Abstract
Vegetables have been part of human food since prehistoric times and are considered nutritionally necessary and good for health. Vegetables are rich natural resource of biological antioxidants and possess capabilities of maintaining glucose homeostasis. When taken before starch-rich diet, juice also of vegetables such as ridge gourd, bottle gourd, ash gourd, chayote and juice of leaves of vegetables such as radish, Indian Dill, ajwain, tropical green amaranth, and bladder dock are reported to arrest significantly the rise in postprandial blood glucose level. Juice of vegetables such as ash gourd, squash gourd, and tropical green amaranth leaves are observed to tone-down sweet-beverages such as sucrose, fructose, and glucose-induced postprandial glycemic excursion. On the other hand, juice of egg-plant and juice of leaves of Ceylon spinach, Joyweed, and palak are reported to augment starch-induced postprandial glycemic excursion; and juice of leaves of Ceylon spinach, Joyweed, and radish supplement to the glucose-induced postprandial glycemia. Vegetables possess multifaceted antihyperglycemic activities such as inhibition of pancreatic α-amylase and intestinal α-glucosidase, inhibition of protein-tyrosine phosphatase 1β in liver and skeletal muscles, and insulin mimetic and secretagogue activities. Furthermore, they are also reported to influence polyol pathway in favor of reducing development of oxidative stress, and consequently the development of diabetic complications. In the wake of emergence of modern maladaptive diet-induced hyperglycemic epidemic therefore, vegetables may offer cost-effective dietary regimen to control diet-induced glycemic over load and future development of diabetes mellitus. However, for vegetables have been reported to do both, mitigate as well as supplement to the diet-induced postprandial glycemic load, care is required in selection of vegetables when considered as medicament.
Keywords: Aldose reductase, antioxidant, glucose homeostasis, hyperglycemia, oxidative stress, type 2 diabetes mellitus, vegetables
INTRODUCTION

The term vegetable refers to the edible parts of herbaceous plants, be it the roots, stem, leaves, flowers, or the fruits. Traditionally, common fruits (Latin verb frui, meaning to enjoy, to delight in) containing higher sugars and more acidic in nature are perceived as closer to the dimensions of luxury; vegetables (Latin verb vegere meaning to enliven, to invigorate), however, are inscribed in English as a useful fuel for humans and are considered more soberly as “nutritionally necessary” and “good for health.”[2] These attributes conferred to vegetables find testimony with the first ever recorded controlled human trial of dietary intervention traced in Bible.[3] Daniel and his friends while getting trained at court determined to take only vegetables to eat and water to drink rather than the daily allowance of food and wine from the Royal table for 10 days. It is mentioned that at the end of 10 days, they looked healthier and better nourished than other young men who lived on the foods assigned to them by the King.[4]
Historical accounts of nutrition transition
Archaeological evidences indicate that as mankind evolved, the nature of diet changed.[5] Some 30,000 years ago, climatic changes and population growth followed by overhunting led Cro-Magnons in Europe increase intake of vegetable foods.[6] Recent archaeological studies also indicate that Neanderthals who lived some 30,000-24,000 years ago had the knowledge of nutritional and medicinal values of vegetables.[7]
In the course of evolution however, high-quality diet became the prerequisite for developing large brain of modern human, the Homo sapiens.[8] In fact, on the metabolic ground, human brain is a very expensive organ and requires high-quality nutrients in order to function, survive, and sustain. Therefore, the shift toward agriculture from hunter-gatherer lifestyle of primitive human might have been one of the reasons to select and cultivate carbohydrate-rich cereal grains (rice, potatoes, barley, maize, millet, etc).[9] Advent of agriculture and animal husbandry practices was hence the brilliant dawn that shaped modern civilisation some 10,000 years ago. Selection of staple crops in the beginning of agriculture era was dependent on vegetations growing around the habitat of human living and varied according to the climatic conditions and soils.[8] It was later influenced by diverse ethnobotanical and cultural practices.[10] In the course of time, populations got adapted to such food items which became part of their traditional and cultural practices.
