Abstract
Background:
The rhizomes of Acorus calamus and their essential oil are widely used in the flavoring industry and production of alcoholic beverages in Europe. Recent reports have confirmed the presence of several pharmacological components in the rhizomes of A. calamus.
Objective:
The objective of this study was to find out the efficacy of topical administration of ethanolic extract of A. calamus on dermal wound healing in rats. Wound healing is a natural process occurring in living organisms, which results in a complete or partial remodeling of injured tissue and ultimately progresses to the formation of a fibrous scar. Several natural products have been reported to augment the wound healing process.
Materials and Methods:
An ethanolic extract of A. calamus was prepared and its wound-healing efficacy was studied. An excision wound was made on the back of the rat and 200 μL (40 mg/kg body weight) of the A. calamus extract was applied topically once daily for the treated wounds. The control wounds were treated with 200 μL of phosphate buffered saline.
Results:
The granulation tissues formed were removed at 4, 8 and 12 days and biochemical parameters such as deoxyribonucleic acid, total protein, total collagen, hexosamine and uronic acids were measured. The amount of type I/III collagen formed in control and treated wound tissues was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The epithelialization time, tensile strength and histological examination of the wounds were also studied. Biochemical analyses of the granulation tissues revealed a significant increase in collagen, hexosamine and uronic acid when compared with the control. The tensile strength of extract treated wounds was found to increase by 112%. A significant reduction in lipid peroxide levels suggested that A. calamus possesses antioxidant components.
Conclusions:
The results strongly confirm the beneficial effects of A. calamus in augmenting the wound healing process.
Keywords: Acorus calamus, collagen, epithelialization, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, tensile strength, wound healing
INTRODUCTION
Healing is a multifaceted and intricate process initiated in response to tissue injury that restores the function and integrity of damaged tissues.[1] Wound healing involves continuous cell–cell and cell-matrix interactions that allow the process to proceed in three overlapping phases such as inflammation (0-3 days), cellular proliferation (3-12 days) and remodeling (3-6 months).[2] Several other natural products and medicinal plants enhance the process of wound healing.[3,4] The therapeutic benefits of secondary metabolites of plants have also been focused in many investigations.[5] We have been screening topical evergreen plants as agents for wound healing since two decades. Here, we report the efficacy of alcoholic extract of A. calamus on dermal wound healing in rats.
A. calamus (Family: Acoraceace) is a perennial plant, the rhizome has many pharmacological activities; the ethanol extract has anticellular and immunosuppressive properties.[6] The rhizomes of A. calamus and their essential oil (calamus oil) are widely used in the flavoring industry and production of alcoholic beverages in Europe. Recent reports have confirmed the presence of several pharmacological components in the alcoholic extract of rhizomes of A. calamus.[7]
Conventionally, it is used as a constituent for many mixture preparations used for the treatment and management of headache, migraine, body ache, severe inflammatory pain and vincristine-induced painful neuropathy.[8] Phytochemical analyses have shown that A. calamus possesses glycosides, flavonoids, saponins, tannins, polyphenols, mucilage, volatile oil and few bitter principles.[8] The aqueous and hydro-alcoholic extracts have also been shown to possess lipid lowering and neuropharmacological actions.[9]
Recently, the role of ί-asarone, a component of A. calamus, has been reported for its inhibitory effect on adipogenesis in 3T3-L1 cells.[10] Wound healing activity of ethanolic extract of A. calamus leaves has been studied and reported wherein, the wounds were treated with the plant extract in an ointment form and the results were compared with a standard drug, povidone-iodine.[11] However, in this investigation, experiments were carried out to find out the efficacy of ethanolic extract of the rhizomes of A. calamus on wound repair process by measuring various biochemicals, biophysical parameters related to wound healing. Histological evaluation of wounds was also studied to confirm the results. The efficacy of the extract on collagen characteristics has also been carried out. The ratio of Type I/III collagen has also been studied and found out that the alcoholic extract of A. calamus enhances all phases of wound healing.
MATERIALS AND METHODS
Chemicals
Acrylamide, ammonium per sulfate, bovine serum albumin, calf thymus deoxyribonucleic acid (DNA), chloramine-T D-glucuronic acid and L-hydroxyproline were from Sigma Chemical Company, St. Louis, USA. p-dimethyl aminobenzaldehyde and Folin's Phenol reagent were from Loba Chemie, Mumbai, India. Methyl cellosolve was obtained from Merck, Darmstadt, Germany. All other reagents were high analytical grade.
