Abstract
Background:
Paris quadrifolia L. is a medicinal plant which contains steroidal saponins. The present study reports isolation and structural identification of six pennogenyl saponins obtained from P. quadrifolia rhizomes. The four spirostan saponins were obtained from P. quadrifolia for the first time. The cytotoxic effects of the sub-fractions and six compounds isolated from the plant extract were evaluated on tumour cells.
Materials and Methods:
Ethanol extract from the rhizomes of P. quadrifolia were partinioned using column chromatography. The saponins were isolated from the obtained sub-fractions by isocratic RP HPLC and their structures were determined by means of 1D and 2D NMR spectroscopy and MALDI TOF MS. The cytotoxic effects of the sub-fractions and the isolated compounds were tested against human promyelocytic leukaemia cells (HL-60), human cervical adenocarcinoma cells (HeLa) and human breast cancer cells (MCF-7) using the [(3-(4,5-dimethylthiazol-2-yl)]-2,5-diphenyltetrazolium bromide (MTT) assay.
Results:
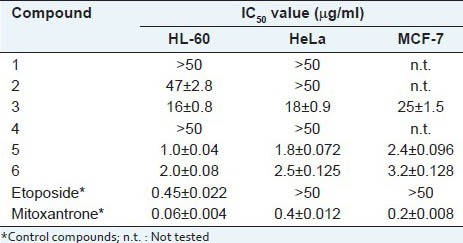
Six pennogenyl saponins were isolated from P. quadrifolia rhizomes: pennogenin 3-O-β-D-glucopyranoside (1), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (2), pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (3), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (4), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (5), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (6). Pennogenyl saponins 5 and 6 exhibited cytotoxic activity against HL-60, HeLa and MCF-7 tumour cells with IC50 values of 1.0 ± 0.04 μg/ml, 1.8 ± 0.072 μg/ml and 2.4 ± 0.096 μg/ml respectively, and 2.0 ± 0.08 μg/ml, 2.5 ± 0.125 μg/ml and 3.2 ± 0.128 μg/ml respectively.
Conclusion:
Compounds 1-4 were isolated from this species for the first time.
Keywords: Cytotoxicity, Paris quadrifolia, pennogenin, saponins, structure elucidation
INTRODUCTION
The genus Paris (Melanthiaceae) includes 24 species of plants, which grow in an extensive area from Europe to Asia. Apart from the European Paris quadrifolia and the Caucasian P. incompleta, the other 22 species of Paris grow in western Asia, western Siberia and the Himalayas.[1] The plant occurs in deciduous forests in Poland and studies of this species are conducted at present.[2,3]
The Paris species contain a wide range of steroidal compounds which are potential cytotoxic agents. Many articles describe species of Paris widespread in the Far East as plants of traditional Chinese medicine. The objects of those studies were mainly P. polyphylla var. chinensis and P. polyphylla var. yunnanensis.[4,5,6,7,8]
In this work, we present isolation of six pennogenyl saponins from P. quadrifolia rhizomes and determination of their structures: pennogenin 3-O-β-D-glucopyranoside (1), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (2), pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (3), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (4), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (5), pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (6). Earlier, these substances had been identified in other species of Paris[9,10,11] and other genera/species: Trillium,[12,13,14,15,16,17,18] Heloniopsis orientalis,[19,20] Polygonatum kingianum,[21] Trachycarpus wagnerianus,[22] Dioscorea,[23,24] Triteleia lactea,[25] Majanthemum dilatatum,[26] Ophiopogon japonicus,[27] Dracaena mannii,[28] Ypsilandra thibetica.[29] But the six saponins mentioned above have not been isolated at the same time from one species of Paris. Except saponins 5 and 6,[30,31] the spirostan saponins 1, 2, 3, 4 from P. quadrifolia rhizomes were obtained from this plant for the first time.
The cytotoxic effects of fractions obtained from P. quadrifolia extract and the six mentioned compounds were examined in vitro on HL-60, HeLa and MCF-7 tumour cells. Saponins 5 and 6 showed significant cytotoxic activity against the cells.
MATERIALS AND METHODS
General experimental procedures
Column chromatography was performed on a column (35 × 4.8 cm) with silica gel (Kieselgel 60; 0.05-0.2 mm; Macherey-Nagel). Thin layer chromatography (TLC) was carried out on precoated Kieselgel 60 (Merck). Semi-preparative high-performance liquid chromatography (HPLC), isocratic separations were run with the use of a Gradient Pump (Pharmacia LKB), Econosil C18 column (250 × 10 mm; 10 μm; Alltech) connected to a VYDAC C18 guard column; KNAUER injector with a loop of 200 μl. The elution profile was monitored with a differential refraction detector RIDK 102 (Laboratorni Pristroje Praha).
D and L configurations of sugar components were assigned as previously described.[32,33,34]
Gas liquid chromatography (GLC) analyses were performed on a TOP GC 8000 (CE Instruments) gas chromatograph equipped with a flame ionization detector (FID) and a DB-23 fused-silica capillary column (60 m, 0.3 mm I.D., 0.15 μm film thickness, JandW scientific).
