Abstract
Background:
Our earlier study on the antiproliferative (APF) activity of leaf extracts of ten Apocynaceae species showed that leaves of Vallaris glabra possessed strong and broad-spectrum properties.
Materials and Methods:
In this study, sequential extracts of leaves, flowers and stems, and fractions and isolated compounds from dichloromethane (DCM) leaf extract of V. glabra were assessed for APF activity using the sulphorhodamine B (SRB) assay. Apoptotic effect of MDA-MB-231 cancer cells treated with DCM leaf extract of V. glabra was studied using Hoechst 33342 dye and caspase colorimetry.
Results:
Both DCM extracts of leaves and flowers possessed broad-spectrum APF activity against HT-29, MCF-7, MDA-MB-231 and SKOV-3 cancer cells. From DCM leaf extract, stearic acid (SA) and ursolic acid (UA) were isolated by column chromatography, and identified by NMR and MS analyses. APF activity of SA from DCM leaf extract displayed weak inhibitory activity and scientific literature showed UA has anticancer properties against those cancer cells used in this study. MDA-MB-231 cancer cells treated with DCM leaf extract and stained with Hoechst 33342 dye provided evidence that the extract had an apoptotic effect on the cells. Caspase colorimetry showed that the apoptotic effect involved activation of caspase-8, -9 and -3, but not caspase-6.
Conclusion:
The potential of V. glabra as a candidate species for anticancer drugs warrants further investigation.
Keywords: Antiproliferative activity, apoptosis, caspase colorimetry, Hoechst nuclear staining, Vallaris glabra
INTRODUCTION
The family Apocynaceae consists of about 250 genera and 2000 species of tropical trees, shrubs and vines.[1] Almost all species of the family produce milky sap. Other characteristic features are simple, opposite or whorled leaves; large, colorful and slightly fragrant flowers with five contorted lobes; and fruits are in pairs. With inclusion of species of Asclepiadaceae, the family has now been enlarged from two to five subfamilies.[2]
In traditional medicine, Apocynaceae species are used to treat gastrointestinal ailments, fever, malaria, pain and diabetes.[1] Apocynaceae species have also been reported to show anticancer properties. Our earlier study on the antiproliferative (APF) activity of leaf extracts of ten Apocynaceae species showed that Alstonia angustiloba, Calotropis gigantea, Catharanthus roseus, Nerium oleander, Plumeria obtusa and Vallaris glabra displayed positive inhibition.[3,4] Allamanda cathartica, Cerbera odollam, Dyera costulata and Kopsia fruticosa did not display any APF activity. Dichloromethane (DCM) and DCM: Methanol (MeOH) extracts of V. glabra inhibited all six cancer cell lines of MCF-7, MDA-MB-231, HeLa, HT-29, SKOV-3 and HepG2, while the MeOH extract inhibited MCF-7 and HepG2 cells. Results showed that leaves of V. glabra possessed strong and broad-spectrum properties. Recently, the botany, uses, phytochemistry and pharmacology of selected Apocynaceae species including V. glabra have been reviewed.[5]
In this study, the leaves, flowers and stems of V. glabra were sequentially extracted with solvents of increasing polarity. The resultant fractions and compounds isolated from DCM leaf extract from V. glabra were assessed for APF activity. Apoptosis of MDA-MB-231 breast cancer cells treated with DCM leaf extract was shown and possible mechanisms were elucidated by caspase colorimetry.
MATERIALS AND METHODS
Plant materials
Leaves, flowers and stems of V. glabra were collected from Kepong (N 3° 12’14”; E 101° 37’50”) in Selangor, Malaysia and identified by Dr. Chan Hung Tuck, former Division Director of Forest Research Institute Malaysia. V. glabra is a woody climber that produces clusters of white flowers [Figure 1] with a scent characteristic of Pandanus amaryllifolius (pandan) leaves or fragrant rice. With brief description of its morphology and location, a voucher specimen of V. glabra was deposited in the herbarium of Monash University Sunway Campus in Malaysia.
Figure 1.

Flowers and leaves of Vallaris glabra
Extraction of plant material
Leaves (40 gm), stems (10 gm) and flowers (10 gm) of V. glabra were freeze-dried overnight, ground and sequentially extracted with hexane (Hex), DCM, DCM: MeOH (1:1) and MeOH. For each solvent (50 mL/gm of plant sample), the suspension of ground samples was shaken for 1 h on the orbital shaker. After filtering, the samples were extracted two more times for each solvent. Solvents were removed with a rotary evaporator and the dried extracts were stored at -20° C for further analysis.
Antiproliferative activity
Antiproliferative (APF) activity of extracts, fractions and isolated compounds was assessed for growth inhibitory (GI50) activity against four types of human cancer cells (HT-29, MCF-7, MDA-MB-231 and SKOV-3) using the sulphorhodamine B (SRB) assay, following the procedures described earlier.[6,7] The measured quantity, GI50, is the concentration of extract that causes 50% reduction in cell growth.
