Abstract
Simple and reliable genotyping technology is a key to success for high-throughput genetic screening in the post-genome era. Here we have developed a new real-time PCR genotyping approach that uses displacement hybridization-based probes: displacing probes. The specificity of displacing probes could be simply assessed through denaturation analysis before genotyping was implemented, and the probes designed with maximal specificity also showed the greatest detection sensitivity. The ease in design, the simple single-dye labeling chemistry and the capability to adopt degenerated negative strands for point mutation genotyping make the displacing probes both cost effective and easy to use. The feasibility of this method was first tested by detecting the C282Y mutation in the human hemochromatosis gene. The robustness of this approach was then validated by simultaneous genotyping of five different types of mutation in the human β-globin gene. Sixty-two human genomic DNA samples with nine known genotypes were accurately detected, 32 random clinical samples were successfully screened and 114 double-blind DNA samples were all correctly genotyped. The combined merits of reliability, flexibility and simplicity should make this method suitable for routine clinical testing and large-scale genetic screening.
INTRODUCTION
Disease-related genetic variations, such as single nucleotide polymorphisms (SNPs), deletions and insertions, are currently being identified at a remarkable pace, providing a rich resource with vast potential for clinical diagnostics and pharmacogenetics (1,2). High-throughput, cost-effective genotyping methods are essential for making the most advantageous and immediate use of these mutation data. Although DNA sequencing has been successfully used for the discovery of genetic variations, simpler, faster and more automated genotyping methods are needed for routine use and population studies, especially when only a small number of hot spots are focused (3).
Homogenous genotyping that requires little post-manipulation is becoming one of the leading techniques to fulfill the above goal (4,5). Such a trend has been accelerated with the availability of real-time PCR that combines amplification with on-line detection (6). To date, a variety of probe-based real-time PCR genotyping strategies have been developed. Most of these methods involve PCR amplification of a short DNA fragment containing the polymorphism sites to be detected in the presence of specific detection probes. These probes are labeled with given fluorophores that allow the recognition of different alleles in a single tube. Currently, widely used probes include 5′-nuclease probes (7), fluorescent resonance energy transfer (FRET) probes (8) and molecular beacons (9). Though more or less satisfied in practical use, the main disadvantage of these probe-based methods is that design, synthesis and purification of the probes are difficult and expensive. Moreover, extensive optimization of reaction conditions is often required when simultaneous detection of multiple alleles is involved. Therefore, their applications in simultaneous genotyping of different kinds of mutations have not been well established so far.
We recently reported a new class of homogeneous nucleic acid probe based on specific displacement hybridization (10). These so-called displacing probes are composed of two complementary oligonucleotides of different lengths. The longer positive strand is labeled with a fluorophore at the 5′-end and the shorter negative strand is labeled with a quencher at the 3′-terminus, so that the fluorophore and the quencher groups are in close contact in the duplex probe. Thus, in the absence of a target, the probe is non-fluorescent due to the contact-quenching effect between the fluorophore and the quencher. In the presence of a target, the negative stand can be displaced and the fluorophore becomes fluorescent. We found that such displacing probes are readily capable of discriminating among targets that differ by only a single nucleotide. Particularly, displacing probes are much easier to design and cheaper and simpler to synthesize in comparison with other types of probes currently in use.
The advantageous features of the displacing probes prompted us to explore their potential utilizations in real-time PCR genotyping. We expect that the specificity of displacing probes will be further enhanced at annealing temperatures in PCR and should be able to discriminate single nucleotide variations as well as all other types of mutation, such as deletions and insertions. Two examples were used to validate the displacing probe-based genotyping methods. One is the genotyping of the human hemochromatosis (HFE) gene harboring a C282Y mutation that is responsible for hereditary hemochromatosis; the other is the simultaneous genotyping of the human β-globin (HBB) gene that involves five representative causative mutations for β-thalassemia among Chinese populations. Our results showed that the displacing probe-based real-time PCR method is not only able to detect the difficult G→A mutation in the HFE, but also able to simultaneously detect five different mutations that include deletion, insertion, transition and transversion in the HBB gene. The reliability of this approach was also demonstrated by testing human genomic samples of known genotypes as well as random screening and a double-blind analysis.