Rapid progressions in modern scientific knowledge followed by technological advancements post-world war further affected lifestyle of modern human. Physical activities decreased followed by more mental involvement, work pressure, and 24/7 work-packages led to introduction of modern food products. Resultantly, fast energy releasing, calorie-dense, refined starch and fat-rich easily available and digestible food products, and sugar-sweetened beverages were developed. Industrialized nations and man in modern globalized world is now foraging in supermarkets.[11]
Diseases of modern civilization and importance of vegetables
Nutrition transitions from traditional to the modern diets[12,13] accompanied with coca-colonization[14] are now being held responsible for the outbreak of diseases of modern civilization, namely, type 2 diabetes, cardiovascular diseases, obesity, cancers, and other chronic diseases as leading contributors to death and disability. These challenges force modern scientific community revisit and evaluate importance of so-called traditional Palaeolithic,[15] Mediterranean,[16] Okinawan,[17] and Indian[18,19,20] diets to combat modern diseases originating due to the consumption of maladaptive hyperglycemic and hyperlipidemic diet. Examination of such traditional diets reveals extensive use of vegetables as a common strand among majority of traditional diets. It becomes important therefore to revisit, identify, scientifically evaluate, and highlight vegetables in the light of their multidimensional health-promoting values in general and medicinal properties in correcting disturbed glucose homeostasis in particular.
In 1865, Claude Bernard observed that the “constancy of the internal milieu was the essential condition to a free life.”[21] Walter Cannon (1932) coined the term “homeostasis” (resistance to change) to describe the control of physiological equilibrium.[5] Seminal works published in the early 20th century reported that vegetables contain substances that reduce blood sugar upon injection into normal rabbits.[22,23] It was identified by Collip (1923) as glucokinin, a hormone-like substance which upon subcutaneous injection to animals produced hypoglycaemia and demonstrated insulin-like activity when injected into depancreatized dogs.[24] Concurrently, Dubin and Corbi (1923) observed that vegetables contain substances that can increase as well as decrease blood glucose level, and hence possess properties to correct imbalanced glucose homeostasis.[25] However, discovery of insulin[26,27] and emergence of synthetic antihyperglycemic drugs[28,29] in concurrent years gloomed research on nutritional and medicinal values of the culinary vegetations that grew around us.
Late-20th century's realization that majority of chronic diseases of modern civilization are the result of metabolic overload and postprandial hyperglycemic/hyperlipidemic excursions induced redox imbalance again highlighted the importance of culinary plants that furthered research identifying their role in prevention and management of diseases of metabolic overload.[30] Vegetables were identified as potent cost-effective natural resources of antioxidants to correct redox imbalance. Several studies approved potentials of vegetables in mitigating oxidative stress and maintaining antioxidant defence in the body.[31,32,33,34,35]
Modernization and urbanization has not only seen marked rise in the incidence of type 2 diabetes only in developed nations but also is taking shape of epidemic in many developing worlds.[36,37] Recent observations reveal that there are marked differences in dietary patterns among different population groups around the world. Studies have identified that the reason for increased incidence of type 2 diabetes and prolonged postprandial hyperglycemic excursion in South Asians (those who live in or have their roots in India, Pakistan, Sri Lanka, Bangladesh, Nepal, or the Maldives)[38] are due to the more consumption of refined carbohydrate diet, polished white rice in particular[38,39] than white Caucasian and other populations around the world. The higher incidences and prevalence of hyperglycemia, particularly in South Asians, may be explained by epigenetic mechanism[40] which states that a diet highly rich in calories be it the simple carbohydrates, sugary drinks, or sweets (very typical to Indian food habits), exposure to persistent organic pollutants in the course of modernization[41,42] leads to the development of latent diabetes in subjects genetically predisposed through depletion of insulin due to constant stimulation. It is being opined therefore that utilization of pools of nutrients may prove effective dietary therapeutic strategies in meeting such clinical situations.[40]
Balancing disturbed glucose metabolism by vegetables
The age-old classics of traditional Indian medicines, the Ayurveda had recognized food items not only as source of biological energy but also advocated their medicinal properties. The ancient compendia of Indian medicinal plant called Nighantu were scripted in Sanskrit language between 7th and 17th centuries AD.[43] These classics advocated beneficial therapeutic effect of Indian vegetable Karela (Momordica charantia L. Bitter gourd English). However, in the modern times significance of its hypoglycaemic activity was not recognized until the publication of research of Sharma et al. in 1960.[44] Later scientific investigations disclosed its multifaceted beneficial effect in the management of dysglycemia. Bitter gourd has been researched out to regulate disturbances of carbohydrate metabolism; important natural resource possessing insulin mimetic and secretagogue activities, a valuable culinary item that helps normalize physiological imbalances in glucose uptake, improves vitiated lipid profiles in diabetics and relieves oxidative stress.[20] However, its bitter taste limits its universal liking, and adverse effects such as hypoglycaemic coma, convulsion, and effect on fertility warrant its cautious use as a therapy for diabetic individuals.[20]
The schematic representation in Figure 1 provides evidences that vegetables possess multifaceted activities in correcting imbalance in glucose homeostasis induced due to the starch-rich diet or sweetened beverages. A simple meal plan of “eating vegetables before carbohydrate” is emerging as more effective dietary management strategy in achieving glycemic control in Japanese patients with type 2 diabetes mellitus. Results indicate that dietary carbohydrates consumed after vegetables are digested slowly and require less insulin for subsequent metabolic disposal. Increased postmeal satiety and decreased hunger were some of the other benefits patients experienced when they consumed more amount of vegetable.[45,46]
Figure 1.