Human cancer cell lines, obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), were seeded 24 h prior to treatment in 96-well plates at appropriate densities (30,000 cells/mL for MCF-7; 60,000 cells/mL for MDA-MB-231 and HT-29; and 120,000 cells/mL for SKOV-3) with each well containing 100 μL of cell culture. Extracts (5 mg/mL) were dissolved in 100% DMSO and serially diluted two-fold to concentrations ranging from 3.13-50.0 μg/mL with appropriate medium. As 100 μL of the diluted samples were introduced to the existing 100 μL of cell cultures, the final concentration of extracts ranged from 1.56-25.0 μg/mL. The control cultures were treated with the same volume of DMSO (0.5%). The concentration of DMSO was kept within 1% to avoid any interference with cell viability. After the addition of extracts, the plates were incubated for 48 h. After incubation, the cells were fixed with 50 μL of cold 50% TCA (w/v) and incubated for 1 h at 4°C. The plates were then washed with water and air dried. Cells were stained with 100 μL of 0.4% (w/v) SRB solution diluted with 1% acetic acid followed by incubation for 10 min at room temperature. Unbound dye was removed by washing with 1% acetic acid. Bound stain was then solubilised with 200 μL of 10 mM trizma base.
Absorbance of each well at 505 nm was obtained using a microplate reader. Dose-response curves were plotted to obtain GI50, calculated using the formula [(Tz- Ti)/(Tc- Ti)] ×100 = 50 where Tz is the absorbance of cells treated with extracts or drugs at the end of incubation, Ti is the absorbance of cells prior to treatment with extracts or drugs and Tc is the absorbance of untreated cells treated with vehicle (0.5-1.0% DMSO) at the end of incubation. APF activity is considered to be effective when GI50 value ≤20 μg/mL.[8]
Isolation of compounds
DCM leaf extract (26 gm) of Vallaris glabra was subjected to column chromatography over Silica gel 60. A solvent system with an increasing polarity gradient was used: Hexane-ethyl acetate (Hex-EtAc) and ethyl acetate-methanol (EtAc-MeOH) to give 13 fractions (D1 to D13).
Fraction D11 (3.26 gm) was passed through Sephadex LH-20 with gradient H2O-MeOH (50→100% MeOH) to give five sub-fractions (D11-1 to D11-5). Sub-fraction D11-1 (1.38 gm) was then subjected to Chromatorex C18 with gradient H2O-MeOH (10→100% MeOH) to give six sub-fractions (D11-1-1 to D11-1-6). Sub-fraction D11-1-5 (0.27 gm) was passed through Sephadex LH-20 with gradient CHCl3-EtAc (0→100% EtAc) to give three sub-fractions (D11-1-5-1 to D11-1-5-3). Sub-fraction D11-1-5-2 (0.16 gm) was passed through Sephadex LH-20 with gradient CHCl3-EtAc (0→50% EtAc) to give six sub-fractions (D11-1-5-2-1 to D11-1-5-2-6). Sub-fraction D11-1-5-2-2 (86 mg) was subjected to Chromatorex C18 with gradient H2O-MeOH (10→100% MeOH) to obtain Compound 1 (12 mg).
Fraction D8 of the DCM leaf extract (2.61 gm) was passed through Silica gel 60 using a solvent system of Hex-DCM (20→100% DCM) followed by DCM-Acetone (0→100% Acetone) and finally Acetone-MeOH (0→100% MeOH) to give 27 sub-fractions (D8-1 to D8-27). Sub-fraction D8-19 (0.71 gm) was subjected to Silica gel 60 chromatography by eluting with DCM-EtAc (0→100% EtAc), followed by EtAc-Acetone (0→100% Acetone) and finally Acetone-MeOH (0→100% MeOH) to give 16 sub-fractions (D8-19-1 to D8-19-16). Sub-fraction D8-19-6 (0.09 gm) was again chromatographed on Silica gel 60 with Hex-EtAc (10→100% EtAc) to obtain six sub-fractions (D8-19-6-1 to D8-19-6-6). Compound 2 (0.01 gm) was precipitated from sub-fraction D8-19-6-4 (0.02 gm) by dissolving in chloroform with sonication and heat (40°C) followed by chilling overnight at -20°C. The precipitate was recovered by filtration.
Identification of compounds
Compounds were dissolved in a deuterated solvent and subjected to 1H and 13C NMR analysis using a Bruker DRX 300 MHz spectrometer (300 MHz for 1H and 75.4 MHz for 13C) and a Varian Unity Inova 500 MHz spectrometer (500 MHz for 1H and 125.7 MHz for 13C). Chemical shifts were recorded in ppm (δ) using TMS as internal standard. MS analysis was performed by APCI-MS in negative mode using a Thermo Finnigan LCQ Deca spectrometer and EI-MS using a Thermo Finnigan Trace GC-PolarisQ spectrometer.