MATERIALS AND METHODS
Probe preparation
The positive and the negative strands of displacing probes were synthesized and PAGE-purified by Shanghai Shenyou Ltd (Shanghai, China). Displacing probes were prepared by mixing 5.0 nmol positive strand and 6.0 nmol negative strand in 50 µl of 10 mM Tris–HCl (pH 8.0) containing 1.5 mM MgCl2. The concentration of the positive strand was used to represent the concentration of the probes.
Denaturation analysis
Denaturation analysis was carried out by recording the fluorescence intensity of a 50 µl solution (20 mM Tris–HCl pH 8.0, 2.0 mM MgCl2) containing 0.1 µM displacing probes in the presence of 0.2 µM oligonucleotide targets at temperatures ranging from 72 to 35°C. On the iCycler (Bio-Rad), the solution was denatured at 95°C for 10 s, followed by annealing at 72°C for 15 s. This procedure was repeated for 37 cycles with 1°C decrease in the annealing temperature at each cycle. Fluorescence was recorded during the annealing stage. For comparison, the fluorescence values of each probe were normalized by the equation: (F – Fmin)/(Fmax – Fmin), where F is the measured fluorescence intensity, Fmin is the lowest fluorescence intensity and Fmax is the highest fluorescence intensity detected.
Real-time PCR genotyping protocol
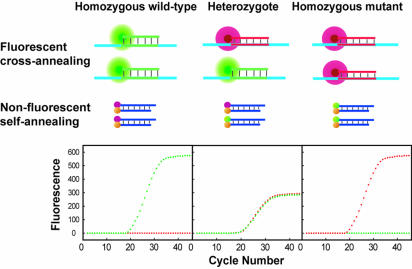
The principle of real-time PCR genotyping using displacing probes is illustrated in Figure 1. For genotyping of each allele, one pair of displacing probes was used. The one specific for the wild-type allele was labeled with a green fluorophore, Fam, and the other one specific for the mutant allele was labeled with a red fluorophore, Texas Red. The generation of green fluorescence during amplification indicates homozygous wild type, red fluorescence indicates homozygous mutants, and both green and red fluorescence indicates heterozygotes. For scatter plot analysis, the automatically generated threshold cycle values from each sample were plotted at coordinates that correspond to the signal of either Fam or Texas Red.
Figure 1.
Principle of real-time PCR genotyping using displacing probes. A pair of displacing probes is included in the reaction. In the presence of wild-type templates, only Fam-labeled wild-type probes hybridize to the amplicons and generate green fluorescence (fluorescent cross-annealing), whereas the Texas Red-labeled mutant probes retain their duplex and cannot produce red fluorescence (non-fluorescent self-annealing). In the presence of heterozygous templates, both wild-type and mutant probes hybridize to amplicons and generate both green and red fluorescence. In the presence of mutant templates, only the Texas Red-labeled mutant probes hybridize to the amplicons, generating red fluorescence, whereas Fam-labeled wild-type probes remain dark.
Real-time PCR was performed in the IQ iCycler. PCR conditions were identical for all reactions unless noted elsewhere. The 25 µl reactions contained 5 µl of DNA template (1.0 × 106 copies of plasmid or 75.0 ng of genomic DNA), 0.2 µM (5.0 pmol) of each forward and reverse primer, 0.1 µM (2.5 pmol) of wild-type and mutant probes, 3.0 mM MgCl2 in a PCR master mix (10 mM Tris–HCl pH 8.0, 2.0 U Taq DNA polymerase, 200 µM dNTPs and 50 mM KCl). The cycling was initiated by heating at 95°C for 3 min; followed by 40 cycles of 95°C for 15 s, 52°C for 20 s and 72°C for 25 s. Fluorescence from both Fam and Texas Red channels was recorded at the annealing step.