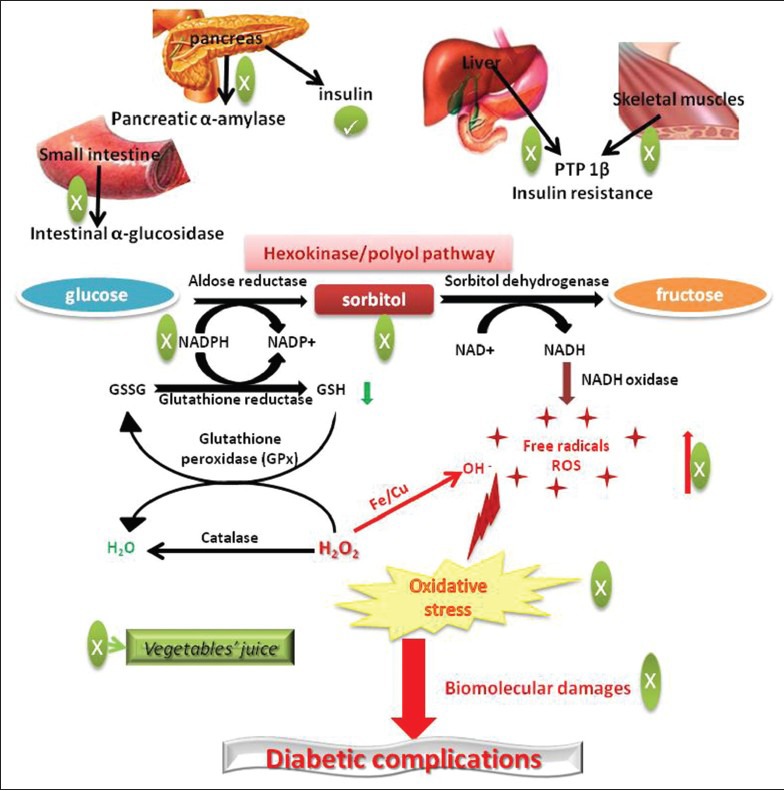
Multifaceted antihyperglycemic and antioxidative mechanisms of vegetables juice
Pancreatic α-amylase and intestinal α-glucosidase are important enzymes that breakdown complex carbohydrates into disaccharides and subsequently into monosaccharide glucose, during digestion of the food. In a recent study, it was found that juice of green leafy vegetables such as bladder dock (Rumex vesicarius L. Chooka in Hindi) and ajwain (Trachyspermum ammi L.) reduced starch-induced postprandial glycemic excursion in rats due to their potent pancreatic α-amylase and intestinal α-glucosidase inhibitory activities and juice of Indian Dill (Anethum sowa Roxb. Sowa in Hindi) and tropical green amaranth (Amaranthus viridis L. Chaulai in Hindi) leaves mitigated starch induced postprandial glycemic load by virtue of their intestinal α-glucosidase inhibitory activities [Figure 1].[47]
Calystegines are polyhydroxylated nortropane alkaloids commonly found in plants of Convolvulaceae, Brassicaceae, and Solanaceae families. Several commonly consumed vegetables such as sweet peppers (Capsicum annum L., Shimla Mirch in Hindi, Fam. Solanaceae), egg plant (Solanum melongena L., Brinjal in Hindi, Fam. Solanaceae), radish (Raphanus sativus L., Muli in Hindi, Fam. Brassicaceae), and sweet potatoes (Ipomoea batatas (L.) Lam., Sakarkand in Hindi, Fam. Convolvulaceae) belong to these families. Compound calystegine B2 is recently reported to inhibit human intestinal α-glucosidase which may become potent antihyperglycemic agent in reducing steep increase in blood glucose after a carbohydrate-rich meal.[48]
It is worth pointing here that several such compounds present in these vegetables represent only micro quantities. Although their development as medicine can serve one-dimensional therapeutic purpose, they may not be the sole and principal contributor of antihyperglycemic activities present in dietary materials. For, the fundamentals of traditional medicines are based on the concept of polypharmacological synergies and that of the traditional foods based on total sum of nutrients and balanced-diet rather than mere number of calorie counts. Therefore, application of holistic approach would be a more intelligent and appropriate one in revisiting and defining medicinal values of vegetables. For example, juice of radish leaves could not display pancreatic α-amylase and intestinal α-glucosidase inhibition however; significantly diminished starch induced-postprandial inhibition glycemic load.[47] Similarly, juice of ridge gourd (Luffa acutangula L. Roxb., Taroi in Hindi), bottle gourd (Lagenaria siceraria Molina Standl., Lauki in Hindi), ash gourd (Benincasa hispida Thunb. Cong., Raksaul Konhda, Petha in Hindi), and squash gourd, chayote (Sechium edule L., Chow Chow in Hindi) has been reported to significantly reduce increase in starch-induced postprandial glycemic load even in the absence of carbohydrate digestive enzymes’ inhibitory activities.[49] Radish, ridge gourd, bottle gourd, ash gourd, and chayote have been found to potently inhibit protein-tyrosine phosphatase 1β (PTP 1β) in liver and skeletal muscle [Figure 1],[50] an important enzyme responsible for the development of insulin resistance, type 2 diabetes mellitus, and obesity.[51] These observations, therefore, underline consideration of multifaceted polypharmacological approach during investigation and evaluation of medicinal properties of culinary vegetations.