Hoechst nuclear staining
MDA-MB-231 breast cancer cells treated with DCM leaf extract and stained with Hoechst 33342 dye (Sigma Chemical Co., St. Louis, MO, USA) was performed following protocols previously described.[7] Visualisation of nuclear morphology was performed using Hoechst 33342, a DNA-binding fluorescent dye. MDA-MB-231 breast cancer cells were cultured in 3.5 mm cell-culture dishes with a cover slip placed into each of the dishes and sterilized under UV radiation for 15 min. After culturing of cells overnight, they were treated for 48 h with 1% DMSO as control and with varying concentrations of leaf extract at 1.56, 3.13, 6.25, 12.5, 25.0 and 50.0 μg/mL. After removing the cell culture media, the dishes and cover slips were washed twice with 10% PBS followed by fixing the cells to the cover slips with 3.7% paraformaldehye (v/v) for 10 min. The fixing solution was removed and the dishes and cover slips were washed twice with 10% phosphate-buffered saline (PBS). Hoechst 33342 (1 μg/mL) was added directly to the affixed cells and stained in the dark for 10 min before washing twice with 10% PBS. The cover slips were carefully lifted from the dishes, placed onto glass slides with 10 μL of Gel Mount aqueous mounting medium (Sigma) and sealed with nail varnish. The stains cells were visualized at ×40 magnification using an inverted microscope (Olympus BX41) and a UV filter. Images of cells were photographed by a digital Nikon DS-Fi1c camera using NIS-Elements F software version 3.00.
Caspase colorimetry
MDA-MB-231 cells were treated with varying concentrations (1.56-25.0 μg/mL) of DCM leaf extract. The floating and trypsinised-adherent cells were collected and washed in cold PBS. The cells were lysed by adding 50 μL chilled lysis buffer (BioVision, USA) and incubated on ice for 10 min. The suspension was then centrifuged for 1 min at 10,000 × g. The supernatant containing the cytosolic extract was transferred to a fresh tube and put on ice for assay or stored in aliquots at -80°C for future use.
The protein concentration was determined using the microassay procedure adopted by the manufacturer (Bio-Rad). Protein standards (125-1500 μg/mL) using bovine serum albumin (BSA) were prepared. The standards and samples of protein cell lysates in various dilutions (5 μL) were added into each well of a 96-well microtiter plate. Bio-Rad dye reagent concentrate (250 μL) was added into each well. Control consisted of water in place of the protein sample. The plate was incubated in the dark at room temperature for 30 min before reading absorbance at 595 nm.
Activities of caspase-3, -6, -8 and -9 were determined using the Caspase Colorimetric Assay Kit (BioVision Research, Mountain View, CA, USA) according to the instructions of the manufacturer. Cytosolic protein (200 μg) was diluted to 50 μL cell lysis buffer for each assay in a 96-well plate. To each well, 50 μL of reaction buffer containing 10 mM dithiothreitol (DTT) was added followed by 5 μL of substrate containing 4 mM Asp-Glu-Val-Asp (DEVD)-pNA substrate (caspase-3), 4 mM Val-Glu-Ile-Asp (VEID)-pNA substrate (caspase-6), 4 mM Ile-Glu-Thr-Asp (IETD)-pNA substrate (caspase-8), or 4 mM Leu-Glu-His-Asp (LEHD)-pNA substrate (caspase-9). The plate was then incubated at 37°C for 2 h. At the end of incubation, the plate was read in a microtitre plate reader at 412 nm. Fold-increase in caspase activity was determined by comparing the results of treated samples with those of the control.