Mass spectra of all saponins were recorded in ethanol solutions on a Bruker BIFLEX III MALDI TOF mass spectrometer equipped with a nitrogen laser (λ = 337 nm) in a DHB matrix (2,5-dihydroxybenzoic acid). The spectra were recorded in positive mode in range of m/z 400-2500 amu (averages of 250 to 450 acquisitions) with a pulse width of 3ns, and an energy density of 106 to 107 W cm-2. A mixture of peptides was used as the calibration standard. All nuclear magnetic resonance (NMR) spectra were acquired on a Bruker Avance III 700 MHz spectrometer at 27°C in C6D5N, and calibrated according to Transcranial magnetic stimulation tetramethylsilane (TMS).
Plant material
P. quadrifolia L. was collected at Gdańsk (Poland, 54° 21’69”N, 18° 33’24”E). The fresh rhizomes of the plant were dried at room temperature. A voucher specimen has been deposited in the Herbarium of the Medical University of Gdańsk (GDMA herbarium).
Extraction and isolation
Dried plant rhizomes (410 g) were incubated with distilled water at 40°C for 24 h, extracted with 96% ethanol for 25 h at room temperature, then ethanol was evaporated using a vacuum evaporator (40°C). Next, water was added to the residue and the whole was frozen and lyophilised yielding 80.1 g of extract. Obtained sample was defatted by petroleum ether yielding 78.2 g of degreased material. n-Butanol/water extraction was performed for part of this material (71 g). Each 10 g of lyophilisate were mixed with 250 ml of distilled water and extracted with 80 ml of n-butanol three times. Butanol layers were collected and n-butanol was removed at low pressure using a rotatory evaporator at 35°C. The extract was placed in water, frozen, and lyophilised giving 7.5 g of material. A solution of the extract (40 ml) in a mixture of CHCl3/CH3OH/H2O (72 v: 18 v:1.8 v) was transferred to the silica gel Kieselgel 60 column and separated into fractions by gradient flash chromatography. Elution was conducted with a mixture of CHCl3/CH3OH/H2O with gradually increasing volumes of methanol (72v: 18v: 1.8v; 63v: 27v: 2v; 54v: 36v: 2.5v; 45v: 45V: 3v; 27v: 63v: 4v; 0v: 100v: 0v respectively) with the flow rate of the mobile phase 15-18 ml/min.
The presence of saponins in the eluents was monitored by TLC on precoated Kieselgel 60 plates developed with CHCl3/MeOH/H2O (7v/3v/0.5v). The chromatograms were visualised with Liebermann-Burchard reagent (Ac2O/CHCl3/H2SO4 at 20v/50v/1v) and heated at 90°C for 10 min.
Single eluents of 10 ml from the column chromatography of similar composition were combined, which resulted in 11 sub-fractions. Organic solvents were removed at low pressure in a rotatory evaporator at 35°C. Distilled water was added to each sub-fraction and the aqueous suspensions obtained were frozen and freeze-dried, which yielded the following: 7-25-954.3 mg, 26-71-1934.1 mg, 72-85-247 mg, 86-92-129.7 mg, 93-103-415.8 mg, 104-109-149.1 mg, 110-117-86.5 mg, 118-136-15.2 mg, 137-159-100.5 mg, 160-186-288.3 mg, 187-198-96.8 mg. The saponins from each fraction were isolated by isocratic reversed-phase high-performance liquid chromatography (RP HPLC) using mobile phase MeOH/CH3CN/H2O 32v: 25v: 25v and flow rate 3.8 ml/min. The mobile phase was removed (low pressure evaporation at 40°C), then the saponins were suspended in water, frozen and lyophilised. No pennogenyl saponins were found in the last four sub-fractions. MALDI TOF and NMR spectra of all saponins were recorded [Figures A.1. and B.1. Appendix].
Figure A.1.
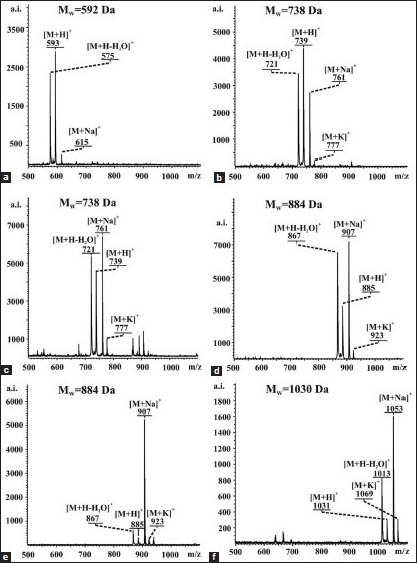
Maldi toff ms spectra of the saponins isolated from rhizomes of Paris quadrifolia L.: a)-1, b)-2, c)-3, d)-4, e)-5, f)-6
Figure B.1.