Genotyping of HFE gene
The sequences of the two allele-discriminating displacing probes for genotyping of HFE involving C282Y mutation were 5′-Fam-AGATATACGTGCCAGGTGGAG-PO4-3′/5′- CCACCTGGCACGTATATCT-3′-Dabcyl (wild-type probe, the underlined nucleotide denotes the variant site) and 5′-Texas Red-AGATATACGTACCAGGTGGAG-PO4-3′/5′-CCACCTGGTACGTATATCT-3′-Dabcyl (mutant probe), respectively. The sequences of the forward and the reverse primers for PCR amplification of the polymorphic region were 5′-TGGCAAGGGTAAACAGATCC-3′ and 5′-CTCAGGC ACTCCTCTCAACC-3′, respectively.
For PCR–restriction fragment length polymorphism (RFLP) analysis, PCRs were performed on a T3 thermocycler (Biometra) with the same reaction mixture and conditions, but omitting the probes in the reactions. Twenty microliters of PCR product were mixed with RsaI digestion solution (Promega) containing 2 µl of 10× reaction buffer, 1 µl of 10 U/µl RsaI and 0.5 µl of 10 mg/ml BSA, incubated first at 37°C for 50 min and then at 50°C for 30 min. The products were analyzed on a 3.0% agarose gel stained with ethidium bromide.
Wild-type DNA templates were extracted from different hair samples provided by volunteers from the lab. Mutant DNA templates were artificial plasmids prepared by PCR-mediated in vitro mutagenesis (11). The heterozygotes were produced by mixing wild-type genomic DNA templates in equimolar with mutant plasmid DNA templates.
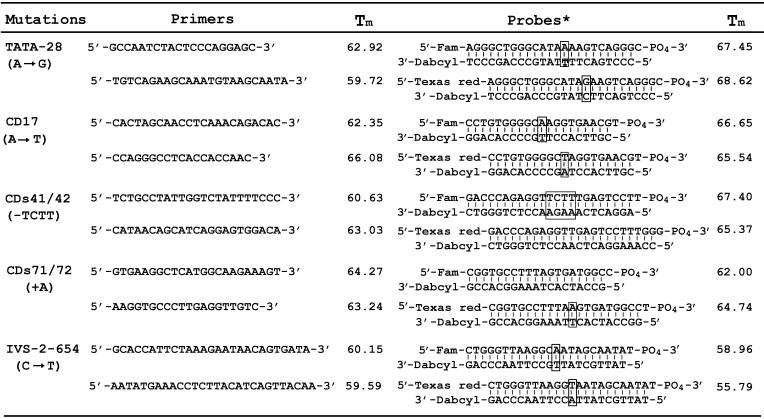
Multiplex genotyping of HBB
Five pairs of allele-discriminating probes and five pairs of primers flanking the variant sites were listed in Table 1. Each DNA sample was divided into five reaction tubes containing different pairs of allele-discriminating probes and the amplification primers. The five reactions were run in parallel in the iCycler. In a standard experiment, a total of 95 reactions containing 18 samples (each sample has five reactions) and five negative controls were run in one round.
Table 1. Primer and probe sequences used in the HBB genotyping assay.
Blocked bases denote mutation sites. The Tm of the negative strand denotes the Tm of the probe.
Artificial DNA templates corresponding to each allele were prepared by PCR-mediated in vitro mutagenesis and their sequences were confirmed by bi-directional sequencing. Human genomic DNA samples from 62 patients of known genotype were kindly provided by Professor Xiangmin Xu of the First Military Medical University, Guangzhou. Thirty-two clinical samples were from No.1 City Hospital of Xiamen and informed consent was obtained from the individual patients regarding the random screening for β-thalassemia. Human genomic DNA was isolated from leukocytes in peripheral blood using a modification of salting-out procedure (12). Purified DNA was dissolved in TE buffer (10 mM Tris–HCl, 1 mM EDTA pH 8.0) and stored at –20°C until use.
Final validation of the multiplexing assay was accomplished by a double-blind analysis of 114 patients samples. The genotypes of these samples were determined previously either by reverse dot-blot analysis or by dHPLC. Samples were coded and assayed by different individuals, and results were scored independently by the person performing the assay and by a third individual.