Extract preparation
The rhizome of A. calamus obtained locally were minced, weighed, powdered and homogenized in 10-20 volumes (by weight) of 70% ethanol and filtered to yield a viscous supernatant. This was used as the crude extract. An aliquot of the extract was lyophilized and weighed. About 75% by weight of the starting dry material was recovered in this fraction. The lyophilized powder was reconstituted in phosphate buffered saline (PBS).
Animals
Male rats of Wistar strain weighing 180-200 g were chosen and divided into two groups of 6 each for the present study. The animals were maintained on clean, sterile, polyvinyl cages and fed with commercial rat feed from M/S Hindustan Lever Ltd., India (mixed with wheat flour in the ratio of 1:1 (w/w)). Food and water were provided ad libitum to the animals. All procedures were carried out according to the Institutional Animal Care and Use Committee. A formal approval from the Animal Ethical Committee of our institute has also been obtained.
Wound creation and drug administration
A 2 cm2 (4 cm) full thickness open excision wound was made on the back of the rat as reported in our earlier studies.[12] A total number of 48 animals were used for the whole study. The animals were divided into two groups (control and treated), each group containing six rats. Each animal was given a light ether anesthesia and shaved on its back under aseptic conditions. Wound was created with a sterile scalpel as per the rules of Committee for the Purpose of Control and Supervision of Experiments on Animals under sterile conditions. About 200 μl of the plant extract (lyophilized powder reconstituted in PBS at a concentration of 40 mg/Kg body weight) was applied topically on the wounds once daily until the wounds healed completely. The control wounds were treated with 200 μl of PBS.
The animals were sacrificed at different time point intervals such as 4th, 8th and 12th day. The wound tissues formed were removed and used for various biochemical estimations. Separate groups of animals, 6 each for control and experiment were maintained to find out the period of epithelialization and rate of wound contraction.
For tensile strength determination, a 6 cm long linear full thickness incision wound was made on either side of the midline. After the wounds had been wiped off to remove blood, if any, intermittent sutures were placed 1 cm apart. The sutures were removed after the 7th day post-wounding and the tissues were removed on 10th day to determine the tensile strength of the wound tissues according to the method of Vogel.[13]
Biochemical estimations
Protein and DNA of wet granulation tissues were extracted in 5% trichloroacetic acid (TCA) as per Porat et al.[14]. Briefly, 10 mL of 5% TCA was added to the tissue (100 mg wet weight of tissue) maintained at 90°C for 30 min in a water bath to extract protein and DNA. The solution was centrifuged and the supernatant was used to estimate DNA by the method of Burton[15] and protein by the method of Lowry et al.[16] To estimate the collagen and hexosamine, the tissue samples were defatted in chloroform: methanol (2:1) and dried in acetone, before use. Weighed tissues was first hydrolyzed in 6 N HCl for 18 h at 110°C, evaporated to dryness and then made up with a known volume of water. Collagen and hexosamine were estimated by the method of Woessner[17] and Elson and Morgan,[18] respectively. Lipid peroxide levels in granulation tissues were determined by the thiobarbituric acid reaction.[19]
Fractionation of collagen was performed by the method of Piez.[20] Briefly, for the neutral salt soluble collagen, the wound tissue was minced well, homogenized in 10 volumes of a neutral salt solvent (1.0 mol/L NaCl, 0.05 mol/L Tris, pH 7.5) containing 20 mmol/L ethylenediaminetetraacetic acid and 2.0 mmol/L N-ethyl maleimide and stirred for 24 h. The suspension was then centrifuged at 35,000 g for 1 h at 4°C and the extraction was repeated with the pellet. The supernatants were pooled and an aliquot was used for the assay of hydroxyproline[17] and for the acid-soluble collagen, the residue obtained was resuspended in 10 volumes of 0.5 mol/L acetic acid and extracted for 24 h with constant stirring, after which the contents were centrifuged at 35,000 g for 1 h at 4°C. The pellet was re-extracted with acetic acid, the supernatants were pooled and an aliquot was used for the determination of hydroxyproline.[17] Finally, for the pepsin-soluble collagen, the residue obtained after acid extraction was resuspended in 0.5 mol/L acetic acid containing 100 mg pepsin/g wet tissue. Digestion was performed for 24 h, followed by centrifugation at 35,000 g for 1 h at 4°C and reextraction. Aliquots of pooled supernatants were used for hydroxyproline measurement.[17] The susceptibility of insoluble collagen to denaturing agents like urea and potassium thiocyanate was analyzed by the method of Adam et al.[21]
Interrupted sodium dodecyl sulfate-polyacrylamide gel electrophoresis
The sub-unit composition of the isolated collagen was investigated by interrupted sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE.[22] Briefly, collagen bands were separated by SDS-PAGE) using 3% stacking gel with 5% separating gel and Coomassie brilliant blue staining.