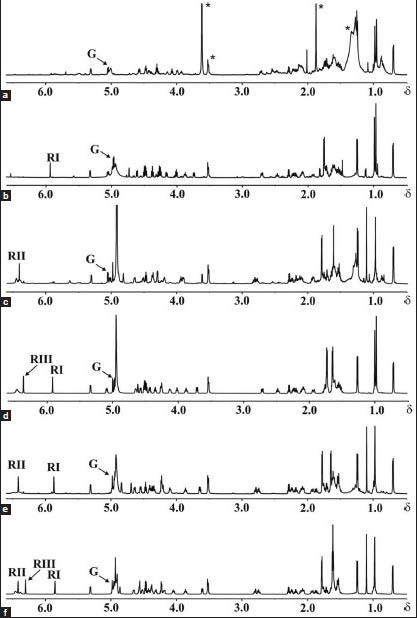
1H NMR spectra of the saponins isolated from rhizomes of Paris quadrifolia L.: a)-1, b)-2, c)-3, d)-4, e)-5, f)-6
Pennogenin 3-O-β-D-glucopyranoside (1): White powder; MALDI TOF MS m/z: 593 [M + 1]+, 575 [M + 1-18]+, 615 [M + 23]+, 631 [M + 39]+, molecular formula C33H52O9.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (2): White powder; MALDI TOF MS m/z: 739 [M + 1]+, 721 [M + 1-18]+, 761 [M + 23]+, 777 [M + 39]+, molecular formula C39H62O13.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1 →2)-β-D-glucopyranoside (3): White powder; MALDI TOF MS m/z: 739 [M + 1]+, 721 [M + 1-18]+, 761 [M + 23]+, 777 [M + 39]+, molecular formula C39H62O13.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (4): White powder; MALDI TOF MS m/z: 885 [M + 1]+, 867 [M + 1-18]+, 907 [M + 23]+, 923 [M + 39]+, molecular formula C45H72O17.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (5): White powder; MALDI TOF MS m/z: 885 [M + 1]+, 867 [M + 1-18]+, 907 [M + 23]+, 923 [M + 39]+, molecular formula C45H72O17.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (6): White powder; MALDI TOF MS m/z: 1031 [M + 1]+, 1013 [M + 1-18]+, 1053 [M + 23]+, 1069 [M + 39]+, molecular formula C51H82O21.
Cytotoxicity assay
Cell culture
The HL-60 human promyelocytic leukaemia cell line was cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. The HeLa human cervical adenocarcinoma and MCF-7 human breast cancer cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% foetal bovine serum, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Cultures were maintained in an incubator in a humidified atmosphere with 5% of CO2 at 37°C (Heraceus, Hera cell).
Evaluation of cytotoxicity
Cell viability was determined using the MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.[35,36,37] Cells were seeded in 96-well plates at a density of 5 × 103 cells/well and treated for 24 h with the isolated pennogenyl saponins in the concentration range 0-50 μg/ml. Next, MTT (0.5 mg/ml) was added directly to the medium and cells were further incubated for 3 h at 37°C. The optical density of the formazan solution was measured at 550 nm with a plate reader (Victor, 1420 multilabel counter). Results are expressed as IC50 values. ± SD was calculated from at least three independent experiments.
RESULTS AND DISCUSSION
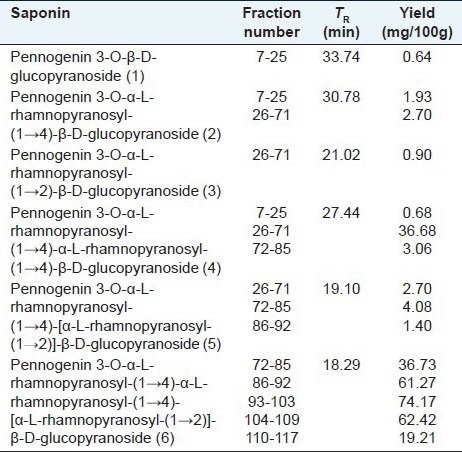
The isolation of pennogenyl saponins from P. quadrifolia rhizomes was completed in a few steps described in the experimental part. The procedure involved drying, ethanol extraction, degreasing and n-butanol/water extraction. The n-Bu-OH soluble fraction was subjected to column chromatography on silica gel in the eluent mixture ingredients gradient mode concentration (with growing concentration of MeOH). Eluates were monitored by TLC and 11 sub-fractions were obtained. Six pennogenyl saponins 1-6 (0.002, 0.019, 0.005, 0.194, 0.017 and 0.158% of the rhizome dry mass respectively) were isolated from 1-7 sub-fractions by semi-preparative isocratic RP HPLC. An especially large amount of saponin 6 was found in three sub-fractions: 93-103 (74.17%), 104-109 (62.42%) and 86-92 (61.27%) [Table 2].
Table 2.
Contents of pennogenyl saponins in P. quadrifolia L. fractions
Structural studies
The structures of pennogenyl saponins 1-6 [Figure 1] were elucidated by analyses of their molecular mass (MALDI TOF MS) and 1D and 2D NMR spectra (1H, COSY, TOCSY, ROESY, HMQC, HMBC).
Figure 1.
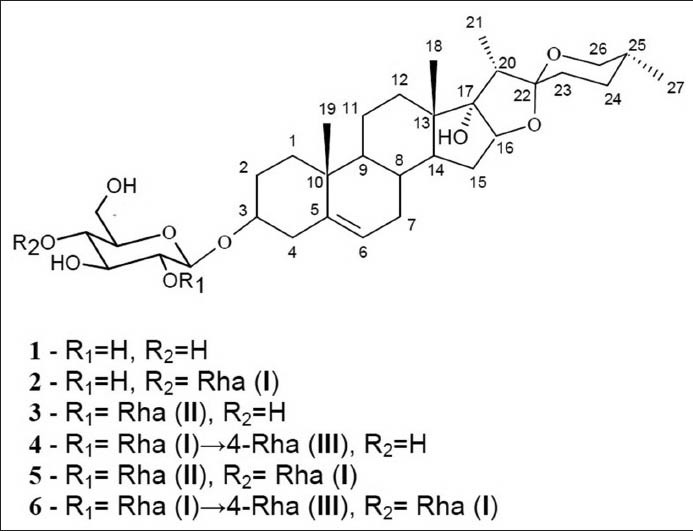
The structures of the saponins isolated from rhizomes of P. quadrifolia L.