RESULTS AND DISCUSSION
APF activity of extracts
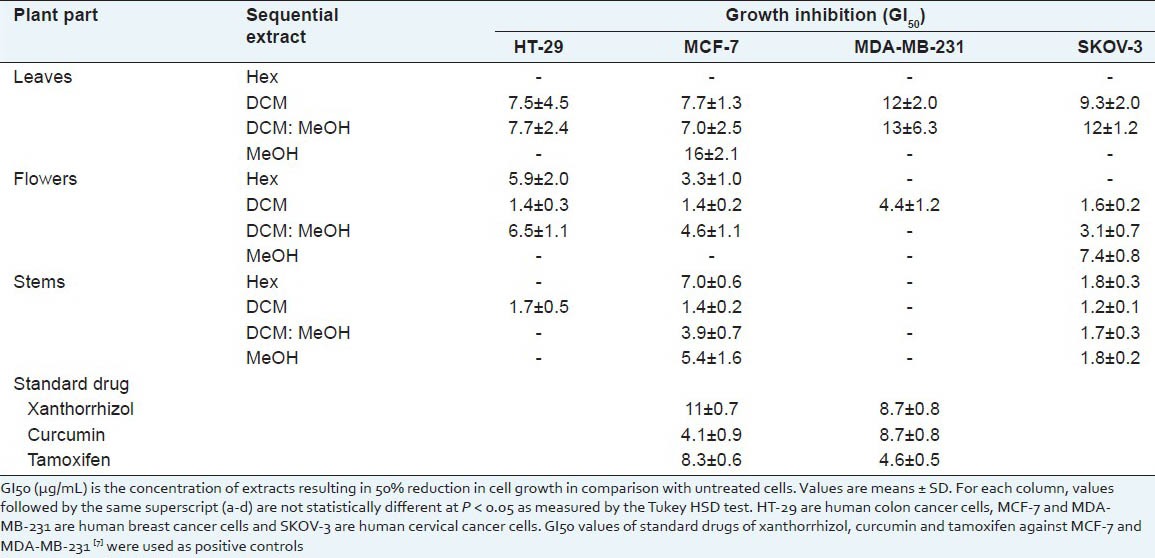
Table 1 shows that both DCM extracts of leaves and flowers possessed more broad-spectrum APF activity than stems against HT-29, MCF-7, MDA-MB-231 and SKOV-3 cancer cells. DCM extract of stems and flowers were the most potent against MCF-7 cells compared to the other three sequential extracts. GI50 of DCM extract of stems and flowers (both 1.4 ± 0.2 μg/mL) for MCF-7 cells was lower than that of the positive controls. Hexane extract of stems (7.0 ± 0.6 μg/mL) and flowers (3.3 ± 1.0 μg/mL) were more potent against MCF-7 cells than tamoxifen (8.3 ± 0.6 μg/mL) and xanthorrhizol (11 ± 0.7 μg/mL). Hexane flower extract was as effective as curcumin (4.1 ± 0.9 μg/mL). The inhibition of stem extracts was more specific to MCF-7 and SKOV-3 cells. Overall, the screening results show that extracts from three different parts of V. glabra possessed effective APF activity.
Table 1.
Antiproliferative activity of sequential extracts of leaves, flowers and stems of Vallaris glabra against four human cancer cell lines
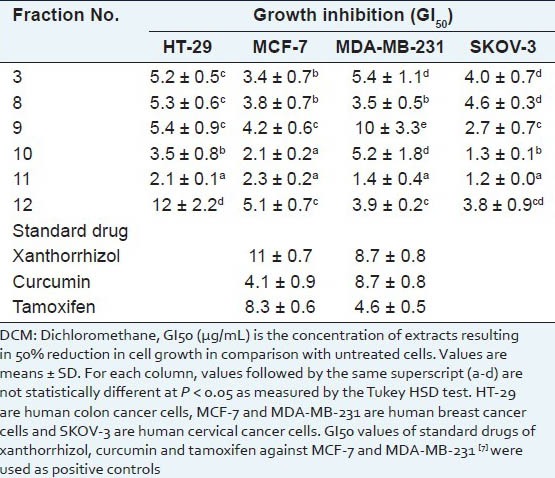
It was found that further fractionation of the DCM extract using silica gel 60 column and a mobile phase with increasing polarity resulted in much more potent APF activity against the four cancer cell lines [Table 2]. Strongest APF activity was observed in Fractions 10 and 11. Against MCF-7 cells, GI50 of Fractions 10 and 11 (2.1 ± 0.2 and 2.3 ± 0.2 μg/mL) was significantly lower than xanthorrhizol (11 ± 0.7 μg/ml), curcumin (4.1 ± 0.9 μg/mL) and tamoxifen (8.3 ± 0.6 μg/mL). Against MDA-MB-231 cells, GI50 of Fraction 11 (1.4 ± 0.4 μg/mL) was significantly lower than xanthorrhizol (8.7 ± 0.8 μg/mL), curcumin (8.7 ± 0.8 μg/mL) and tamoxifen (4.6 ± 0.5 μg/mL). GI50 of Fraction 10 (5.2 ± 1.8 μg/mL) was lower than xanthorrhizol and curcumin, but comparable to tamoxifen.
Table 2.
Antiproliferative activity of fractions from DCM leaf extract
Identification of compounds
Two compounds were isolated from DCM leaf extract of V. glabra. Their appearance, MS and 1H and 13C NMR spectral data are as follows:
Compound 1: stearic acid
Amorphous waxy white solid; EI-MS m/z 284 [M+]; 1H NMR (CDCl3, 300 MHz) δ: 2.37 (2H, t, J = 7.5 Hz, H-2), 1.54 (2H, m, H-3), 1.25–1.33 (28H, m, H4 to H17), 0.85 (3H, t, J = 6.9 Hz, H-18); 13C NMR (CDCl3, 75.4 MHz) δ: 180 (COO), 34.2 (C-2), 24.7 (C-3), 29.4 (C-4), 29.2–32.2 (C-5 to C-16), 22.7 (C-17), 14.1 (C-18).
