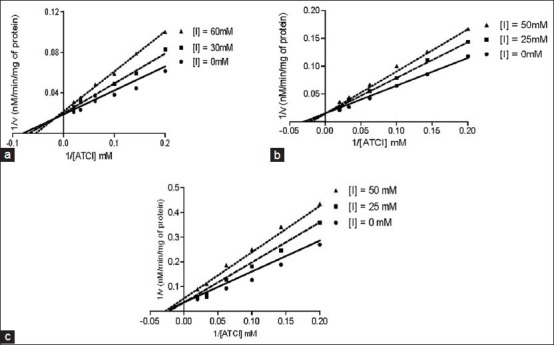
Lineweaver-Burk plot of acetylcholinesterase inhibitory activity (a) Methanol extract (b) Clove oil and (c) Eugenol
Figure 7.
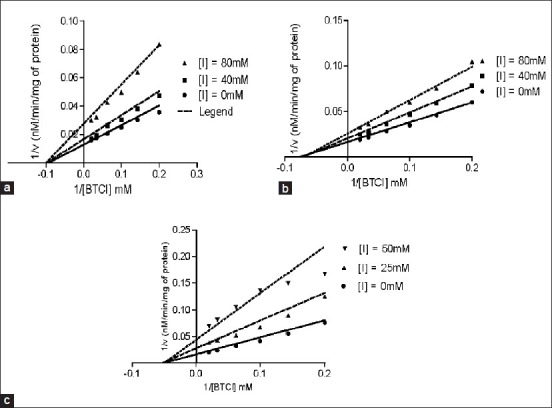
Lineweaver-Burk plot of butyrylcholinesterase inhibitor activity (a) Methanol extract (b) Clove oil and (c) Eugenol
DISCUSSION
It has been hypothesized that cognition and behavior of AD patients closely associated with a decrease in concentration of brain ACh level. The hypothesis was supported by the reports suggested that inhibition of AChE along with BChE remains important target for the treatment of neurodegenerative disorders. Therefore, substrate specific AChE and BChE inhibitors are important options for treatment of AD.[15,16] Until recently, large numbers of naturally-occurring compounds have been found to inhibit AChE/BChE.[17,18] Most of the prescribed ChIs such as huperzine, rivastigmine and galantamine are obtained from plant sources. This provides an opportunity to explore new ChIs from plant sources.[19]
ChE inhibition potential of S. aromaticum was assessed by TLC bioautography method and found to be positive. For further confirmation of the inhibitory potential of clove extract, oil, eugenol and galantamine 96-well microplate method was applied. Enzyme inhibition efficiency of clove oil was better than the extract. Eugenol demonstrated highest AChE inhibitory among the extract and oil, but less active than the reference compound galantamine. All the tested compounds exhibited weak affinity toward BChE.
Enzyme kinetics of AChE inhibitory activities of methanol extract of S. aromaticum extract, clove oil, eugenol and galantamine was screened. The Vmax value of clove extract, oil and eugenol were decreased by comparing with the control group, while the Km values of above constituents were increased, which indicated a reversible and mixed type of inhibition.[20] However, Vmax value of reference compound galantamine did not showed any significant change with respect to control group, but Km values decreased, which suggested the type of inhibition would be reversible and competitive type.[21] In the case of BChE, extract, oil and eugenol were showed reversible and non-competitive type of inhibition,[22] while galantaine possessed mixed type of inhibition. Results also indicated that galantamine have a significant (P < 0.05) value than the control group. The phytoconstituents of the S. aromaticum extract and its oil may be explored as potential lead for the treatment of AD.
CONCLUSION
The present study suggested that S. aromaticum extract and its oil, along with eugenol has potential anti-cholinesterase property. Therefore, this culinary spice may be explored further for its use in the management of AD and related cognitive disorders.
ACKNOWLEDGMENTS
Thanks are also due to the AICTE for providing QIP research scholarship to the first author. The authors also wish to express their gratitude to the Department of Science and Technology, Government of India for financial support.
Footnotes
Source of Support: The authors also wish to express their gratitude to the Department of Science and Technology, Government of India for financial support
Conflict of Interest: None declared.