Biophysical analyses
The wounds were also inspected for the extent of epithelialization and calculated as days required for shedding of eschar without any raw wound left behind. The excision wounds were traced on a transparent graph paper and changes in wound size were measured planimetrically. Reduction in wound size (reflecting the rate of wound contraction) was calculated as a percentage of the original wound size. The tensile strength of wound tissues was measured by the method of Vogel.[13]
Histology
The rats were sacrificed and tissues in the wound site of the individual animal were removed. These samples were then separately fixed in 10% formalin, dehydrated through graded alcohol series, cleared in xylene and embedded in paraffin wax (melting point 56°C). Serial sections of 5 μm were cut and stained with hematoxylin and eosin and Van Gieson's. The sections were examined under light microscope and photomicrographs were taken.
Statistics
Data are expressed as mean ± SD and the results were statistically evaluated using Students paired and unpaired t-test and nonparametric Mann-Whitney test. All statistical analyses were executed using GraphPad Prism (version 5.0; GraphPad software Inc. San Diego CA, California, USA).
RESULTS
Biochemical and biophysical analyses
The biochemical evaluations such as DNA, total protein, hexosamine and total collagen content in the wound tissues of control and treated rats are depicted in Table 1. The DNA content was significantly elevated to 46% on day 4 further increased to 60% on day 8 and then decreased. A similar pattern (29% increase on day 4, 34% on day 8) was observed in protein content also. The collagen content was 43% on day 4, which further rose up to 73% for day 8 and then reduced to 66% on day 12. Level of hexosamine in the wound tissues of treated rats was also elevated by 34% on day 4 and decreased to 16% and 15% on day 8 and 12 respectively.
Table 1.
Effect of A. calamus on various biochemical parameters (mg/100 mg tissue)
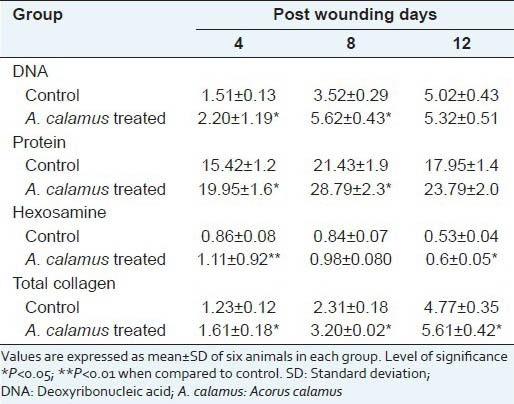
Table 2 and Figure 1 show the percentage wound contraction on various days of control and A. calamus treated rats on different days. Wounds that received A. calamus extract contracted a very fast and healed completely by day 14 whereas, the control wounds took 21 days for complete healing [Figure 2a]. Figure 2b illustrates the tensile strength of control and treated wound tissues measured on day 10. There is more than two fold increase in tensile strength in the A. calamus treated wounds.
Table 2.
Rate of wound contraction as percentage of original wound size
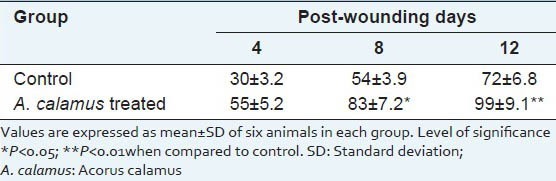
Figure 1.

Photographical illustration of wound contraction rate on different days of control and Acorus calamus treated wounds. Scale bar 1 cm
Figure 2.
