Abstract
Background:
The slough shed of Cryptotympana atrata Fabricius is widely used to treat skin diseases in China, Japan, and Korea.
Objective:
To investigate the anti-inflammatory effects of C. atrata on contact dermatitis.
Materials and Methods:
We investigated the effects of C. atrata methanol extract (MECA) on ear swelling, histophathological changes and cytokine production in 1-fluoro-2,4-dinitrofluorobenzene (DNFB)-induced contact dermatitis (CD) mice.
Results:
Topical application of MECA effectively inhibited enlargement of ear swelling (30 and 100 μ/ear, P < 0.05; 300 μg/ear, P < 0.01). MECA treatment also inhibited hyperplasia, spongiosis (100 and 300 μg/ear, P < 0.001), and immune cell infiltration (30 μg/ear, P < 0.05; 100 and 300 μg/ear, P < 0.001) induced by DNFB. In addition, treatment with MECA suppressed the increase in the levels of TNF-α (P < 0.05), IFN-g (3, 100 μg/ear, P < 0.05; 300 μg/ear, P < 0.01), and IL-6 (100 μg/ear, P < 0.05; 300 μg/ear, P < 0.01) production.
Conclusion:
These data suggest that MECA has the potential for use in the treatment of inflammatory skin diseases, including CD. Moreover, the results presented herein indicate that anti-inflammatory actions of MECA are mediated by decreasing production of TNF-α, IFN-γ, and IL-6 in inflamed tissues.
Keywords: Cryptotympana atrata, contact dermatitis, inflammation, traditional Chinese medicine
INTRODUCTION
The slough shed of Cryptotympana atrata Fabricius, family Cicadidae, is widely used for the treatment of skin diseases such as eczema, pruritus, and urticaria in China, Japan, and Korea. In traditional medicine, the slough shed of C. atrata is believed to expel wind and heat, let out skin eruptions, and alleviate the lung and liver meridians.[1] Additionally, it is used to treat unsmooth eruption of measles and rubella with itching.[1] Recently, N-acetyldopamine dimers[2] and N-acetyldopamine tetrapolymers[3] were isolated from the slough shed of C. atrata. The slough shed of C. atrata. and its isolated components have anticonvulsive, sedative and hypothermic,[4] and antiallergic effects.[5]
Contact dermatitis (CD) is the most common cause of occupational skin diseases, which are one of the most common work-related disorders worldwide.[6] CD is very important in terms of social and economic impact.[6] The primary treatment of CD is avoidance of the offending agent; however, this is often difficult in cases of occupational diseases.[7] As a result, treatments of CD tend to consist of the repeated use of immuno-modulatory and anti-inflammatory agents such as corticosteroids.[7] Corticosteroids are very effective when applied for treatment of allergic and inflammatory diseases, but the dosage and schedule of corticosteroids are frequently restricted. As a result, it is necessary to minimize or alter corticosteroid usage, especially in cases of continuous application. Traditional medicines have emerged as complementary and alternative medicines for corticosteroids owing to their low cost and safety.[8] For these reasons, we investigated the possibility of using traditional medicines as complementary and alternative medicines for corticosteroids. We have already reported the anti-inflammatory effects of traditional medicines such as Sophora flavescens Aiton and Dictamnus dasycarpus Turcz. on CD. In this study, we evaluated the anti-inflammatory effects of C. atrata slough shed using a mouse model of CD. Specifically, the effects of C. atrata on ear thicknesses, weights, histopathological changes in ear tissues and cytokine levels of inflamed tissues were assessed in vivo.
MATERIALS AND METHODS
Chemicals and reagents
1-Fluoro-2,4-dinitrofluorobenzene (DNFB), dimethyl sulfoxide (DMSO), and dexamethasone (DEX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). A protein extraction kit was obtained from Intron Bio (Daejeon, Korea) and a cytometric bead array kit was acquired from BD Bioscience (Franklin Lakes, NJ, USA).
Preparation of MECA
The slough shed of Cryptotympana atrata Fabricius was purchased from Hwalim Medicinal Herbs (Pusan, Korea) and extraction processes were conducted according to our previously described standard extraction process.[9] Briefly, 50 g of C. atrata slough shed were immersed in 1,000 mL of methanol and sonicated for 30 minutes, after which they were extracted for 24 hours. The extract was then filtered through Whatman filter paper No. 20 and evaporated under reduced pressure using a vacuum evaporator (Eyela, Japan). Next, the condensed extract was lyophilized using a freeze dryer (Labconco, Kansas City, MO, USA). Finally, 0.78 g of lyophilized powder were obtained (yield, 1.56%). The methanol extract of C. atrata slough shed (MECA, Voucher No. MH2011-004) and a specimen of the crude herb were deposited at the Division of Pharmacology, School of Korean Medicine, Pusan National University.