Compound 2: ursolic acid
Amorphous white powder; APCI-MS m/z 456 [M-H]−; 1H NMR (CD3OD, 500 MHz) δ: 5.23 (1H, t, J = 3.5 Hz, H-12), 3.15 (1H, dd, J = 11.5, 4.5 Hz, H-3), 2.21 (1H, d, J = 11.0 Hz, H-18), 1.08 (3H, s, H-23), 0.98 (3H, s, H-27), 0.97 (3H, s, H-26), 0.96 (3H, s, H-24), 0.89 (3H, d, J = 6.5 Hz, H-29), 0.85 (3H, d, J = 6.5 Hz, H-30), 0.78 (3H, s, H-25); 13C NMR (CD3OD, 125.7 MHz) δ: 182 (C-28), 140 (C-13), 127 (C-12), 79.7 (C-3), 56.8 (C-5), 54.4 (C-18), 48.5 (C-9 and C-17), 43.3 (C-14), 40.8 (C-8), 40.4 (C-19 and C-20), 40.0 (C-4), 39.9 (C-1), 38.1 (C-10 and C-22), 34.4 (C-7), 31.8 (C-21), 29.2 (C-15), 28.8 (C-23), 27.9 (C-2), 25.3 (C-16), 24.4 (C-11), 24.1 (C-27), 21.6 (C-30), 19.5 (C-6), 17.8 (C-29), 17.7 (C-26), 16.4 (C-25), 16.1 (C-24).
The MS and the 1H and 13C NMR data of the above two compounds matched those of the previously reported data on stearic acid[9,10] and ursolic acid.[11,12] Stearic acid or octadecanoic acid (IUPAC name) is a saturated fatty acid with an 18-carbon chain. Its molecular formula is C18H36O2 and molecular weight is 284.5. Ursolic acid is a pentacyclic triterpene acid. Its molecular formula is C30H48O3 and molecular weight is 456.7.
Stearic acid (SA) isolated from leaves of Vallaris glabra is a major essential oil found in Apocynaceae species such as leaves and flowers of Catharanthus roseus,[13] and leaves of Alstonia boonei.[14] Ursolic acid (UA), widely found in plants, is a compound that possesses many biological activities such as antioxidative, anti-inflammatory, anticancer and hepatoprotective, as well as the ability to induce apoptosis.[15] In Apocynaceae, it has been isolated from species such as Alstonia scholaris[16] and Plumeria rubra.[17]
Other compounds isolated from leaf extracts of V. glabra included three caffeoylquinic acids (CQAs) and two cardiac glycosides.[18,19] Isolated from MeOH leaf extract of V. glabra, the CQAs were identified as 3-CQA, 4-CQA and 5-CQA or chlorogenic acid (CGA).[18] Compared to flowers of Lonicera japonica (the commercial source of CGA), 5-CQA content was two times higher and 3-CQA and 4-CQA content was about 16 times higher. One of the two cardiac glycosides isolated wasidentified as acoschimperoside P, 2’-acetate. This compound was active in Hedgehog signaling inhibition and showed strong cytotoxicity against human pancreatic (PANC1) and human prostate (DU145) cancer cells.[19] The other compound was a new cardiac glycoside.
APF activity of isolated compounds
Results showed that SA isolated from DCM leaf extract displayed weak inhibitory activity against HT-29 (59 ± 6.0 μg/mL), MCF-7 (102 ± 16 μg/mL), MDA-MB-231 (73 ± 8.8 μg/mL) and SKOV-3 (78 ± 4.8 μg/mL) cancer cells. Against MCF-7 and MDA-MB-231, SA had much higher GI50 values of 49 ± 6 and 31 ± 4 μg/mL, respectively than standards such as curcumin (4.1 ± 0.9 and 8.7 ± 0.8 μg/mL) and tamoxifen (8.3 ± 0.6 and 4.6 ± 0.5 μg/mL), respectively.[7] Previous studies on the APF properties of SA have reported variable results. SA had no APF effect on 14 human leukemic cell lines.[20] Using clonogenic assay, SA was found to be toxic against five human cancer cell lines,[21] one of which was HT-29 with IC50 of 7.65 μg/mL, which is in variance with our value of 59 μg/mL. The discrepancy could be due to the different methods used in assessing APF activity. Against HOG-1 cervical cancer cells, SA at 21.3 μg/mLinhibited the growth of HOG-1 cells after 48 h in culture and at 35.6 μg/mL, cell death occurred.[22] SA isolated from aerial parts of Oldenlandia diffusa inhibited the growth of human hepatoma cells of HepG2 and Hep3B, but not human normal cells of WRL-68.[23] Against HepG2 (IC50 of 25.6 μg/mL) and Hep3B (IC50 of 37.0 μg/mL), SA was more effective than other fatty acids.
The yield of UA isolated from DCM extract (only 0.01 gm) was too low for analysis of its inhibitory activity. Nevertheless, there is a wealth of literature reports on the anticancer properties of UA, including those of the cancer cells used in this study. Most of these reports concluded that UA is a potent chemo-preventive agent for cancer.