The following cationised ions of the saponins under study were observed in the MALDI TOF MS spectra: [M + H]+ (quasimolecular), [M + Na]+, [M + K]+ and [M + H-H2O]+, which are consistent with their molecular formulae and molecular weights for the following compounds: 1-C33H52O9, 592 Da, 2-C39H62O13, 738 Da, 3-C39H62O13, 738 Da, 4-C45H72O17, 884 Da, 5-C45H72O17, 884 Da, 6-C51H82O21, 1030 Da [Figure 1 and Figure A.1-Appendix].
All 1H and 13C chemical shifts of compounds 1-6 were assigned using 1H, DQF-COSY, TOCSY, and HMQC experiments [Table 1]. Detailed analysis of the 1H and 13C NMR data of all the isolated compounds [Table 1] enabled the saponins to be identified: they consisted of a pennogenin residue as an aglycone part and sugar moieties.[4,30,31,38] Since our elucidations of all the aglycone parts were in very good agreement with the literature data, only the analysis of the sugar part of the saponins is described in detail here. The constituent monosaccharides of all six saponins possessed six-membered rings: They were assigned by the lack of carbon atom signals in the δ ~83-88 region of the 13C NMR spectrum [Table 1].[39,40]
Table 1.
1H and 13C NMR data of compounds 1.6 (700 MHz, C6D5N)
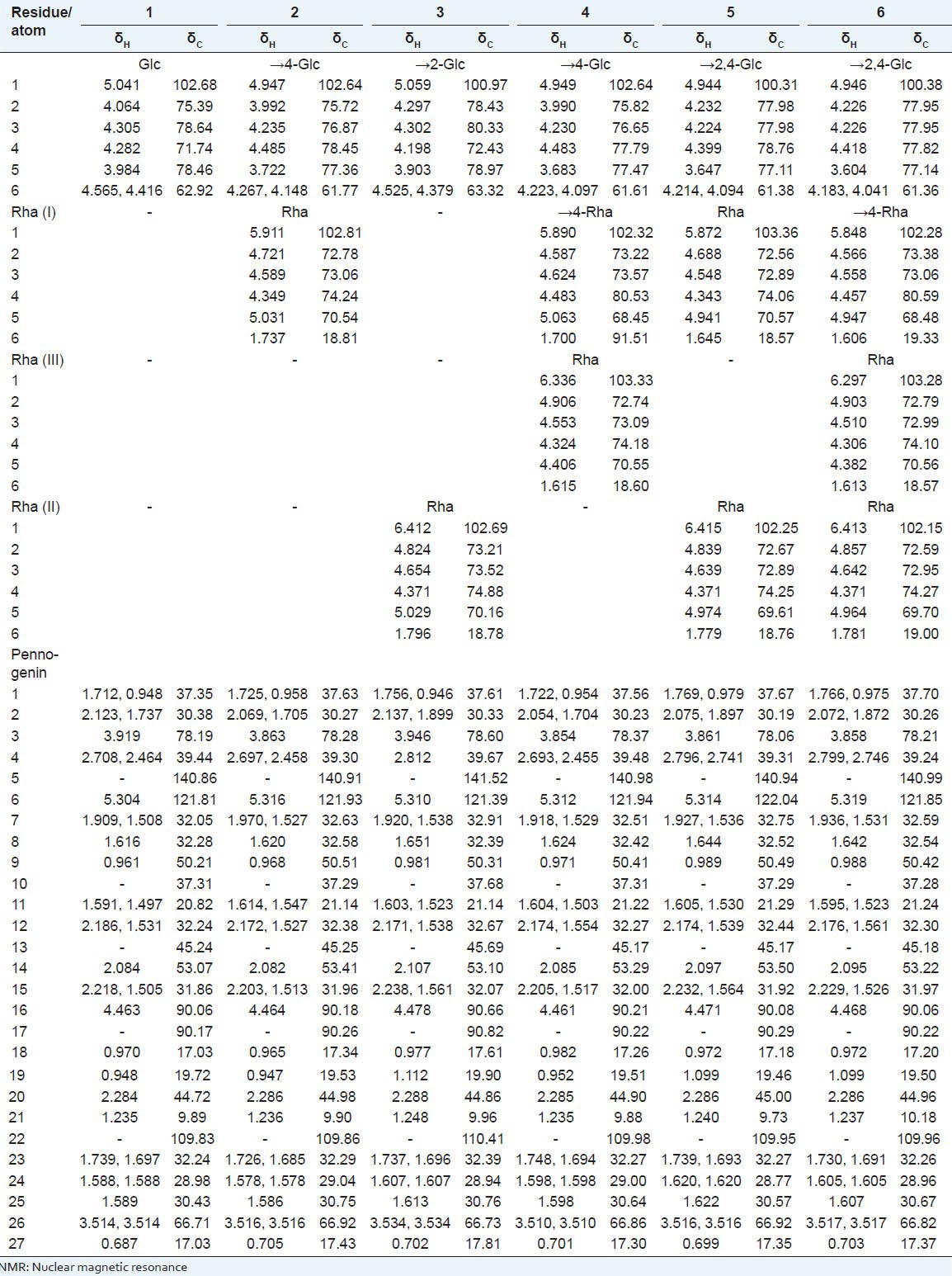
L configurations of all rhamnose residues and D configuration of glucose were identified by GLC of their (S)-(+)- and (±)-2-butyl glycosides.