RESULTS
Design of displacing probes
Two basic principles were followed in the design of the allele-discriminating displacement probes. Firstly, the variant site should be placed in the centre of the probes, as we previously found that mismatch discrimination tends to be more specific in the centre of the probe than near either end of the probe. Secondly, the melting temperature (Tm) of the probe should be higher than that of the duplex formed between the probe and the mismatched target, but be lower than the Tm of the duplex formed between the probe and the matched target. This principle is based on the assumption that the displacing probe has three energy states in the presence of the matched and mismatched target. The duplex formed between the probe and the matched target is at the lowest energy level, the probe itself is at the middle energy level, and the duplex formed between the probe and the mismatched target is at the highest energy level. Therefore, the probe will thermodynamically prefer to react with the matched target or choose to stay self-annealed, yet least likely to hybridize with the mismatched target.
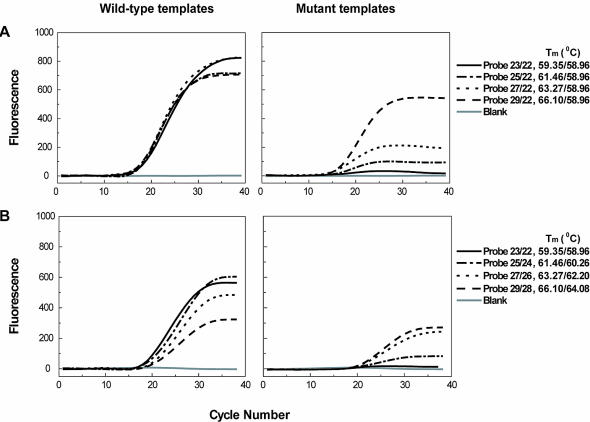
Obviously, the relative length of the negative strand and the positive strand in the displacing probe plays a crucial role in determining the specificity of the probes. To study the length effect, we first prepared a series of displacing probes made up of one common negative strand of fixed length and positive strands of different length. We checked their allele-discriminating ability using both wild-type and mutant templates. As shown in Figure 2A, a proportional decrease in allele-discriminating ability was observed as length difference increased. Interestingly, we also found that while it showed the highest allele-discriminating ability, probe 22/23 also gave relative high end-fluorescence intensity, which is a determinant factor in detection sensitivity. To further investigate the length effect of displacing probes, we prepared another series of probes with fixed length difference but varying in total length. Figure 2B shows that both the end-fluorescence intensity and allele-discriminating ability increase with the decrease of the total length of the displacing probes. These probes were also studied with a series of wild-type template concentrations regarding their detection sensitivity. It was found that, at the same concentration, the end-fluorescence intensities of the probes followed the order of probe 23/22 > probe 25/24 > probe 27/26 > probe 29/28 and the threshold cycle values followed the reverse order. Taken together, these observations demonstrated that probes with smaller length difference and total length have greater allele-discriminating ability and also higher detection sensitivity. The significance of this finding is that sensitivity and specificity of the displacing probe are in accordance with the length effect, which greatly simplifies the probe design. Detailed design guidelines of displacing probes for SNP genotyping are provided as Supplementary Material.
Figure 2.
The effect of the length difference between the negative strand and the positive strand (A), and the effect of the length of the probe (B) on the detection sensitivity and specificity of the displacing probe-based real-time PCR. Real-time PCR was carried out using the primer pair for IVS-2–654 site in the HBB gene with both wild-type and mutant templates. Water was used as the blank template. The probes of varied length used were based on the wild-type probe for the IVS-2–654 site. The probe was named according to the numbers of nucleotide of its positive (before) and negative strands (after), which were separated by a slash (e.g. probe 29/28, 5′-Fam-CTGGGTTAAGGCAATAGCAATATCTCTGC-PO4-3′/5′-CAGAGATATTGCTATTGC CTTAACCCAG-Dabcyl-3′, has a positive strand of 29 nt and a negative strand of 28 nt). The corresponding Tm values of both positive and negative strands are shown separately and are separated by a slash. The length change was made starting from the 3′-end of the positive strand, and accordingly from the 5′-end of the negative strands. Nucleotide changes were based on the original HBB sequence line.