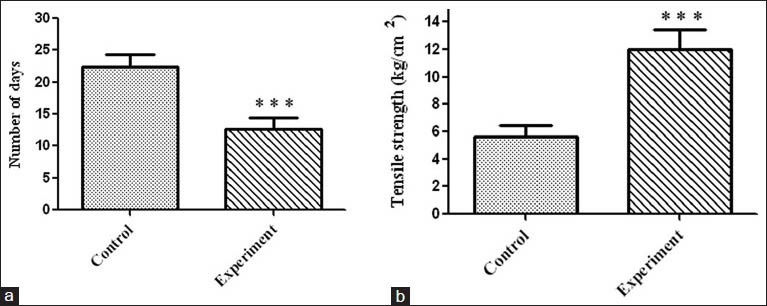
(a) Period of epithelialization (measured as the number of days required for shedding of eschar without any raw wound left behind) in control and treated rats. Values are expressed as mean ± standard deviation (SD) for six animals ***P < 0.001 was significant compared with the control. (b) Tensile strength of tissues from control and treated wounds. Values are expressed as mean ± SD for six animals ***P < 0.001 was significant when compared with the corresponding control
Figure 3 imparts levels of lipid peroxide in control and treated rats. A. calamus treated rats showed lower free radical levels and oxidative tissue damage (as represented as malondialdehyde level) than control.
Figure 3.
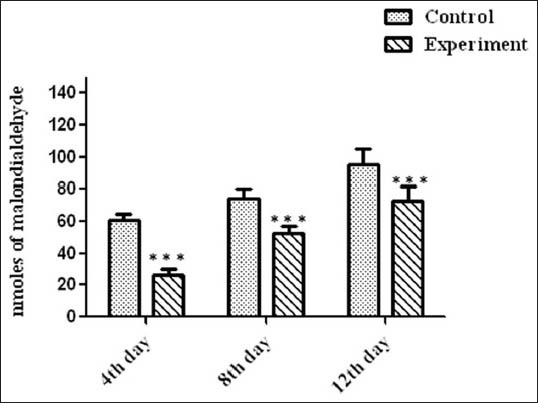
Levels of lipid peroxidation in wound tissues on various days. Values are expressed as mean ± standard deviation for six animals and level of significance is expressed as ***P < 0.001 with the respective control
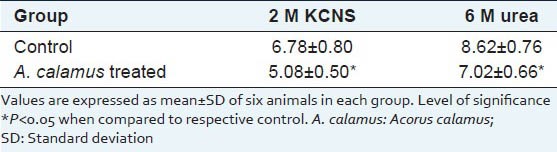
Solubility pattern of collagen and susceptibility of insoluble collagen in denaturing agents are depicted in Table 3a and b. There is about 39% increase in the insoluble collagen in A. calamus treated wounds, which confirm that collagen is more cross-linked than the collagen from control wounds. Similarly, when the susceptibility of insoluble collagen to denaturing agents was measured in the presence of 2 M KCNS and 6 M urea, there was about 33% and 22% decrease respectively in the A. calamus treated wound collagen further confirming the increased cross-linking of collagen.
Table 3a.
Solubility pattern of collagen from 8th day wound tissue (μg/100 mg tissue)
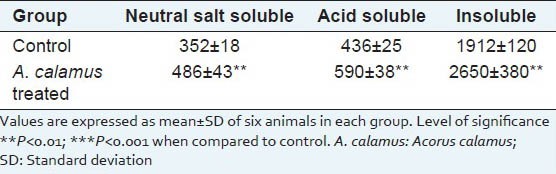
Table 3b.
Susceptibility of insoluble collagen (8th day tissue) to denaturing agents (mg/100 mg tissue)
Histopathological analysis
The histological examination displayed that the tissue regeneration was much faster in the A. calamus treated rats. On day 4, less number of cellular infiltration and proliferating blood capillaries was observed along with lesser fibroblasts in control wound tissue [Figure 4a]. Whereas, A. calamus treated wound tissue showed increased fibroblasts and macrophages with reasonable collagen fibers. Budding of new blood capillaries in wound tissues and prominent re-epithelialization were also detected in A. calamus treated wound tissues [Figure 4b]. On day 8, in control, irregularly arranged collagen fibers and less fibrosis could be seen with incomplete epithelialization [Figure 4c]. But A. calamus treated wound tissues showed complete epithelialization, increased fibrosis, uniform thick collagen deposition and macrophages at the wound site [Figure 4d].
Figure 4.