Pennogenin 3-O-β-D-glucopyranoside (1): Examination of the 1H NMR spectrum [Figure B.1.A- Appendix] revealed the presence of only one anomeric proton signal at δ 5.041, which was identified on the basis of the HMQC cross peak at δ 5.041/102.68. The gluco configuration of this residue was assigned on the basis of the 3JH, H coupling constant pattern. Moreover, 3JH-1, H-2 = 8.2 Hz clearly revealed a β-configured Glc residue. The HMBC experiment (not shown) identified the linkage between Glc and pennogenin residues as 1→3. Strong cross-peaks H-1 of Glc/C-3 of pennogenin (δ 5.041/78.19), as well as of H-3 of pennogenin/C-1 of Glc (δ 3.919/102.68) were observed in the spectrum.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (2): The 1H NMR spectrum of compound 2 [Figure B.1.B-Appendix] was very similar to one described above. The spectrum contained two anomeric proton signals at δ 4.947 and 5.911. The first residue was identified as β-Glc (3JH-1, H-2 = 8.1 Hz) substituted at C-4 (δ 78.45), and the second one as terminal α-Rha (I). The α configuration of the rhamnopyranosyl unit (3JH1, H2 < = 1.5Hz) was deduced from the absence of strong intraresidual ROESY correlations between protons H-1 and H-3/H-5. This was also confirmed by 1JH1, C1 = 169 Hz, measured from the residual direct correlation observed in the HMBC spectrum, which is in agreement with that reported for the alpha anomer of rhamnopyranose.[41] The HMBC experiment confirmed the 1→3 linkage of Glc to pennogenin (H-1 of Glc/C-3 of pennogenin at δ 4.947/78.28, and H-3 of pennogenin/C-1 of Glc at δ 3.863/102.64). Furthermore, the substitution of Glc by the Rha (I) residue at C-4 (H-1 of Rha (I)/C-4 of Glc at δ 5.911/78.45, and H-4 of Glc/C-1 of Rha (I) at δ 4.485/102.81) was demonstrated.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (3): The 1H NMR spectrum of compound 3 [Figure B.1.C- Appendix] also showed two anomeric proton signals (δ 5.059, and 6.412). The first one belonged to β-Glc (3JH-1, H-2 = 7.3 Hz) substituted at C-2 (δ 78.43), and the second to a terminal α-Rha (II) (1JH1, C1 = 171 Hz). The HMBC spectrum revealed the 1→3 linkage of Glc to pennogenin (H-1 of Glc/C-3 of pennogenin at δ 5.059/78.60, and H-3 of pennogenin/C-1 of Glc at δ 3.946/100.97), as well as the substitution of Glc by the Rha (II) residue at C-2 (H-1 of Rha (II)/C-2 of Glc at δ 6.412/78.43, and H-2 of Glc/C-1 of Rha (II) at δ 4.297/102.69).
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (4): Three anomeric signals were identified in the anomeric region of the 1H NMR spectrum of compound 4 [Figure B.1.D - Appendix]. The signal with the lowest chemical shift (δ 4.949) was identified as H-1 of β-Glc (3JH-1, H-2 = 7.7Hz) substituted at C-4 (δ 77.79), then the signal at δ 5.890 belonging to α-Rha (I) (1JH1, C1 = 170Hz) substituted at C-4 (δ 80.53), and finally the signal at δ 6.336 represented the terminal α-Rha (III) (1JH1, C1 = 171Hz). In the HMBC spectrum, the cross-peaks at δ 4.949/78.37 (H-1 of Glc/C-3 of pennogenin) and at δ 3.854/102.64 (H-3 of pennogenin/C-1 of Glc) indicated glycosylation of the aglycone at the C-3 position. Another cross-peak between the second anomeric proton at δ 5.890 and the carbon at δ 77.79 (H-1 of Rha (I)/C-4 of Glc), and the cross-peak at δ 4.483/102.32 (H-4 of Glc/C-1 of Rha (I)) revealed the 1→4 linkage of Rha (I) to Glc. Finally, the cross-peak between the third anomeric proton at δ 6.336 and the carbon at δ 80.53 (H-1 of Rha (III)/C-4 of Rha (I)), and the cross-peak at δ 4.483/103.33 (H-4 of Rha (I)/C-1 of Rha (III)) revealed the 1→4 linkage of Rha (III) to Rha (I).