In the case of single nucleotide substitution genotyping, there is only one nucleotide difference between the negative strands used in both wild-type and mutant probes. We checked whether one shared degenerated negative strand could be used to replace the two negative strands. For example, the negative strand of the wild-type probe and the mutant probe is 5′-TATTGCTATTACCTTAACCCAG-Dabcyl-3′ and 5′-TATT GCTATTGCCTTAACCCAG-Dabcyl-3′, respectively. These two strands differ only in one nucleotide at the underlined position. They can be replaced with one strand of 5′-TAT TGCTATTRCCTTAACCCAG-Dabcyl-3′ accordingly. It turned out to be that, using one such degenerated negative strand with doubled concentration, the same genotyping results could be obtained as using two different negative strands (data not shown). Successful substitution of one degenerated negative strand for the two different negative strands is helpful for reducing the synthesis cost as well as the labor in probe labeling and quality control.
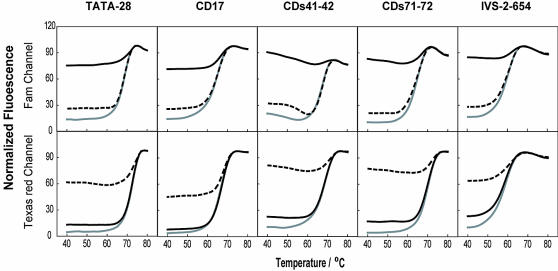
Specificity evaluation of displacing probes with denaturation analysis
Denaturation analysis is a characteristic evaluation procedure for displacing probes. From the denaturation curves, the exact Tm values can be obtained, and the overall quality of a displacing probe can be evaluated as well. In addition, by conducting denaturation analysis in the presence of the wild-type and the mutant oligonucleotide targets, the temperature ranges allowing good allele discrimination can be drawn. The probes listed in Table 1 for HBB genotyping were subjected to denaturation analysis. As shown in Figure 3, the wild-type probes only hybridized with the wild-type but not with mutant targets below their melting temperature, thus only wild-type targets produced a signal from the Fam channel. Likewise, the mutant probes hybridized only with mutant target but not with wild-type sequences, thus only mutant targets generated signal from the Texas Red channel. The denaturation analysis indicates that displacing probes can recognize single nucleotide variations as well as insertions and deletions. It is important to emphasize that all of the probes can achieve allelic discrimination over a wide range of temperatures (ranging from 40–60°C) despite their different Tms. These observations form a solid basis for simultaneous genotyping of different alleles.
Figure 3.
Thermal denaturation curves of the five pairs of displacing probes used for HBB genotyping. The fluorescence intensity of solutions containing wild-type probes (upper panel) and mutant probes (lower panel) was plotted as a function of temperature in the absence of targets (gray solid lines), in the presence of the wild-type target (black solid lines) and in the presence of the mutant targets (dashed lines). The wild-type targets are oligonucleotides perfectly complementary to the positive strand of the wild-type probes, and the mutant targets are oligonucleotides perfectly complementary to the positive strand of the mutant-type probes.
The sensitivity of displacing probes in real-time PCR
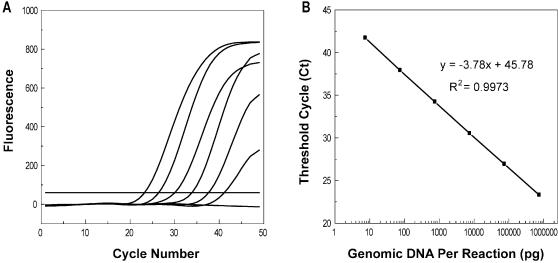
The ability to detect DNA template in wide concentration ranges is important for practical genotyping that may involve samples from various resources. We checked the sensitivity of the designed probes using human genomic DNA. The results showed that all probes could detect human genomic DNA in the range of 7.5 × 10–3–7.5 × 102 ng per 25-µl reaction (Fig. 4).