REFERENCES
- 1.Mukherjee PK, Ahamed KF, Kumar V, Mukherjee K, Houghton PJ. Protective effect of biflavones from Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion induced oxidative stress. Behav Brain Res. 2007;178:221–8. doi: 10.1016/j.bbr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Giacobini E. Drugs that target cholinesterase. In: Buccafusco JJ, editor. Cognitive Enhancing Drugs. Basel: Springer; 2004. pp. 11–36. [Google Scholar]
- 3.Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2:32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee PK, Houghton PJ. The worldwide phenomenon of increased use of herbal products: Opportunities and threats. In: Mukherjee PK, Houghton PJ, editors. Evaluation of Herbal Medicinal Products-Perspectives of Quality, Safety and Efficacy. London: Pharmaceutical Press; 2009. pp. 3–12. [Google Scholar]
- 5.Banerjee S, Panda CK, Das S. Clove (Syzygium aromaticum L.). a potential chemopreventive agent for lung cancer. Carcinogenesis. 2006;27:1645–54. doi: 10.1093/carcin/bgi372. [DOI] [PubMed] [Google Scholar]
- 6.Halder S, Mehta AK, Kar R, Mustafa M, Mediratta PK, Sharma KK. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011;77:830–4. doi: 10.1055/s-0030-1250605. [DOI] [PubMed] [Google Scholar]
- 7.Viuda-Martos M, Ruíz-Navajas Y, Fernández-López J, Perez-Alvarez J. Chemical composition of the clove oils obtained from some spices widely used in Mediterranean region. Acta Chim Slov. 2007;54:921–6. [Google Scholar]
- 8.Yoshimura M, Amakura Y, Yoshida T. Polyphenolic compounds in clove and pimento and their antioxidative activities. Biosci Biotechnol Biochem. 2011;75:2207–12. doi: 10.1271/bbb.110491. [DOI] [PubMed] [Google Scholar]
- 9.Nangle MR, Gibson TM, Cotter MA, Cameron NE. Effects of eugenol on nerve and vascular dysfunction in streptozotocin-diabetic rats. Planta Med. 2006;72:494–500. doi: 10.1055/s-2005-916262. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee PK. 1st ed. New Delhi: Business Horizons; 2002. Quality Control on Herbal Drugs. [Google Scholar]
- 11.Bhadra S, Mukherjee PK, Kumar NS, Bandyopadhyay A. Anticholinesterase activity of standardized extract of Illicium verum Hook. f. fruits. Fitoterapia. 2011;82:342–6. doi: 10.1016/j.fitote.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine. 2010;17:292–5. doi: 10.1016/j.phymed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 14.Bhadra S, Mukherjee PK, Bandyopadhyay A. Cholinesterase inhibition activity of Marsilea quadrifolia Linn. an edible leafy vegetable from West Bengal, India. Nat Prod Res. 2012;26:1519–22. doi: 10.1080/14786419.2011.565006. [DOI] [PubMed] [Google Scholar]
- 15.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–7. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 16.Greig NH, Utsuki T, Yu Q, Zhu X, Holloway HW, Perry T, et al. A new therapeutic target in Alzheimer's disease treatment: Attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17:159–65. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- 17.Perry N, Court G, Bidet N, Court J, Perry E. European herbs with cholinergic activities: potential in dementia therapy. Int J Geriatr Psychiatry. 1996;11:1063–9. [Google Scholar]
- 18.Mukherje PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee PK, Kumar V, Mal M, Houghton PJ. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007;73:283–5. doi: 10.1055/s-2007-967114. [DOI] [PubMed] [Google Scholar]
- 20.Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, et al. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60:6131–8. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- 21.Benie T, Thieulant ML. Interaction of some traditional plant extracts with uterine oestrogen or progestin receptors. Phytother Res. 2003;17:756–60. doi: 10.1002/ptr.1208. [DOI] [PubMed] [Google Scholar]
- 22.Nawaz SA, Ayaz M, Brandt W, Wessjohann LA, Westermann B. Cation-πand π-πstacking interactions allow selective inhibition of butyrylcholinesterase by modified quinine and cinchonidin alkaloids. Biochem Biophys Res Commun. 2011;404:935–40. doi: 10.1016/j.bbrc.2010.12.084. [DOI] [PubMed] [Google Scholar]