Animals
Male 6-week-old Balb/c mice were purchased from Samtaco (Incheon, Korea) and housed under specific pathogen-free conditions with a 12-hour light/dark cycle and free access to standard rodent food and water. All animal experiments were approved by our Animal Care and Use Committee and conducted according to institutional guidelines (PNU-2011-000406).
Induction of CD and experimental design
CD induction was performed using DNFB as previously described.[9] Briefly, mice were sensitized by painting 50 μL of DNFB (0.1%, v/v) in acetone: Olive oil (AOO, 4:1) onto the shaved dorsum of each animal for 3 consecutive days. Four days after sensitization, each mouse was challenged by painting 30 μL of DNFB (0.2%, v/v) in AOO onto the dorsum of both ears every 2 days. For topical application of drugs, MECA and DEX were dissolved in ethanol, filtered using a 0.45 μm pore size syringe filter and finally diluted in AOO (ethanol: AOO, 1:4). MECA solution (30, 100 or 300 μg/ear) was applied onto the dorsum of both ears every two days. All animals except naïve mice were sensitized and challenged with DNFB. The naïve animals (NOR) were treated with vehicle (for sensitization and challenge, AOO) and painted with vehicle (for treatment, ethanol and AOO) (n = 6). Control animals (CTL) were sensitized and challenged with DNFB in AOO, after which they were painted with vehicle (ethanol and AOO) (n = 8). MECA-treated animals were sensitized and challenged with DNFB, then painted with 30, 100, or 300 μg/ear of MECA (n = 8). DEX-treated animals were sensitized and challenged with DNFB and then painted with 75 μg/ear of DEX in ethanol and AOO (n = 6) as a positive control. The experimental design is summarized in Figure 1.
Figure 1.
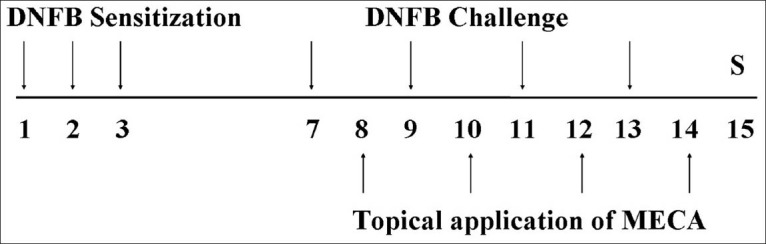
Experimental design The experimental groups, except for the naïve group, were sensitized by painting with DNFB on days 1, 2, and 3. Mice were challenged by DNFB on days 7, 9, 11, and 13. The naïve group was treated with vehicle (AOO) in the same way. MECA and DEX groups were topically treated with MECA (30, 100, or 300 μg/ear in ethanol and AOO) or DEX (75 μg/ear in ethanol and AOO) on days 8, 10, 12, and 14. All animals were sacrificed on day 15
Measurement of ear thicknesses and weights
Mice were anesthetized with 30 mg/kg of zoletil (Virbac, Carros, France) and the thicknesses of both ears was measured using vernier calipers (Mitutoyo, Carros, Japan) at the end of experiment. The weights of ear pieces (5 mm in diameter) were also determined at the same time.
Histopathological examination
After measurement of ear thicknesses and weights, ear tissues were resected and paraffin embedded. Sections were then stained with hematoxylin and eosin (H and E) for histopathological observation of immune cell infiltration and spongiosis. Stained tissues were observed using a light microscope (×200).