Increasing evidences suggest that rise in consumption of sugar-sweetened beverages is one among the important contributing factors exaggerating development of dysglycemia.[52] Such beverages are identified as important causative factors responsible for increasing insulin resistance, decreasing insulin sensitivity, and consequently development of impaired glucose tolerance.[53] Impaired glucose tolerance (also defined as pre-diabetes) represents intermediate-state toward progression of overt diabetes in few years and is associated with development of microvascular and macrovascular complications before a person is diagnosed diabetic.[54] Feeding rats with juice of either ash gourd or the chayote for 3 months along with sucrose or fructose-sweetened beverages not only tone-down the progression of impaired glucose tolerance but also significantly prevented development of oxidative stress by maintaining total antioxidant potentials in the blood.[55] Furthermore, 15 days treatment with these vegetable juice of sugar-sweetened beverage induced hyperglycemic rats, normalized their glucose tolerance ability, reduced oxidative stress, and improved impaired platelet aggregation function and glycated haemoglobin levels.[55] Juice of tropical green amaranth leaves was also found to significantly decrease glucose-induced postprandial glycemic load in rats.[47]
Vegetables in mitigating hyperglycemia-induced oxidative stress
In fact, managing euglycemic levels in diabetics and delaying development of hyperglycemia-induced complications is not only a serious public health concern but also disturbing socioeconomic issues in developed countries as well as fast developing nations. Hyperglycemia-induced redox imbalance and resultant oxidative stress have been identified as one of the important cause ensuing development of microvascular and macrovascular diabetic complications.[56] Therefore, strategies that lower hyperglycemia-induced oxidative stress, improve antioxidant defence mechanisms, and correct imbalanced physiological functions may open new avenues in preventing development of diabetic complications not only in hyperglycemic individuals but also in prediabetics.
Several new mechanism-based strategies have been proposed recently for controlling oxidative stress in diabetics that can help prevent development of diabetic complications.[57] One of the important mechanisms by which hyperglycemia induces oxidative stress is polyol pathway. Under euglycemic condition, only trace amounts (~3%) of glucose enter polyol pathway. However, increased flux (>30%) of glucose through polyol pathway has been noticed under hyperglycemic condition.[58,59] The rate-limiting step of polyol pathway is reduction of glucose into sorbitol by enzyme aldose reductase (ALR) at the expense of reduced nicotinamide adenosine dinucleotide phosphate (NADPH).[59] Sorbitol, in turn, is converted into fructose by sorbitol dehydrogenase (SDH) with the help of cofactor nicotinamide adenine dinucleotide (NAD+).[59] Depletion of NADPH by ALR impedes regeneration of an important intracellular antioxidant, the reduced glutathione (GSH) and hence compromising effective scavenging of reactive oxygen species (ROS) and aggravating development of oxidative stress [Figure 1].[57] Furthermore, during conversion of sorbitol into fructose by SDH, the cofactor NAD+ is converted into NADH.[59] NADH is a substrate for NADH oxidase which is responsible for the generation of superoxide anions [Figure 1].[60] Therefore, increased formation of NADH during this process aggravates generation of free radicals such as superoxide anions. Taken together, reduction in antioxidant enzyme GSH and increased generation of free radicals (ROS) through polyol pathway contribute to the overt development of oxidative stress [Figure 1]. Oxidative stress and free-radical-induced damage to biomolecules results imbalance in normal physiological functions, consequently leading to the development of diabetic complications.