UA was reported to be a potent inhibitor of MCF-7 cell proliferation, exerting an early cytostatic response at G1 phase of cell cycle followed by cell death.[24] UA induced apoptosis, PARP cleavage and also decreased Bcl-2 protein in MCF-7 cells, supporting the hypothesis that UA induces apoptosis through the intrinsic mitochondrial pathway.[25] Against MDA-MB-231 breast cancer cells, UA was reported to exert a dose- and time-dependent inhibitory effect on the migration and invasion of MDA-MB-231 breast cancer cells at non-cytotoxic concentrations.[26] UA isolated from rosemary has been shown to inhibit cell proliferation and induce apoptosis in MDA-MB-231.[27] The study showed that apoptosis was induced through both mitochondrial death pathway and extrinsic death receptor dependent pathway in MDA-MB-231 cells. Against HT-29 colon cancer cells, UA has been reported to have strong antiproliferative and apoptotic effects.[28] Another study showed that UA inhibited cell growth by inhibiting the EGFR/MAPK pathway.[29] Against SKOV-3 ovary cancer cells, UA exerted cytotoxicity with IC50 of 50 μM.[30] Apoptotic bodies were observed in treated SKOV-3 cells. The cytotoxic activity of some UA derivatives was stronger than UA against SKOV-3 cancer cells.[31]
Hoechst nuclear staining
To examine the nuclear morphological changes in response to DCM leaf extract, which contained SA and UA, both control and treated MDA-MB-231 breast cancer cells were stained with fluorescent Hoechst 33342 dye and visualised under a fluorescent microscope. MDA-MB-231 cells were chosen as a candidate for apoptotic studies due to its fast doubling rate and have been demonstrated to undergo apoptosis. MDA-MB-231 cells have high invasive ability and tend to establish resistance against programmed cell death stimuli.[7] Thus, the ability of plant extracts to arrest their proliferation is beneficial for the prevention and treatment of invasive cancer.
Results of Hoechst staining of control and treated cells showed that the dye was able to diffuse through intact membranes of the cells and stain their DNA. At 1.56, 3.13 and 6.25 μg/mL of leaf extract, the cells remained uniformly stained and showed no fluorescence in the nucleus. The cells were neither apoptotic nor exhibit fragmentation of DNA.
MDA-MB-231 cancer cells treated with higher concentrations of DCM leaf extract (12.5, 25.0 and 50.0 μg/mL) began to display apoptotic morphology [Figure 2]. As the concentration of leaf extract was increased, there was stronger and more intense fluorescence. Fluorescence was seen in the nuclear region of the cells, indicating the presence of DNA fragmentation. Cell shrinkage, chromatin condensation, nuclear fragmentation and apoptotic bodies (small highly fluorescent spherical masses) were visualised. These observations provided evidence that the DCM leaf extract had an apoptotic effect on MDA-MB-231 cells.
Figure 2.
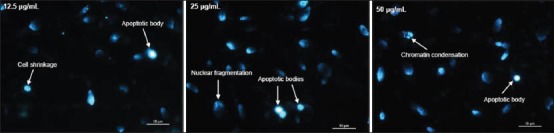
DCM leaf extract of Vallaris glabra at 12.5, 25.0 and 50.0 ìg/mL showed apoptotic effects on treated MDA-MB-231 breast cancer cells
Caspase colorimetry
MDA-MB-231 cells were treated with varying concentrations (1.56-25.0 μg/mL) of DCM leaf extract. Fold-increase in activity of caspase-3, -6, -8 and -9 was determined by comparing the results of treated samples with the control. A graph plotting concentration of DCM leaf extract against fold-increase in caspase activity is shown in Figure 3.
Figure 3.
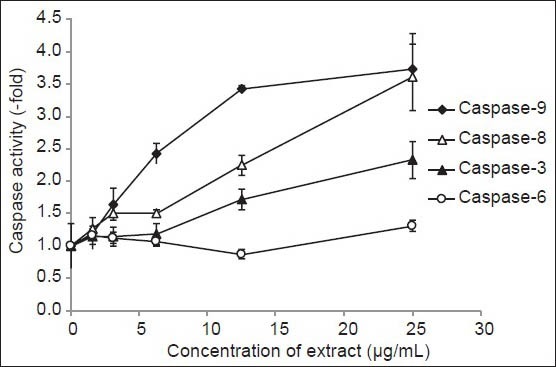
Graph of fold-increase in the activity of caspase-3, -6, -8 and 9 vs. concentration of DCM leaf extract of Vallaris glabra
Two caspases analysed showed fairly strong fold-increase activity with increasing concentration of DCM leaf extract. They were caspase-8 and -9. At 3.13 and 6.25 μg/mL of extract, caspase-8 remained at 1.50 fold. At 12.5 and 25.0 μg/mL of extract, activity increased 2.25 and 3.61 fold, respectively. Caspase-9 showed a slightly different trend of increase. At 3.13, 6.25 and 12.5 μg/mL of extract, activity strongly increased 1.64, 2.42 and 3.42 fold, respectively. Activity culminated to 3.73 fold at 25.0 μg/mL of extract. Fold-increase activity of caspase-3 was weak with caspase-6 hardly showing any activity. Caspase-3 showed no discernable activity at 3.13 and 6.25 μg/mL of extract. Activity was 1.72 and 2.33 fold at 12.5 and 25.0 μg/mL of extract. Caspase-6 showed slight decline in activity from 3.13-12.5 μg/mL of extract. Activity was only 1.31 fold at 25.0 μg/mL of extract.