These slides were examined and scored in a blind manner. Five nonoverlapping fields per slide were randomly selected and images were captured with a light microscope. To evaluate the immune cell (IC) index, infiltrated immune cells were counted using cell counting grid. Then, the number of immune cells was categorized into five easily reproducible subgroups as follows: point 0 (no detectable cell), point 1 (1-20 cells), point 2 (21-40 cells), point 3 (41-60 cells), or point 4 (60 + cells). This categorization for the IC index was developed by a slightly modified method of Milne et al.[10] The severity of connective tissue edema (CTE) index was evaluated as follows: the proportion of area showing edema to the total connective tissue area was calculated by percentage and categorized into seven groups. It was scored as point 0 (0%), point 1 (1-9%), point 2 (10-29%), point 3 (30-49%), point 4 (50-69%), point 5 (70-89%), or point 6 (90-100%). We established the scoring system for CTE index by slight modification of Cha et al.'s method.[11]
Measurement of cytokine production
At the end of the experiment, resected ear tissues were lysed and homogenized with protein extraction solution (Intron Bio, Daejeon, Korea) using a bullet blender (Next Advance, NY, USA) to obtain tissue lysates. Fifty micrograms of each lysate were then used to measure the levels of tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-g interleukin-10 (IL-10) and interleukin-6 (IL-6). Cytokine levels were measured using a cytometric bead array kit (BD Bioscience). All experimental procedures were conducted according to the manufacturer's protocols.
Statistical analysis
Normality of the data distribution was evaluated by the Kolmogorov-Smirnov test. The Kruskal-Wallis test and Mann-Whitney U test were used for all analyses. All data were presented as the mean ± SD. Differences with a value of P < 0.05 were considered significant. All statistical analyses were performed using Window PASW (Predictive Analytics SoftWare) version 18.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
MECA inhibited increases in ear swelling in CD mice
Repeated application of DNFB increased ear thickness and weight, which is a major feature of CD. Marked increases in the thickness and weight of ear tissues were observed in the control group (CTL). Topical treatment with MECA effectively inhibited increases in the thickness and weight of ear tissues dose-dependently. Inhibitory effects on ear thickness and weight in the dexamethasone (DEX)-treated group were more effective than those in the MECA-treated group [Figure 2].
Figure 2.
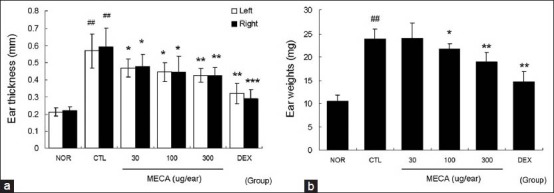
Effects of MECA on ear swelling in CD mice. Inhibitions of ear thickness and weight in response to topical application of MECA were analyzed using vernier calipers and a microbalance on day 15. Every 2 days, 30, 100, or 300 μg of MECA was painted onto each ear (four times). NOR, nontreated naïve mice; CTL, nontreated CD mice; DEX, 75 μg/ear of dexamethasone painted CD mice. (a) Ear thicknesses; (b) ear weights. All values are presented as the mean ± SD.##P < 0.01 vs. nontreated naïve mice (NOR); *P < 0.05, **P < 0.01, ***P < 0.001 vs. nontreated CD mice (CTL)
MECA prevented hyperplasia, spongiosis, and immune cell infiltration in CD mice
Repeated painting with DNFB induced hyperplasia as well as significant edema and spongiosis, which is a hallmark of skin inflammation. Marked infiltration of immune cells was observed [Figure 3A] and the highest IC index was also observed in CTL group [Figure 3b], while MECA treatment inhibited immune cell infiltration in a dose-dependent manner [Figure 3A and B]. In addition, severe edema and spongiosis were observed in the CTL group [Figure 3A and C]. Treatment with more than 100 μg/ear of MECA prevented edema and spongiosis significantly [Figure 3A and C]. Treatment with DEX most effectively prevented spongiosis and immune cell infiltrations [Figure 3].
Figure 3.