Vegetables are potent natural resource of biological antioxidants. Although the effectiveness of natural antioxidants in preventing diabetic complications is still uncertain, recent research discloses that vegetables’ juice possess capabilities of influencing polyol pathway by various mechanisms in favour of reducing burden of oxidative stress and hence, may be helpful in preventing development of diabetic complications.[61] This study finds that among 10 vegetables’ juice analysed to investigate their influence on polyol pathway, the juice of ivy gourd (Coccinia grandis L. J.Voigt, Kunduru in Hindi) followed by green cucumber (Cucumis sativus L., Kheera in Hindi) and ridge gourd displayed potent activities on various steps of polyol pathway independent of their antioxidant activities [Figure 1].[61] These vegetables’ juice, therefore, may become part of the highly sought mechanism-based complementary antioxidant therapy to prevent development of diabetic complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disorders.
Future perspectives
Albeit vegetables represent potent natural source of biological antioxidant and possess activities of correcting disturbed glucose metabolism, there are certain issues that need to be addressed.
Modern globalized world is facing problems associated with population explosion, increasing urbanization, expanding size of cities, disturbing food linked demand/supply ratio, and shrinking the size of cultivable agriculture land. Furthermore, modernization of agriculture and increased and indiscriminate use of chemical fertilisers and agrochemicals are polluting and adversely affecting natural matrix of agricultural land and resultantly the quality of food crops. All these activities have been found associated with aggravating increase in the incidences of diabetes development.[41]
During early 1930s, agricultural chemicals were hardly used. Manure and compost were the main fertilizers used. These practices changed, however, post world war and farmers became more reliant on the use of chemical fertilizers and other agrochemicals as well as heavy farm machineries.[62] These changes during modernization of agriculture also called “green revolution” post 1960s have no doubt increased the yields of food crops manifold in many countries[63] but have also drastically affected the nutrient qualities as well as quantities in cultivated food crops. For example, minerals play important role in aligning several biochemical and physiological processes. They are the essential cofactors for many enzymes and important contributors in efficient energy management, fertility, mental stability, and immunity empowerment.[62] It has been noticed that since 1940s while yield of food crops has increased manifold due to the increased use of synthetic chemical fertilisers, agrochemicals, and improved irrigation facilities, it has drastically diluted[64] and decreased biologically important minerals and nutrients concentrations particularly in vegetables.[62,64] Efforts are required therefore to improve and increase important essential mineral and nutrients in vegetables by encouraging application of organic cultivation practices and applications of biotechnological tools.[65]
Recently, health effects of antioxidant-rich natural materials have raised certain concerns due to their pro-oxidant effects when used at high concentrations.[66,67,68] Polyphenol contents (the major phytochemicals responsible for antioxidant activities) in vegetables’ juice have been found augmenting postprandial blood glucose load while reverse was the case observed with total protein content in vegetables’ juice.[49] Juice of egg-plant fruit,[49] and leaves of Ceylon spinach (Basella alba L. Upodica or Poi in Hindi), Joyweed (Alternanthera sessilis L. Garundi in Hindi), and palak (Spinacea oleracea L.) augmented postprandial glycemic load when administered before starch feeding in rats.[47] Ceylon spinach, Joyweed, and radish leaves juice supplemented to the glucose induced postprandial glycemic load.[47] These observations support 90 years ago published report that vegetables contain substances that can do both, increase as well as decrease the blood glucose levels.[25]
It is warranted recently that consumption of high dose of antioxidants may adversely affect other physiological processes in the direction of disease progression, and hence more is not always better.[69] It is interesting to mention here that the order of eating antioxidant-rich vegetables requires cautious investigation. Because antioxidants have been observed to affect appetite when taken on an empty stomach and satiety when consumed along with meal.[70] Also, vegetables have been reported to affect postprandial glycemic status in type 2 diabetes patients for, when consumed before carbohydrate-rich meal, it reduces postprandial glycemic load than when taken later.[71]
CONCLUSION
In the modern era of economic instability and increasing medical expenditures, vegetables can become cost-effective antioxidant-rich food materials equipped to correct disturbed glucose homeostasis. Consumption of vegetables can do both, augment as well as reduce carbohydrate/glucose-induced blood glucose level and can provide health benefits in various disease conditions. Research is required, therefore, to segregate such food materials based on their abilities of influencing various biochemical and physiological mechanisms when desired for therapeutic purpose under different disease conditions.