Figure 4.
Real-time PCR quantification using displacing probes. PCR was conducted using the primer pair and the wild-type probe for IVS-2–654. Ten-fold serial dilutions of each genomic DNA template, ranging from 7.5 × 102–7.5 × 10–3 ng (A, from left to right) per 25-µl reaction and the negative control were amplified. The linear relationship (B) between the number of threshold cycle values and the logarithm of the mass amount of genomic DNA present in a sample demonstrated that quantitative determinations could be made over a target concentration range of at least six orders of magnitude.
Real-time PCR genotyping of HFE using displacing probes
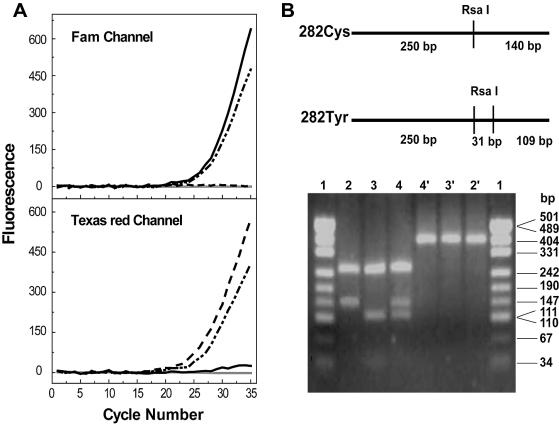
In our initial evaluation of the possibility of displacing probes in real-time PCR genotyping, we used the C282Y mutation in the HFE gene as a model. The C282Y (G→A) mutation in the HFE gene is one of the most common single gene disorders that causes hereditary hemochromatosis in Caucasians (13). The difficulty in detecting this mutation with classical single-stranded probes lies in the fact that the G/T is the least destabilized mismatch, and thus requires the probes to be extremely specific. Using a pair of displacing probes, we performed real-time PCR on 20 artificial DNA templates with known genotypes. The results of PCRs with wild-type, mutant and heterozygous templates are shown in Figure 5. The color of the fluorescence that developed in real-time PCR clearly identified all the genotypes. Real-time PCR genotyping results were also confirmed by the classical PCR–RFLP method.
Figure 5.
(A) Real-time PCR genotyping of HFE. Typical genotyping results were from four PCRs with wild-type (black solid line), mutant type (dashed line), heterozygous templates (dash-dotted line) and negative control (gray solid line) respectively. The color of the fluorescence that developed during PCR identified each of the three genotypes. (B) PCR–RFLP results. The upper panel showed the restriction sites located in the wild-type amplicon (282Cys) and mutant amplicon (282Tyr). The lower panel shows the electrophoresis results of different amplicons treated or not treated with RsaI. The wild-type PCR product (lane 2) was cleaved into two fragments, 250 and 140 bp; the mutant product (lane 3) was cut to three fragments, 250, 111 and 29 bp (note: the 29 bp fragment band is hardly visualized in this figure); and the heterozygous product (lane 4) was digested into four fragments. Lanes 2′, 3′, and 4′ showed the corresponding undigested product of the wild-type, mutant and heterozygous type, all of which were 390 bp. Lane 1 and 1′ were pUC19-MspI (HapII) markers.