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (5): The 1H NMR spectrum of compound 5 [Figure B.1.E - Appendix] also showed three anomeric proton signals (δ 4.944, 5.872, and 6.415). The first one belonged to β-Glc (3JH-1, H-2 = 6.9 Hz) substituted at C-2 (δ 77.98) and C-4 (δ 78.76), as well as two other signals of two terminal α-Rha residues (1JH1, C1 = 169 Hz, and 1JH1, C1 = 171 Hz respectively). The HMBC spectrum confirmed the 1→3 linkage of Glc to pennogenin (H-1 of Glc/C-3 of pennogenin at δ 4.944/78.06, and H-3 of pennogenin/C-1 of Glc at δ 3.861/100.31). Moreover, the cross-peaks at δ 5.872/78.76 (H-1 of Rha (I)/C-4 of Glc) and at δ 4.399/103.36 (H-4 of Glc/C-1 of Rha (I)) revealed the linkage 1→4 between Rha (I) and Glc, and the cross-peaks at δ 6.415/77.98 (H-1 of Rha (II)/C-2 of Glc) and at δ 4.399/102.25 (H-2 of Glc/C-1 of Rha (II)) revealed the linkage 1→2 between Rha (II) and Glc.
Pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1 →4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside (6): Four anomeric signals were identified in the anomeric region of the 1H NMR spectrum of compound 6 [Figure B.1.F - Appendix]. The signal with the lowest chemical shift (δ 4.946) was identified as H-1 of β-Glc (3JH-1, H-2 = 7.0 Hz) substituted at C-2 (δ 77.95) and at C-4 (δ 77.82), then the signal at δ 5.848 belonging to α-Rha (1JH1, C1 = 169 Hz) substituted at C-4 (δ 80.59), and finally two signals at δ 6.297 and 6.413 representing terminal α-Rha residues (1JH1, C1 = 171 Hz, and 1JH1, C1 = 172 respectively).
In the HMBC spectrum, the cross-peaks at δ 4.946/78.21 (H-1 of Glc/C-3 of pennogenin) and at δ 3.858/100.38 (H-3 of pennogenin/C-1 of Glc) indicated glycosylation of the aglycone at C-3. Another cross-peak at δ 5.848/77.82 (H-1 of Rha (I)/C-4 of Glc) and the cross-peak at δ 4.418/102.28 (H-4 of Glc/C-1 of Rha (I)) revealed the 1→4 linkage of Rha (I) to Glc. Finally, the position of glycosylation by the two remaining terminal Rha residues was identified. The cross-peak at δ 6.297/80.59 (H-1 of Rha (III)/C-4 of Rha (I)) and the cross-peak at δ 4.457/103.28 (H-4 of Rha (I)/C-1 of Rha (III)) revealed the 1→4 linkage of Rha (III) to Rha (I), while the cross-peak at δ 6.413/77.95 (H-1 of Rha (II)/C-2 of Glc) and the cross-peak at δ 4.226/102.15 (H-2 of Glc/C-1 of Rha (II)) revealed the 1→2 linkage of Rha (II) to Glc.
Bioassay studies
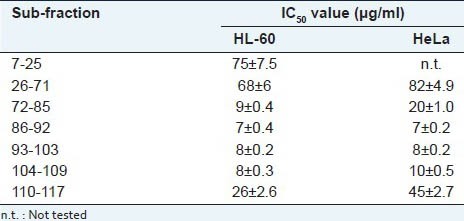
The isolated eleven sub-fractions were evaluated for their cytotoxic activity against HL-60 and HeLa cells. The seven sub-fractions (7-25, 26-71, 72-85, 86-92, 93-103, 104-109, 110-117) showed cytotoxicity below 100 μg/ml [Table 3]. The fractions 86-92, 93-103, 104-109 with large amount of compound 6 [Table 2] were the most potent [Table 3].
Table 3.
Cytotoxic activity of sub-fractions from P. quadrifolia L. extract
The six compounds isolated from the all sub-fractions were tested on HL-60, HeLa and MCF-7 cells. Pennogenyl saponins 5 and 6 exhibited cytotoxic activity against HL-60, HeLa and MCF-7 tumour cells with IC50 values of 1.0 ± 0.04 μg/ml, 1.8 ± 0.072 μg/ml and 2.4 ± 0.096 μg/ml respectively, and 2.0 ± 0.08 μg/ml, 2.5 ± 0.125 μg/ml and 3.2 ± 0.128 μg/ml respectively. Saponins 1, 2 and 4 or without the ramnosyl residue or without the terminal ramnosyl residue linked to C-2 of the glucosyl group did not show any cytotoxic activity [Table 4].
Table 4.
Cytotoxic activity of pennogenyl saponins from P. quadrifolia L. rhizomes
CONCLUSION
The six saponins studied in this paper have been isolated at the same time from rhizomes of one species of Paris and fully structurally characterised using spectroscopic and chemical methods. The spirostan saponins 1, 2, 3 and 4 were obtained from P. quadrifolia rhizomes for the first time. The isolation and the identification of six pennogenyl saponins from P. quadrifolia rhizomes constitute significant contribution into the general knowledge of chemical composition of the Paris family, particularly in the field of saponin substances and P. quadrifolia L. itself.
ACKNOWLEDGMENTS
This research was supported by the Polish Ministry of Research and Higher Education under grant DS 8200-4-0085-1 and grant Nr 538-M031-0736-1 for A. Kawiak and grant Nr N N405 669140 for J.R. Ochocka, J. Stefanowicz-Hajduk.
The authors thank L. Łobocki (UG) for his help with the MS experiments.