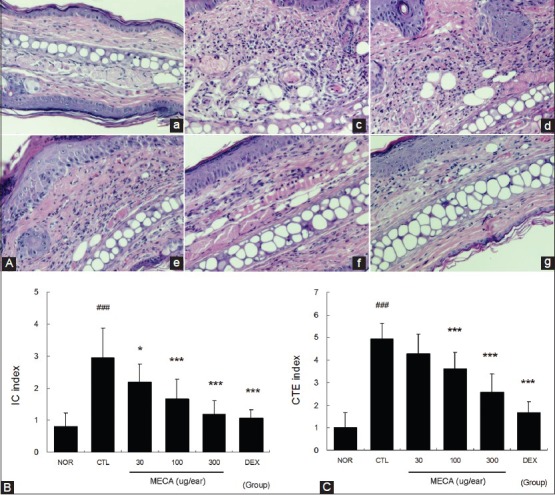
Effects of MECA on histopathological changes in CD mice. (A), Ear tissues were stained with H-E and observed using a light microscope. (a), NOR group; (b), CTL group; (c), 30 μg/ear MECA-treated group; (d), 100 μg/ear MECA-treated group; (e), 300 μg/ear MECA-treated group; (f) 75 μg/ear DEX-treated group. The observations were made at a magnification of ×200. In addition, these sections were evaluated using immune cell (IC) index (B) and connective tissue edema (CTE) index (C). All values are presented as the mean ± SD.###P < 0.001 vs. nontreated naïve mice (NOR); *P < 0.05, ***P < 0.001 vs. nontreated CD mice (CTL)
MECA reduced the levels of TNF-α, IFN-g, and IL-6 in the ear tissues of CD mice
Marked increases in TNF-α, IFN-g, IL-10, and IL-6 production were observed in the CTL group. These increases in TNF-α, IFN-g, and IL-6 production were effectively reduced by topical application of MECA. Treatment with 100 or 300 μg/ear of MECA significantly suppressed the increase in TNF-α, IFN-g, and IL-6 levels. Neither MECA nor DEX affected the IL-10 level. Treatment with DEX was most effective among the experimental groups [Figure 4].
Figure 4.
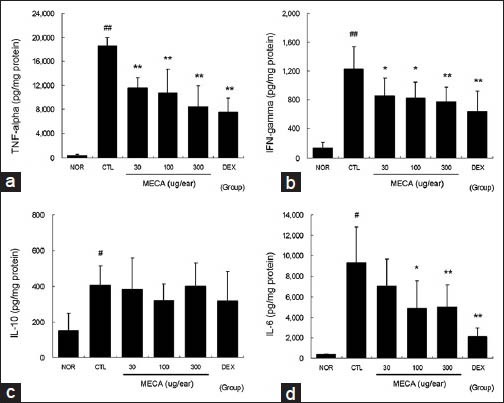
Effects of MECA on cytokine production in CD mice. The levels of TNF-α, IFN-ã, IL-10, and IL-6 in ear tissues were measured using the cytometric bead array method. A total of 50 μg of tissue lysates were used to measure the cytokine levels. Every 2 days 30, 100, or 300 μg of MECA was painted onto each ear (4 times). Abbreviations are the same as in Figure 2. (a), TNF-α; (b), IFN-ã; (c), IL-10; (d), IL-6. All values are presented as the mean ± SD.#P < 0.05 and##P < 0.01 vs. nontreated naïve mice (NOR); *P < 0.05 and **P < 0.01 vs. nontreated CD mice (CTL)
MECA did not affect body weight gain or spleen/body weight ratio in CD mice
Effects of MECA on enlargement of the spleen were estimated in terms of spleen/body weight ratio. Body weight gain in the CTL group was decreased compared to the normal group [Figure 5a]. MECA treatment did not affect body weight gain; however, the DEX-treated group showed significantly lower body weight gain than the CTL group [Figure 5a]. The spleen/body weight ratio was elevated in the CTL group, but significantly lower in the DEX group. Spleen/body weight ratios of the MECA groups were almost the same as in the CTL group [Figure 5b].
Figure 5.
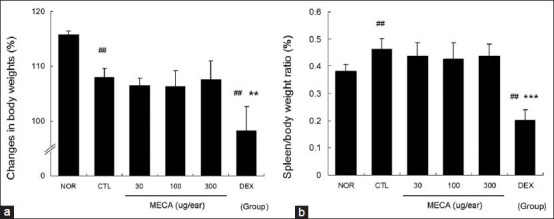
Effects of MECA on body weight gain and spleen/body weight ratio in CD mice. Body weights were measured at the beginning (day 1) and end (day 15) of the experiment. Changes in body weights were based on the average weights on day 15 and expressed as percentages of weight on day 1 (a). Spleen weights were also measured on day 15, at which time the spleen/body weight ratio was calculated (b). Abbreviations are the same as in Figure 2. All values are presented as the mean ± SD.##P < 0.01 vs. nontreated naïve mice (NOR); **P < 0.01 and ***P < 0.001 vs. nontreated CD mice (CTL)
DISCUSSION
In this study, we demonstrated the anti-inflammatory effects of MECA on CD in vivo. MECA effectively prevented enlargement of ear swelling [Figure 2], as well as hyperplasia, spongiosis and infiltration of immune cells in inflamed tissues [Figure 3]. In addition, the production of TNF-α, IFN-g, and IL-6 was reduced in response to MECA in a dose-dependent manner [Figure 4]. These results indicate that MECA can effectively prevent inflammatory reactions, leading to inhibition of ear swelling in CD mice.