Results of the caspase colorimetric assay showed that DCM leaf extract modulated caspase-8, -9 and -3 but not caspase-6, which indicates that the extract induced apoptosis through both the extrinsic (death-receptor) and intrinsic (mitochondrial) pathways. Caspase-8 and -9 are the initiator caspases, while caspase-3 and -6 are the effector caspases.
Similarly, an increase in caspase-8, -9 and -3 activities has been reported after MDA-MB-231 cancer cells were treated with 250 μM of quercetin,[32] and with 40 mM of metformin.[33] Likewise, the induction of apoptosis of MDA-MB-231 cells involving caspase-8, -9 and -3 was also reported for sanguinarine[34] and 2-methoxyestradiol.[35] On the contrary, MDA-MB-231 cancer cells treated with xanthorrhizol (0-20 μg/mL) was reported to modulate caspase-3 and -9 activity but not caspase-6 and -8.[7]
CONCLUSION
Both leaf and flower extracts of V. glabra showed broad-spectrum APF activity. Among the four different leaf extracts of V. glabra, the DCM extract showed strongest APF activity against HT-29, MCF-7, MDA-MB-231 and SKOV-3 cancer cells. The DCM extract had an apoptotic effect on MDA-MB-231 cells which involved activation of caspase-8, -9 and -3, but not caspase-6. The potential of V. glabra as a candidate species for anticancer drugs warrants further investigation.
ACKNOWLEDGEMENTS
This study is partly funded by the Fundamental Research Grant Scheme of the Ministry of Higher Learning of Malaysia. The authors are thankful to the Ministry for providing the research grant. The support of Monash University Sunway Campus, Forest Research Institute Malaysia and UCSI University is gratefully acknowledged.
Footnotes
Source of Support: None
Conflict of Interest: None declared.
REFERENCES
- 1.Wiart C. Boca Raton: CRC Press/Taylor and Francis; 2006. Medicinal Plants of Asia and the Pacific; pp. 245–60. [Google Scholar]
- 2.Endress ME, Bruyns PV. A revised classification of the Apocynaceae. Bot Rev. 2000;66:1–56. [Google Scholar]
- 3.Wong SK, Lim YY, Abdullah NR, Nordin FJ. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Pharmacogn Res. 2011;3:100–6. doi: 10.4103/0974-8490.81957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SK, Lim YY, Abdullah NR, Nordin FJ. Assessment of anti-proliferative and antiplasmodial activities of five selected Apocynaceae species. BMC Complement Altern Med. 2011;11:3. doi: 10.1186/1472-6882-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SK, Lim YY, Chan EWC. Botany, uses, phytochemistry and pharmacology of selected Apocynaceae species: A review. Pharmacogn Commun. 2013;3:2–11. [Google Scholar]
- 6.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 7.Cheah YH, Nordin FJ, Tee TT, Azimahtol HL, Abdullah NR, Ibrahim Z. Anti-proliferative property and apoptotic effect of xanthorrhizol on MDA-MB-231 breast cancer cells. Anticancer Res. 2008;28:3677–90. [PubMed] [Google Scholar]
- 8.Gaidhani SN, Lavekar GS, Juvekar AS, Sen S, Singh A, Kumari S. In-vitro anticancer activity of standard extracts used in Ayurveda. Pharmacogn Mag. 2009;5:425–9. [Google Scholar]
- 9.Dinda B, Guha S. Chemical constituents of Spilanthes paniculata. J Indian Chem Soc. 1988;65:525–6. [Google Scholar]
- 10.Li FS, Yan DL, Liu RR, Xu KP, Tan GS. Chemical constituents of Boswellia carterii (Frankincense) Chin J Nat Med. 2010;8:25–7. [Google Scholar]
- 11.Hamzah AS, Lajis NH. Chemical constituents of Hedyotis herbacea. ASEAN Rev Biodivers Environ Conserv. 1998;2:1–6. [Google Scholar]
- 12.Park YS, Jang HJ, Paik YS. Prolyl endopeptidase inhibitory activity of ursolic and oleanolic acids from Corni fructus. Agric Chem Biotechnol. 2005;48:207–12. [Google Scholar]
- 13.Pandey-Rai S, Mallavarapu GR, Naqvi AA, Yadav A, Rai SK, Srivastava S, et al. Volatile components of leaves and flowers of periwinkle Catharanthus roseus (L.). G. Don from New Delhi. Flavour Frag J. 2006;21:427–30. [Google Scholar]
- 14.Okwu DE, Ighodaro BU. GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild. Der Pharm Chem. 2010;2:261–72. [Google Scholar]