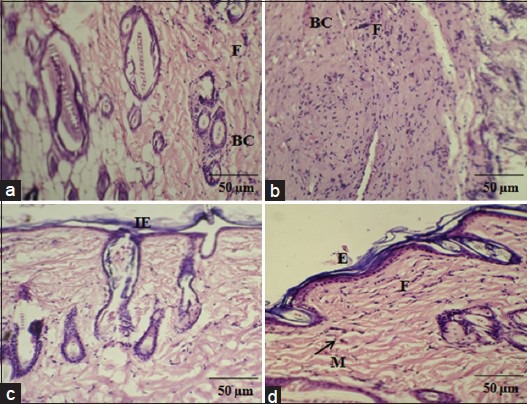
Histological examination showing control and Acorus calamus treated wound tissues on day 4 and day 8 of post wounding, respectively (H and E and Van Gieson's, ×20). On day 4, control showing loosely packed collagen with irregular epithelialization and less fibroblasts (a). Whereas, treated tissue showing new blood vessels formation and high fibroblasts with dense collagen deposition (b). On day 8, control depicts thin epithelial layer with less collagen (c) and treated shows complete epithelialization with regularly arranged dense collagen (d). Scale bar 50 ìm. BC: Blood capillaries; F: Fibroblast; IE: Incomplete epithelialization; E: Epithelialization; M: Macrophages
SDS-PAGE
Figure 5 shows the interrupted SDS-PAGE pattern of acid-soluble collagen of wound tissues. A significant increase in the α1 of type III collagen could be observed in the A. calamus treated wounds.
Figure 5.
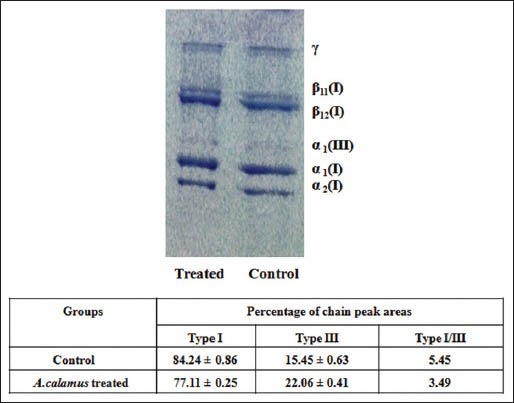
Interrupted sodium dodecyl sulfate-polyacrylamide gel electrophoresis showing the distribution of type I and III collagen of acid soluble fraction of day 8 wound tissues from control and Acorus calamus treated. All lanes were loaded with equal concentration of collagen (25 ìg)
DISCUSSION
Medicinal plants in wound healing are used for disinfection, debridement and the stipulation of appropriate environment for natural healing process. Indeed, alternate medicines are of less toxicity and with fewer side-effects compared with modern medicine and hence, it is significant to introduce a scientific validation for the medicinal values of plants used in traditional medicine.[23]
Wound healing is a complex natural renewal process of skin cells to curtail or eliminate scarring as well to help healing and repairing damage.[24] The major events include cellular migration, proliferation, adhesion and phenotypic differentiation.[25] The present investigation of A. calamus administration on wounds has produced a positive outcome in the healing process. Plant products are potential agents for wound healing and are mainly chosen because of their extensive availability, non-toxicity, ease of administration, absence of unwanted side-effects and their efficiency as crude preparations.[26] A. calamus is widely used as traditional medicine by various cultures world-wide, although applications vary by region.[7,27] In this study, we have evaluated the efficacy of the alcoholic extract of the rhizomes of A. calamus on wound healing in rats.
The increase in DNA and total protein content in the treated wounds reflect mitogenic potential of A. calamus extract and hyperplasia of the cells. Hexosamine is the matrix molecule, present in the extracellular matrix, which acts as the ground substratum for the synthesis of new collagen scaffold. The early increase in hexosamine shows the active synthesis of ground substratum by fibroblasts on which collagen is laid down. The elevated level of hexoasamine in treated rats could provide a strength to regenerated tissues.[28] Collagen deposition is an another important event in the wound tissue formation. The healing process depends to a large extent, on the regulated biosynthesis and deposition of new collagens and their subsequent maturation,[29] which was also increased by A. calamus treatment. Appropriate healing of wounds is a crucial event for the renovation of disrupted skin for its normal functional status. Higher rate of wound contraction in A. calamus treated rats might be attributed to increased proliferation and transformation of fibroblasts into myofibroblasts.[28] This could be attributed by the enhanced contractile property of myofibroblast resulting in the shorter epithelialization period. Histological findings firmly substantiate the increased proliferation of fibroblasts and reepithelialization in A. calamus treated rats.