A number of cytokines including IL-1, IL-6, TNF-α, and IL-12 play a significant role in the development of acute or chronic inflammatory response. IL-6 and TNF-α, which are produced by activated tissue macrophages, induce acute inflammatory response. In addition, IFN-g contributes to the inflammatory response, acting later in the acute response and making an important contribution to chronic inflammation. During expression of CD, recruitment of immune cells such as T cells and macrophages is driven by mediators released by activated resident skin cells such as keratinocytes and fibroblasts.[12] TNF-α induces increased expression of adhesion molecules on vascular endothelial cells and keratinocytes, such as E-selectin, ICAM-1, and VCAM-1, which bind to integrins on lymphocytes and monocytes and contribute to the influx of neutrophils. In the present study, MECA treatment effectively reduced the pro-inflammatory cytokine productions [Figure 4], and also reduced immune cell infiltration in inflamed tissue [Figure 3]. Overall, these data imply that MECA acts as an anti-inflammatory agent, resulting in reduced hyperplasia, spongiosis, and immune cell infiltration in a dose-dependent manner. In addition, these data imply that the major mechanism of anti-inflammatory action is involved in suppressing production of pro-inflammatory cytokines. In our model, most of the infiltrated cells were neutrophils (data not shown) in the CTL group. However, in MECA-treated groups, neutrophil infiltration decreased in the connective tissue, whereas the infiltration of fibroblasts and the formation of capillaries, which are the characteristic features of tissue repair, increased in a dose-dependent manner (data not shown). These findings imply that MECA can effectively suppress the inflammation induced by DNFB as well as stimulate healing process.
Ma et al.[13] reported that C. atrata suppressed immunoglobulin E (IgE)-mediated reactions, passive cutaneous anaphylaxis in mice, and degranulation of mast cells in rats. Shin et al.[14] also reported that C. atrata extract inhibited the release of histamine from rat peritoneal mast cells and prevented systemic anaphylactic shock induced by compound 48/80 in a dose-dependent manner. Mast cells play a central role in both acute and chronic allergic reactions through the release of a number of mediators and cytokines. It is well known that mast cells are activated by TNF-α and that activated mast cells can release TNF-α.[15] TNF-α and histamine derived from skin mast cells can promote the production of pro-inflammatory cytokines, adhesion molecules, and growth factors in resident cells.[16] In the present study, MECA lowered the TNF-α level significantly [Figure 4]. Taken together, C. atrata suppressed the action of immune cells involved in TNF-α production, resulting in reducing hyperplasia in ear tissues. In addition, the suppression of IgE-mediated reactions by C. atrata[13] may be involved in inhibition of IL-6 production in inflamed tissues.
In the present study, elevated levels of TNF-α and IFN-g, a hallmark of Th1 skewing reaction, were lowered by MECA; however, the level of IL-10 was not affected. In addition, very low concentrations and almost the same levels of IL-4, a hallmark of Th2 skewing reaction, were detected in all experimental groups (data not shown). These findings imply that MECA acts as an anti-inflammatory agent against Th1 skewing. However, this result is not in accordance with the observed IL-6 production, which is one of Th2 skewing cytokines. Accordingly, further studies are needed to understand its exact mechanisms.
DEX was found to be more effective than MECA based on most data collected in this experiment. However, treatment with dexamethasone reduced body weight gain and spleen/body weight ratio. Furthermore, spleen/body weight ratio in the DEX group was significantly lower than that in the normal group. In contrast, MECA did not affect body weight gain or spleen/body weight ratio [Figure 5]. Nagao et al.[17] reported that corticosteroids induced weight loss in experimental animals, and weigh loss was the result of adverse reactions.[18] In addition, the reduced mass of the spleen in the DEX group may have been involved in general immune suppression, which is one of the major side effects of corticosteroids.
Taken together, these data indicate that MECA has the potential for use in the treatment of inflammatory skin diseases including CD. In addition, they suggest that MECA acts as an anti-inflammatory agent, decreasing the production of TNF-α, IFN-g, and IL-6 in inflamed tissues.