- 15.Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, et al. Inhibition of cytochro P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004;74:2769–79. doi: 10.1016/j.lfs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Shetty P, Mangaonkar K, Sane RT. HPTLC determination of ursolic acid in Alstonia scholaris R Br. J Planar Chromatogr Mod TLC. 2007;20:65–8. [Google Scholar]
- 17.Silva JA, Silva AG, Alves AS, Reis R, Nascimento CC, Diré GF, et al. Plumeria rubra (Apocynaceae):A good source of ursolic acid. J Med Plant Res. 2013;7:892–6. [Google Scholar]
- 18.Wong SK, Lim YY, Ling SK, Chan EW. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn Res. 2013 doi: 10.4103/0974-8490.122921. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rifai Y, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Acoschimperoside P, 2’-acetate: A hedgehog signaling inhibitory constituent from Vallaris glabra. J Nat Med. 2011;65:629–32. doi: 10.1007/s11418-011-0530-1. [DOI] [PubMed] [Google Scholar]
- 20.Finstad HS, Wik Myhrstad MC, Heimli H, Lømo J, Kiil Blomhoff H, Kolset SO, et al. Multiplication and death-type of leukemia cell lines exposed to very long-chain polyunsaturated fatty acids. Leukemia. 1998;12:921–9. doi: 10.1038/sj.leu.2401030. [DOI] [PubMed] [Google Scholar]
- 21.Fermor BF, Masters JR, Wood CB, Miller J, Apostolov K, Habib NA. Fatty acid composition of normal and malignant cells and cytotoxicity of stearic, oleic and sterculic acids in vitro. Eur J Cancer. 1992;28:1143–7. doi: 10.1016/0959-8049(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson RP, Ayub M, Wright JT, Wood CB, Habib NA, Soutter WP, et al. Fatty acid control of growth of human cervical and endometrial cancer cells. Br J Cancer. 1990;61:500–3. doi: 10.1038/bjc.1990.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang PM, Zhang LD, Chan JY, Lee JK, Liu C, Au SW, et al. Apoptotic effects of stearic acid, an active compound isolated from Oldenlandia diffusa, on human hepatoma cells. Res J Med Sci. 2007;1:30–8. [Google Scholar]
- 24.Es-Saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C. MCF-7 cell cycle arrested at G1 through ursolic acid and increased reduction of tetrazolium salts. Anticancer Res. 1996;16:481–6. [PubMed] [Google Scholar]
- 25.Kassi E, Sourlingas TG, Spiliotaki M, Papouts Z, Pratsinis H, Moutsatsou P. Ursolic acid triggers apoptosis and Bcl-2 down-regulation in MCF-7 breast cancer cells. Cancer Invest. 2009;27:723–33. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 26.Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res. 2010;54:1–11. doi: 10.1002/mnfr.200900414. [DOI] [PubMed] [Google Scholar]
- 27.Kim KH, Seo HS, Choi HS, Choi IH, Shin YC, Ko SG. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch Pharm Res. 2011;34:1363–72. doi: 10.1007/s12272-011-0817-5. [DOI] [PubMed] [Google Scholar]
- 28.Andersson D, Liu JJ, Nilsson A, Duan RD. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res. 2003;23:3317–22. [PubMed] [Google Scholar]
- 29.Shan JZ, Xuan YY, Zheng S, Dong Q, Zhang SZ. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B. 2009;10:668–74. doi: 10.1631/jzus.B0920149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song YH, Jeong SJ, Kwon HY, Kim BL, Kim SH, Yoo DY. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SKOV-3 ovarian cancer cells. Biol Pharm Bull. 2012;35:1022–8. doi: 10.1248/bpb.b110660. [DOI] [PubMed] [Google Scholar]
- 31.Meng Y, Song Y, Yan Z, Xia Y. Synthesis and in vitro cytotoxicity of novel ursolic acid derivatives. Molecules. 2010;15:4033–40. doi: 10.3390/molecules15064033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, et al. Quercetin-induced apoptosis acts through mitochondrial and caspase-3 dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2009;28:493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 33.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 34.Choi WY, Kim GY, Lee WH, Choi YH. Sanguinarine, a benzophenanthridine alkaloid, induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy. 2008;54:279–87. doi: 10.1159/000149719. [DOI] [PubMed] [Google Scholar]
- 35.LaVallee TM, Zhan XH, Johnson MS, Herbstritt CJ, Swartz G, Williams MS, et al. 2-Methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003;63:468–75. [PubMed] [Google Scholar]