A. Kawiak acknowledges funding from the European Social Fund, the project “Educators for the eliteintegrated training program for PhD students, post-docs and professors as academic teachers at University of Gdańsk” within the framework of Human Capital Operational Programme, Action 4.1.1, Improving the quality of educational offer of tertiary education institutions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jacquemyn H, Brys R, Hutchings MJ. Biological flora of the British Isles Paris quadrifolia L. J Ecol. 2008;96:833–44. [Google Scholar]
- 2.Kosiński I, Stefanowicz-Hajduk J, Ochocka JR. Demographic versus genetic (RAPD) variation between and within two populations of the clonal plant Paris quadrifolia L. (Liliaceae) Pol J Ecol. 2009;57:303–11. [Google Scholar]
- 3.Stefanowicz-Hajduk J, Kawiak A, Gajdus J, Ochocka JR, Paszkiewicz M, Stepnowski P, et al. Cytotoxic activity of Paris quadrifolia extract and isolated saponin fractions on human tumour cell lines. Acta Biol Cracov Bot. 2011;53:127–31. [Google Scholar]
- 4.Mimaki Y, Kuroda M, Obata Y, Sashida Y, Kitahara M, Yasuda A, et al. Steroidal saponins from rhizomes of Paris polyphylla var. chinensis and their cytotoxic activity on HL-60 cells. Nat Prod Lett. 2000;14:357–64. [Google Scholar]
- 5.Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, et al. Effects of polyphyllin D, steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248–54. doi: 10.4161/cbt.4.11.2136. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Gao W, Liu X, Zuo Y, Chen H, Duan H. Anti-tumor constituents from Paris polyphylla. Asian J Tradit Med. 2006;1:7–10. [Google Scholar]
- 7.Yun H, Lijian C, Wenhong Z, Yuhong D, Yongli W, Qiang W, et al. Separation and identification of steroidal compounds with cytotoxic activity against human gastric cancer cell lines in vitro from rhizomes of Paris polyphylla var. chinensis. Chem Nat Comp. 2007;43:672–7. [Google Scholar]
- 8.Man S, Gao W, Zhang Y, Ma C, Yang L, Li Y. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm Res. 2011;34:43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H, Pongpiriyadacha Y, Morikawa T, Kishi A, Kataoka S, Yoshikawa M. Protective effects of steroid saponins from Paris polyphylla var. yunnanensis on ethanol- or indomethacin-induced gastric mucosal lesions in rats: Structural requirement for activity and mode of action. Bioorg Med Chem Lett. 2003;13:1101–6. doi: 10.1016/s0960-894x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Liu H, Liu XT, Chen XQ, Wang Q. Steroidal saponins from the rhizomes of Paris delavayi. Steroids. 2009;74:809–13. doi: 10.1016/j.steroids.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Jun Z. Some bioactive substances from plants of West China. Pure Appl Chem. 1989;61:457–60. [Google Scholar]
- 12.Nohara T, Kumamoto F, Miyahara K, Kawasaki T. Steroid saponins of aerial parts of Paris tetraphylla A. Gray and of underground parts of Trillium tschonoskii Maxim. Chem Pharm Bull. 1975;23:1158–60. [Google Scholar]
- 13.Nohara T, Miyahara K, Kawasaki T. Steroids saponins and sapogenins of underground parts of Trillium kamtschaticum PALL. II. Pennogenin- and kryptogenin 3-O-glycosides and related compounds. Chem Pharm Bull. 1975a;23:872–85. [Google Scholar]
- 14.Man S, Gao W, Zhang Y, Wang J, Zhao W, Huang L, et al. Qualitative and quantitative determination of major saponin in Paris and Trillium by HPLC-ELSD and HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2943–8. doi: 10.1016/j.jchromb.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Hufford CD, Liu S, Clark AM. Antifungal activity of Trillium grandiflorum constituents. J Nat Prod. 1988;51:94–8. doi: 10.1021/np50055a013. [DOI] [PubMed] [Google Scholar]
- 16.Ono M, Takamura C, Sugita F, Masuoka C, Yoshimitsu H, Ikeda T, et al. Two new steroid glycosides and a new sesquiterpenoid glycoside from the underground parts of Trillium kamtschaticum. Chem Pharm Bull. 2007;55:551–6. doi: 10.1248/cpb.55.551. [DOI] [PubMed] [Google Scholar]
- 17.Yokosuka A, Mimaki Y. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry. 2008;69:2724–30. doi: 10.1016/j.phytochem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Hayes PY, Lehmann R, Penman K, Kitching W, De Voss JJ. Steroidal saponins from the roots of Trillium erectum (Beth root) Phytochemistry. 2009;70:105–13. doi: 10.1016/j.phytochem.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Nohara T, Ogata Y, Aritome M, Miyahara K, Kawasaki T. Steroid saponins of Heloniopsis orientalis (THUNB) C TANAKA. Chem Pharm Bull. 1975b;23:925–8. [Google Scholar]