Evaluation of collagen content in wound tissues of control and A. calamus treated wounds, obviously confirms that A. calamus promotes collagen synthesis and deposition. The increase in solubility of collagen is relative to the synthesis of new collagen and leads to greater potential for cross-link formation.[28] Thus, the increased solubility and decreased susceptibility of insoluble collagen in treated wounds, strongly substantiates that A. calamus accelerates the collagen synthesis, then maturation and cross-linking. The increase in tensile strength of treated wounds could be due to the increase in the collagen concentration per unit area and stabilization of the collagen fibers.[6] The early tissue deposition and increased tensile strength may be contributed by terpenoid constituents of A. calamus.[11]
Reactive oxygen species are noxious to wound healing process due to their damaging effects on cells and tissues. The increase in free radical production and diminished antioxidant activities may worsen the condition and account for the delay in healing.[28] A. calamus enhances wound healing by reducing the level of free radicals and hence the oxidative damage. Thiem and Goślińska[30] have reported that the topical administration of compounds with free radical scavenging properties in patients have shown to improve wound healing significantly and protect tissues from oxidative damage.
High amount of type III collagen proportional to type I was observed in A. calamus treated wounds. The ratio between type I/type III is very low in A. calamus treated rats showing that more amount of type III collagen is synthesized. Dermal wound tissue in the early phase of healing resembles embryonic skin in that type III collagen present in elevated proportions relative to type I collagen.[31] The increase in type III collagen is derived from local fibroblasts, which are activated by the wound healing process. The early appearance of type III collagen is connected with an early increase in collagen synthesis that may function in offering wound structure and promote wound healing. Type III collagen has a far greater platelet aggregating activity than type I collagen and it plays a vital role in the configuration of homeostatic plug.[32]
Topically administered drugs are effective in faster wound contraction and healing due to the larger availability of the drug at the wound site.[12] Hence, the rate of wound contraction in treated rats was considerably higher. Furthermore, the period of epithelialization was shorter in the treated wounds. These results firmly support the feasible and productive approach of A. calamus in dermal wound healing.
CONCLUSION
Topical administration of A. calamus accelerates wound repair, which involves different steps such as collagen synthesis and maturation, wound contraction and epithelialization. These results firmly support the feasible and productive approach of A. calamus in dermal wound healing in rats.
ACKNOWLEDGEMENTS
The authors would like to thank the Director, CSIR-Central Leather Research Institute for the facility provided. T. Ponrasu and M. Ganeshkumar acknowledge Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of Senior Research Fellowship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing –Exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–13. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RL, Bernfield M. Proteoglycan and their role in wound repair. In: Clark RA, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 475–92. [Google Scholar]
- 3.Karodi R, Jadhav M, Rub R, Bafna A. Evaluation of the wound healing activity of a crude extract of Rubia cordifolia L. (Indian madder) in mice. Int J Appl Res Nat Prod. 2009;2:12–8. [Google Scholar]
- 4.Sumitra M, Manikandan P, Gayathri VS, Mahendran P, Suguna L. Emblica officinalis exerts wound healing action through up-regulation of collagen and extracellular signal-regulated kinases (ERK1/2) Wound Repair Regen. 2009;17:99–107. doi: 10.1111/j.1524-475X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozgen U, Ikbal M, Hacimuftuoglu A, Houghton PJ, Gocer F, Dogan H, et al. Fibroblast growth stimulation by extracts and compounds of Onosma argentatum roots. J Ethnopharmacol. 2006;104:100–3. doi: 10.1016/j.jep.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 6.Parab RS, Mengi SA. Hypolipidemic activity of Acorus calamus L. in rats. Fitoterapia. 2002;73:451–5. doi: 10.1016/s0367-326x(02)00174-0. [DOI] [PubMed] [Google Scholar]