ACKNOWLEDGEMENTS
The author would like to thank the Director, CSIR-IICT, for constant encouragement and support for this research. This research was financially supported in parts by CSIR-New Delhi (India) grants under Net-work project (NWP-0004), MLP-001 and Natural Products as Affordable Healthcare Agents (NaPAHA, CSC-0130).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mylapur, Madras: The Ramakrishna Math; 1921. Taittiriya Upanishad by Swami Sharvananda. Taittiriya Upanishad II Brahmananda Valli, Chapter 2; p. 58. [Google Scholar]
- 2.McKee F. East of Eden: A brief history of fruit and vegetable consumption. Br Food J. 1995;97:5–9. [Google Scholar]
- 3.The book of Daniel. The Bible. 1:1–16. [Google Scholar]
- 4.Michels KB. Nutritional epidemiology –past, present and future. Int J Epidemiol. 2003;32:486–8. doi: 10.1093/ije/dyg216. [DOI] [PubMed] [Google Scholar]
- 5.Fairweather-Tait SJ. Human nutrition and food research: Opportunities and challenges in the post-genomic era. Philos Trans R Soc Lond B Biol Sci. 2003;358:1709–27. doi: 10.1098/rstb.2003.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoeninger MJ. Diet and the evolution of modern human form in the Middle East. Am J Phys Anthropol. 1982;58:37–52. doi: 10.1002/ajpa.1330580105. [DOI] [PubMed] [Google Scholar]
- 7.Hardy K, Buckley S, Collins MJ, Estalrrich A, Brothwell D, Copeland L, et al. Neanderthal medics?Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften. 2012;99:617–26. doi: 10.1007/s00114-012-0942-0. [DOI] [PubMed] [Google Scholar]
- 8.Sudano M, Gregorio F. Ancestral diets and modern diseases. Mediterr J Nutr Metab. 2011;4:181–9. [Google Scholar]
- 9.Diamond J. Chap. 7. Milano, Italy: Editore Einaudi (collana Super ET); 2006. Armi, acciaio e malattie. Breve storia del mondo negli ultimi tredicimila anni. [Google Scholar]
- 10.Galli C. Bioactive components in Mediterranean diets: From historical and ethnobotanical considerations to nutraceutical applications (Review) Nutrafoods. 2012;11:11–7. [Google Scholar]
- 11.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–7. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 12.Mehio Sibai A, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N. Nutrition transition and cardiovascular disease risk factors in Middle East and North American countries: Reviewing the evidence. Ann Nutr Metab. 2010;57:193–203. doi: 10.1159/000321527. [DOI] [PubMed] [Google Scholar]
- 13.Srinath Reddy K, Katan MB. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004;7:167–86. doi: 10.1079/phn2003587. [DOI] [PubMed] [Google Scholar]
- 14.Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: Can the doomsday scenario be averted? J Intern Med. 2000;247:301–10. doi: 10.1046/j.1365-2796.2000.00625.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindeberg S. Palaeolithic diet (“Stone age” diet) Scand J Nutr. 2005;49:75–7. [Google Scholar]
- 16.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–15. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 17.Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan Diet: Health Implications of a Low-Calorie, Nutrient-Dense, Antioxidant-–Rich Dietary Pattern Low in Glycemic Load. J Am Coll Nutr. 2009;28(Suppl):500S–16. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari AK, Kumar MP, Anand D, Agawane SB, Madhusudana K, Zehra A. Ayurvedic dietary formulations and postprandial glycemia in rats. Int Food Res J. 2012;19:765–73. [Google Scholar]
- 19.Tiwari AK. Invigorated barley in diabetes. Curr Sci. 2008;95:25–9. [Google Scholar]
- 20.Tiwari AK. Karela: A promising antidiabetic vegetable therapy. Curr Sci. 2007;92:1697–701. [Google Scholar]
- 21.Bell GH, Davidson JN, Scarborough H. Edinburgh, U.K: E. and S. Livingston Ltd; 1968. Text book of physiology and biochemistry; p. 2. [Google Scholar]
- 22.Winter LB, Smith W. On the nature of the sugar in blood. J Physiol. 1922;57:100–12. doi: 10.1113/jphysiol.1922.sp002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Best CH, Scott DA. Possible sources of insulin. J Metabol Research. 1923;3:177–9. [Google Scholar]
- 24.Collip JB. Glucokinin: A new hormone present in plant tissue. J Biol Chem. 1923;56:513–43. [Google Scholar]