CONCLUSIONS
In the present study, we demonstrated the anti-inflammatory effects of MECA. MECA inhibited production of TNF-α, IFN-g, and IL-6, resulting in reduced hyperplasia, spongiosis, and immune cell infiltrations. These anti-inflammatory reactions of MECA finally led to inhibition of ear swelling. However, in contrast to dexamethasone, MECA had no effect on body weight gain or spleen/body weight ratio. Overall, these results indicate that MECA can be used as complementary or alternative medicine to treat patients with inflammatory skin diseases with relative safety.
Footnotes
Source of Support: Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012011676)
Conflict of Interest: None declared.
REFERENCES
- 1.Zuo Y, Tang D, Xun J. Sanghai: Sanghai Xinhua Printing Works; 2003. Science of chinese materia medica; pp. 91–2. [Google Scholar]
- 2.Xu MZ, Lee WS, Han JM, Oh HW, Park DS, Tian GR, et al. Antioxidant and anti-inflammatory activities of N-acetyldopamine dimers from Periostracum Cicadae. Bioorg Med Chem. 2006;14:7826–34. doi: 10.1016/j.bmc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Li GY, Li QR, Wang JH. Two new N-acetyldopamine tetrapolymers from periostracum Cicadae. J Asian Nat Prod Res. 2012;14:204–9. doi: 10.1080/10286020.2011.637375. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh MT, Peng WH, Yeh FT, Tsai HY, Chang YS. Studies on the anticonvulsive, sedative and hypothermic effects of Periostracum Cicadae extracts. J Ethnopharmacol. 1991;35:83–90. doi: 10.1016/0378-8741(91)90136-2. [DOI] [PubMed] [Google Scholar]
- 5.Shin TY, Park JH, Kim HM. Effect of Cryptotympana atrata extract on compound 48/80-induced anaphylactic reactions. J Ethnopharmacol. 1999;66:319–25. doi: 10.1016/s0378-8741(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 6.Diepgen TL. Occupational skin diseases. J Dtsch Dermatol Ges. 2012;10:297–313. doi: 10.1111/j.1610-0387.2012.07890.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D, Heidary N. Treatment of irritant and allergic contact dermatitis. Dermatol Ther. 2004;17:334–40. doi: 10.1111/j.1396-0296.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 8.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116:517–24. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Lee MR, Lee GS, An WG, Cho SI. Effect of Sophora flavescens Aiton extract on degranulation of mast cells and contact dermatitis induced by dinitrofluorobenzene in mice. J Ethnopharmacol. 2012;142:253–8. doi: 10.1016/j.jep.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha JD, Li S, Kim HK, Cha IH. Cytoplasmic HuR expression: Correlation with cellular inhibitors of apoptosis protein-2 expression and clinicopathologic factors in oral squamous cell carcinoma cells. Head Neck. 2013 doi: 10.1002/hed.23431. In press. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–9. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 13.Ma SP, Qu R, Hang BQ. Immunologic inhibition and anti-allergic action of Cryptotympana atrata Fabricius. Zhongguo Zhong Yao Za Zhi. 1989;14:490–3. 512. [PubMed] [Google Scholar]
- 14.Shin TY, Park JH, Kim HM. Effect of Cryptotympana atrata extract on compound 48/80-induced anaphylactic reactions. J Ethnopharmacol. 1999;66:319–25. doi: 10.1016/s0378-8741(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 15.Olejnik AK, Brzezińska-Błaszczyk E. Tumor necrosis factor alpha (TNF-alpha) modulates rat mast cell reactivity. Immunol Lett. 1998;64:167–71. doi: 10.1016/s0165-2478(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 16.Kanda N, Watanabe S. Histamine enhances the production of granulocyte-macrophage colony-stimulating factor via protein kinase Calpha and extracellular signal-regulated kinase in human keratinocytes. J Invest Dermatol. 2004;122:863–72. doi: 10.1111/j.0022-202X.2004.22432.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagao K, Akabane H, Masuda T, Komai M, Tanaka H, Nagai H. Effect of MX-68 on airway inflammation and hyperresponsiveness in mice and guinea-pigs. J Pharm Pharmacol. 2004;56:187–96. doi: 10.1211/0022357022548. [DOI] [PubMed] [Google Scholar]
- 18.Smith JG, Jr, Wehr RF, Chalker DK. Corticosteroid-induced cutaneous atrophy and telangiectasia. Experimental production associated with weight loss in rats. Arch Dermatol. 1976;112:1115–7. [PubMed] [Google Scholar]