- 20.Nakano K, Murakami K, Takaishi Y, Tomimatsu T, Nohara T. Studies on the constituents of Heloniopsis orientalis (THUNB.). C TANAKA. Chem Pharm Bull. 1989;37:116–8. [Google Scholar]
- 21.Yu HS, Ma BP, Kang LP, Zhang T, Jiang FJ, Zhang J, et al. Saponins from the processed rhizomes of Polygonatum kingianum. Chem Pharm Bull. 2009;57:1011–4. doi: 10.1248/cpb.57.1011. [DOI] [PubMed] [Google Scholar]
- 22.Hirai Y, Sanada S, Ida Y, Shoji J. Studies on the constituents of palmae plants. III. The constituents of Chamaerops humilis L. and Trachycarpus wagnerianus BECC. Chem Pharm Bull. 1986;34:82–7. [Google Scholar]
- 23.Haraguchi M, Zaccarias AP, Santos D, Young MC, Chui EP. Steroidal prosapogenins from Dioscorea olfersiana. Phytochemistry. 1994;36:1005–8. [Google Scholar]
- 24.Liu H, Chou GX, Wu T, Guo YL, Wang SC, Wang CH, et al. Steroidal sapogenins and glycosides from the rhizomes of Dioscorea bulbifera. J Nat Prod. 2009a;72:1964–8. doi: 10.1021/np900255h. [DOI] [PubMed] [Google Scholar]
- 25.Mimaki Y, Nakamura O, Sashida Y, Nikaido T, Ohmoto T. Steroidal saponins from the bulbs of Triteleia lactea and their inhibitory activity on cyclic AMP phosphodiesterase. Phytochemistry. 1995;38:1279–86. doi: 10.1016/0031-9422(94)00790-z. [DOI] [PubMed] [Google Scholar]
- 26.Woo ER, Kim JM, Kim HJ, Yoon SH, Park H. A cytotoxic pennogenin glycoside from Majanthemum dilatatum. Planta Med. 1998;64:466–8. doi: 10.1055/s-2006-957486. [DOI] [PubMed] [Google Scholar]
- 27.Wang JZ, Ye LM, Chen XB. A new C27 - steroidal glycoside from Ophiopogon japonicus. Chin Chem Lett. 2008;19:82–4. [Google Scholar]
- 28.Tapondjou LA, Ponou KB, Teponno RB, Mbiantcha M, Djoukeng JD, Nguelefack TB, et al. In vivo anti-inflammatory effect of a new steroidal saponin, mannioside A, and its derivatives isolated from Dracaena mannii. Arch Pharm Res. 2008;31:653–8. doi: 10.1007/s12272-001-1208-3. [DOI] [PubMed] [Google Scholar]
- 29.Xie BB, Liu HJ, Ni W, Chen CX. Ypsilandrosides C-G, five new spirostanol saponins from Ypsilandra thibetica. Steroids. 2009;74:950–5. doi: 10.1016/j.steroids.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Jenett-Siems K, Krause N, Siems K, Jakupovic S, Wallukat G, Melzig ME. Chemical composition and biological activity of Paris quadrifolia L. Z Naturforsch C. 2012;67:565–70. doi: 10.1515/znc-2012-11-1206. [DOI] [PubMed] [Google Scholar]
- 31.Nohara T, Ito Y, Seike H, Komori T, Moriyama M, Gomita Y, et al. Study on the constituens of Paris quadrifolia L. Chem Pharm Bull. 1982;30:1851–6. [Google Scholar]
- 32.Gerwig GJ, Kamerling JP, Vliegenthart JF. Determination of the D and L configuration of neutral monosaccharides by high-resolution capillary g.l.c. Carbohydr Res. 1978;62:349–57. [Google Scholar]
- 33.Leontein K, Lindberg B, Lönngren J. Assignment of absolute configuration of sugars by GLC of their acetylated glycosides formed from chiral alcohols. Carbohydr Res. 1978;62:359–62. [Google Scholar]
- 34.Gajdus J, Kaczyński Z, Śmietana J, Stepnowski P. Structural determination of the O-antigenic polysaccharide from Salmonella Mara (O:39) Carbohydr Res. 2009;344:1054–7. doi: 10.1016/j.carres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Ragab EA, Mohammed AS, Abbass HS, Kotb SI. A new flavan-3-ol dimer from Ficus spragueana leaves and its cytotoxic activity. Pharmacogn Mag. 2013;9:144–8. doi: 10.4103/0973-1296.111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales P, Haza AI. Antiproliferative and apoptotic effects of Spanish honeys. Pharmacogn Mag. 2013;9:231–7. doi: 10.4103/0973-1296.113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suresh HM, Shivakumar B, Hemalatha K, Heroor SS, Hugar DS, Rao KR. In vitro antiproliferative activity of Annona reticulata roots on human cancer cell lines. Pharmacogn Res. 2011;3:9–12. doi: 10.4103/0974-8490.79109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu XX, Wang L, Yang J, Wang Q. Steroidal saponins from Paris mairei. Chem Nat Comp. 2008;44:821–3. [Google Scholar]
- 39.Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv Carbohydr Chem Biochem. 1983;41:27–66. [Google Scholar]
- 40.Lipkind GM, Shashkov AS, Knirel YA, Vinogradov EV, Kochetkov NK. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydr Res. 1988;175:59–75. doi: 10.1016/0008-6215(88)80156-3. [DOI] [PubMed] [Google Scholar]
- 41.Kasai E, Ohikara M, Asakawa J, Mizutani K, Tanaka O. 13C NMR study of alpha- and beta-anomeric pairs of D-mannopyranosides and L-rhamnopyranosides. Tetrahedron. 1979;35:1427–32. [Google Scholar]