- 25.Dubin HE, Corbitt HB. On the nature of the action of vegetable extracts on the blood sugar of normal rabbits. Exp Biol Med. 1923;21:16–8. [Google Scholar]
- 26.Best CH, Scott DA. The preparation of insulin. J Biol Chem. 1923;57:709–23. [Google Scholar]
- 27.Simoni RD, Hill RL, Vaughan M. The Discovery of Insulin: The Work of Frederick Banting and Charles Best. J Biol Chem. 2002;227:31–2. [Google Scholar]
- 28.Watanabe CK. Studies in the metabolism changes induced by administration of guanidine bases. J Biol Chem. 1918;33:253–65. [Google Scholar]
- 29.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia. 2013;56:1898–906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JY, Kwon O. Culinary plants and their potential impact on metabolic overload. Ann N Y Acad Sci. 2011;1229:133–9. doi: 10.1111/j.1749-6632.2011.06090.x. [DOI] [PubMed] [Google Scholar]
- 31.Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruits and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res. 1999;30:153–62. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- 32.Weisburger JH. Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem Toxicol. 1999;37:943–8. doi: 10.1016/s0278-6915(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 33.Steimez KA, Potter JD. Vegetables, fruits and cancer prevention: A review. J Am Diet Assoc. 1996;96:1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 34.Prior RL, Cao G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. Hort Science. 2000;35:588–92. [Google Scholar]
- 35.Shetty AA, Magadum S, Managanvi K. Vegetables as sources of antioxidants. J Food Nutr Disord. 2013;2:1–5. [Google Scholar]
- 36.Popkin BM. Urbanization, lifestyle changes and the nutrition transition. World Dev. 1999;27:1905–16. [Google Scholar]
- 37.5th ed. Brussels: International diabetes federation; 2011. International diabetes federation. IDF diabetes atlas. [PubMed] [Google Scholar]
- 38.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukkar SG. Targeting a tailored therapeutic diet by means of nutrigenomics: Future or reality? Mediterr J Nutr Metab. 2013;6:1–2. [Google Scholar]
- 41.Tiwari AK. Diabetes: Time to look beyond gluttony and laziness. Indian J Community Med. 2011;36:253–8. doi: 10.4103/0970-0218.91325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty P. Public health: India's diabetic time bomb. Nature. 2012;485:S14–6. doi: 10.1038/485s14a. [DOI] [PubMed] [Google Scholar]
- 43.Narayana A. Controversies in drug and industry-its measures: A view point. Bull Indian Inst Hist Med Hyderabad. 2003;33:1–16. [PubMed] [Google Scholar]
- 44.Sharma VN, Sogani RK, Arora RB. Some observations on hypoglycaemic activity in Momordica charantia. Indian J Med Res. 1960;48:471–7. [Google Scholar]
- 45.Imai S, Matsuda M, Hasegawa G, Fukui M, Obayashi H, Ozasa N, et al. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac J Clin Nutr. 2011;20:161–8. [PubMed] [Google Scholar]
- 46.Imai S, Fukui M, Ozasa N, Ozeki T, Kurokawa M, Komatsu T, et al. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet Med. 2013;30:370–2. doi: 10.1111/dme.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwari AK, Jyothi AL, Tejeswini VB, Madhusudana K, Kumar DA, Zehra A, et al. Mitigation of starch and glucose-induced postprandial glycemic excursion in rats by antioxidant-rich green-leafy vegetables’ juice. Pharmacogn Mag. 2013;9:S66–73. doi: 10.4103/0973-1296.117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jockovic N, Fischer W, Brandsch M, Brandt W, Drager B. Inhibition of human intestinal α-glucosidases by calystegines. J Agric Food Chem. 2013;61:5550–7. doi: 10.1021/jf4010737. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari AK, Reddy KS, Radhakrishnan J, Kumar DA, Zehra A, Agawane SB, et al. Influence of antioxidant rich fresh vegetable juices on starch induced postprandial hyperglycemia in rats. Food Funct. 2011;2:521–8. doi: 10.1039/c1fo10093a. [DOI] [PubMed] [Google Scholar]
- 50.Tiwari AK, Kumar DA, Sweeya PS, Abhinay KM, Hanumantha AC, Lavanya V, et al. Protein-tyrosine phosphatase 1βinhibitory activity potential in vegetables’ juice. Pharmacologia. 2013;4:311–9. [Google Scholar]