- 7.Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: An evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol. 2011;49:2557–63. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 8.Dong W, Yang D, Lu R. Chemical constituents from the rhizome of Acorus calamus L. Planta Med. 2010;76:454–7. doi: 10.1055/s-0029-1186217. [DOI] [PubMed] [Google Scholar]
- 9.Parap RS, Mengi SA. Evaluation of hypolipidemic activity of Acrous calamus Linn in rats. Indian Drugs. 2003;40:25–9. [Google Scholar]
- 10.Lee MH, Chen YY, Tsai JW, Wang SC, Watanabe T, Tsai YC. Inhibitory effect of β-asarone, a component of Acorus calamus essential oil, on inhibition of adipogenesis in 3T3-L1 cells. Food Chem. 2011;126:1–7. [Google Scholar]
- 11.Jain N, Jain R, Jain A, Jain DK, Chandel HS. Evaluation of wound-healing activity of Acorus calamus Linn. Nat Prod Res. 2010;24:534–41. doi: 10.1080/14786410802531782. [DOI] [PubMed] [Google Scholar]
- 12.Ponrasu T, Suguna L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int Wound J. 2012;9:613–23. doi: 10.1111/j.1742-481X.2011.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel HG. Antagonistic effect of aminoacetonitrile and prednisolone on mechanical properties of rat skin. Biochim Biophys Acta. 1971;252:580–5. doi: 10.1016/0304-4165(71)90162-0. [DOI] [PubMed] [Google Scholar]
- 14.Porat S, Rousso M, Shoshan S. Improvement of gliding function of flexor tendons by topically applied enriched collagen solution. J Bone Joint Surg Br. 1980;62-B:208–13. doi: 10.1302/0301-620X.62B2.6245095. [DOI] [PubMed] [Google Scholar]
- 15.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–23. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 17.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 18.Elson LA, Morgan WT. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27:1824–8. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980;33:973–6. doi: 10.1136/jcp.33.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piez KA. The amino acid chemistry of some calcified tissues. Ann N Y Acad Sci. 1963;109:256–68. doi: 10.1111/j.1749-6632.1963.tb13470.x. [DOI] [PubMed] [Google Scholar]
- 21.Adam M, Fietzek P, Kühn K. Investigations on the reaction of metals with collagen in vivo. 2. The formation of cross-links in the collagen of lathyritic rats after gold treatment in vivo. Eur J Biochem. 1968;3:411–4. doi: 10.1111/j.1432-1033.1967.tb19545.x. [DOI] [PubMed] [Google Scholar]
- 22.Hering TM, Marchant RE, Anderson JM. Type V collagen during granulation tissue development. Exp Mol Pathol. 1983;39:219–29. doi: 10.1016/0014-4800(83)90053-9. [DOI] [PubMed] [Google Scholar]
- 23.Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol. 2002;83:193–9. doi: 10.1016/s0378-8741(02)00195-2. [DOI] [PubMed] [Google Scholar]
- 24.Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermöhlen O, et al. Accelerated wound closure in mice deficient for interleukin-10. Am J Pathol. 2007;170:188–202. doi: 10.2353/ajpath.2007.060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–31. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 26.Sandhya S, Kumar PS, Vinod KR, Banji D, Kumar K. Plants as potent anti diabetic and wound healing agents-A review. Hygeia JD Med. 2011;3:11–9. [Google Scholar]
- 27.Lee MR, Yun BS, Park SY, Ly SY, Kim SN, Han BH, et al. Anti-amnesic effect of Chong-Myung-Tang on scopolamine-induced memory impairments in mice. J Ethnopharmacol. 2010;132:70–4. doi: 10.1016/j.jep.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Upadhyay NK, Sawhney RC, Kumar R. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008;16:784–90. doi: 10.1111/j.1524-475X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RC. Collagen cross-linking. Synthesis of collagen cross-links in vitro with highly purified lysyl oxidase. J Biol Chem. 1976;251:5786–92. [PubMed] [Google Scholar]
- 30.Thiem B, Goślińska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75:93–5. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Barnes MJ, Morton LF, Bennett RC, Bailey AJ, Sims TJ. Presence of type III collagen in guinea-pig dermal scar. Biochem J. 1976;157:263–6. doi: 10.1042/bj1570263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazin S, Delaunay A. Biochemistry of inflammation. VI. Fluctuations in the levels of collagen and nonfibrillar proteins in different types of inflammatory foci. Comparative studies. Ann Inst Pasteur (Paris) 1964;107:163–72. [PubMed] [Google Scholar]