- 51.Popov D. Novel protein tyrosine phosphatase 1βinhibitors: Interaction requirements for improved intracellular efficacy in type 2 diabetes mellitus and obesity control. Biochem Biophys Res Commun. 2011;410:377–81. doi: 10.1016/j.bbrc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Malik VS, Papopkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care. 2010;33:2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik VS, Papopkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus and cardiovascular disease risk. Circulation. 2010;121:1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busserolles J, Zimowska W, Rock E, Rayssiguier Y, Mazur A. Rats fed a high sucrose diet have altered heart antioxidant enzyme activity and gene expression. Life Sci. 2002;71:1303–12. doi: 10.1016/s0024-3205(02)01846-5. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari AK, Anusha I, Sumangali M, Kumar DA, Madhusudana K, Agawane SB. Preventive and therapeutic efficacies of Benincasa hispida and Sechium edule fruit's juice on sweet-beverages induced impaired glucose tolerance and oxidative stress. Pharmacologia. 2013;4:197–207. [Google Scholar]
- 56.Araki E, Nishikawa T. Oxidative stress: A cause and therapeutic target of diabetic complications. J Diabetes Invest. 2010;1:90–6. doi: 10.1111/j.2040-1124.2010.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishikawa T, Araki E. Mechanism based antioxidant therapies promise to prevent diabetic complications? J Diabetes Invest. 2013;4:105–7. doi: 10.1111/jdi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez RG, Barnett P, Aguayo J, Cheng HM, Chylack LT., Jr Direct measurement of polyol pathway activity in the ocular lens. Diabetes. 1984;33:196–9. doi: 10.2337/diab.33.2.196. [DOI] [PubMed] [Google Scholar]
- 59.Yabe-Nishimura C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. [PubMed] [Google Scholar]
- 60.Morre DM, Lenaz G, Morre DJ. Surface oxidase and oxidative stress propagation in aging. J Exp Biol. 2000;203:1513–21. doi: 10.1242/jeb.203.10.1513. [DOI] [PubMed] [Google Scholar]
- 61.Tiwari AK, Kumar DA, Sweeya PS, Chauhan HA, Lavanya V, Sireesha K, et al. Vegetables’ juice influences polyol pathway by multiple mechanisms in favour of reducing development of oxidative stress and resultant diabetic complications. Pharmacogn Mag. 2014 doi: 10.4103/0973-1296.133290. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayer AM. Historical changes in the mineral content of fruits and vegetables. Br Food J. 1997;99:207–11. [Google Scholar]
- 63.Davis DR. Declining fruit and vegetable nutrient composition: What is the evidence? HortScience. 2009;44:15–9. [Google Scholar]
- 64.Jarrell WM, Beverly RB. The dilution effect in plant nutrition studies. Adv Agron. 1981;34:197–224. [Google Scholar]
- 65.Betoret E, Betoret N, Vidal D, Fito P. Functional foods development: Trends and technologies. Trends Food Sci Tech. 2011;22:498–508. [Google Scholar]
- 66.Martin KR, Appel CL. Polyphenols as dietary supplements: A double-edged sword. Nutr Diet Suppl. 2010;2:1–12. [Google Scholar]
- 67.Serrano J, Cipak A, Boada J, Gonzalo H, Cacabelos D, Cassanye A, et al. Double-edged sword behaviour of gallic acid and its interaction with peroxidases in human microvascular endothelial cell culture (HMEC-1). Antioxidant and pro-oxidant effects. Acta Biochim Pol. 2010;57:193–8. [PubMed] [Google Scholar]
- 68.Bouayed J, Bohn T. Exogenous antioxidants –Double-edged swords in cellular redox state: Health beneficial effects at physiological doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–37. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khanal RC, Rogers TJ, Wilkes SE, Howard LR, Prior RL. Effects of dietary consumption of cranberry powder on metabolic parameters in growing rats fed high fructose diets. Food Funct. 2010;1:116–23. doi: 10.1039/c0fo00089b. [DOI] [PubMed] [Google Scholar]
- 70.Diano S. Regulation of free radicals by the brain could play a role in weight control and appetite. Forefront (The American Diabetes Association's Research Magazine) 2009;6:26. [Google Scholar]
- 71.Imai S, Kajiyama S. Eating order diet reduced the postprandial glucose and glycated haemoglobin levels in Japanese patients with type 2 diabetes. J Rehab Health Sci. 2010;8:1–7. [Google Scholar]