Real-time PCR genotyping of HBB using multiple displacing probes
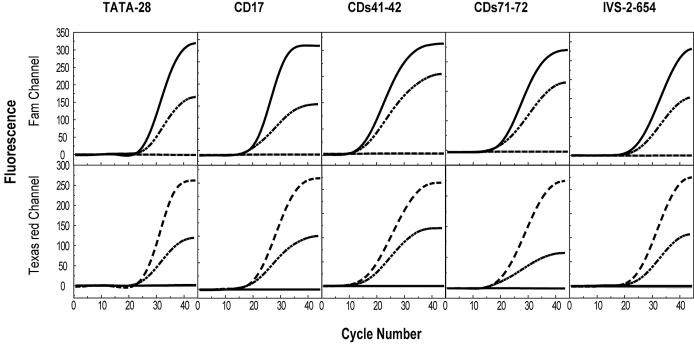
In order to explore the applicability of displacing probes in a complicated case that may involve multiple mutations of different kinds, we chose the five most common causative mutations in HBB for β-thalassemia in the Chinese population (14). Although ∼200 different mutations have so far been reported worldwide for this disorder, each representative ethnic group features its own particular common mutations (15). The five Chinese-featured mutations dispersed within a 1.5 kb region are widely diversified, including one transversion, i.e. CD17 (A→T), two transitions, i.e. TATA-28 (A→G) and IVS-2–654 (C→T), one small deletion, i.e. CDs41–42 (–TCTT) and one insertion, i.e. CDs71–72 (+A). By using five pairs of displacing probes, we performed five PCRs in parallel for each artificial template. The annealing temperature of 52°C was chosen for all reactions, though we noted that the temperatures within the range from 50 to 60°C were all applicable. The color of the fluorescence that developed in real-time PCR unequivocally identified which of the 15 possible genotypes was present (Fig. 6). These results demonstrated the excellent allele-discriminating ability of the displacing probe, as well as their distinguished adaptability to varied assay conditions.
Figure 6.
Simultaneous real-time PCR genotyping of HBB gene containing five mutations. Fluorescence was recorded at both Fam and Texas Red channels corresponding to the wild-type and mutant probes, respectively. For each mutation, typical three PCR results were shown corresponding to the three genotypes of the templates, they were wild-types (black solid lines), mutant types (dashed lines) and heterozygotes (dash-dotted lines). Templates used were artificial plasmid DNA. Negative control is shown as a gray solid line.
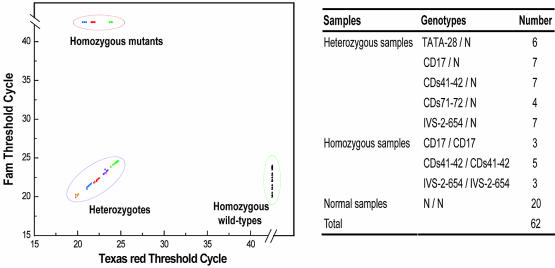
We then determined the genotypes of 62 human genomic DNA samples of nine known alleles and all of the samples were correctly identified. According to the threshold values that were generated automatically at the end of PCR, the ease with which all nine genotypes could be distinguished from each other is shown in Figure 7. In the random screening of 32 patients, we detected three heterozygous samples, i.e. two CD17/N genotypes and one CDs41–42/N genotype. These genotypes were confirmed by sequencing. It is known that these two genotypes are most commonly found in this demographic region, and the incidence of 9.4% (3/32) detected here is consistent with the recently reported number of 10% among the local population (14). When compared with sequencing, real-time PCR genotyping is obviously advantageous in its unambiguity, especially in the case of frameshift mutation.
Figure 7.
Simultaneous genotyping results of HBB gene from 62 human genomic DNA samples. Human genomic DNA samples of known genotype were listed on the right. The results from each sample were plotted at coordinates that correspond to the threshold cycle for the signal in either wild-type (Fam) or mutant probe (Texas Red) (left panel). For the wild-type samples, only one threshold value was plotted for each sample as the five PCRs gave similar results. In the mutant and heterozygous group, mutation types were illustrated with different colors. Note that the three major genotypes were clearly separated into three groups. The 20 wild-type samples were clustered in the group located in the lower right corner. The 11 homozygous mutant samples were clustered in the group located in the upper left corner, including three samples of CD17/CD17 (blue), five samples of CDs41–42/CDs41–42 (red) and three samples of IVS-2–654/ IVS-2–654 (green). The 31 heterozygous samples were clustered and located in the lower left corner, including six samples of –28(A→G)/N (orange), seven samples of CD17 (A→T)/N (blue), seven samples of CDs41–42 (–TCTT)/N (red), four samples of CDs71–72 (+A)/N (yellow) and seven samples of IVS-2–654 (C→T)/N (green).
As a final validation of the simultaneous multiplex HBB genotyping, we performed a double-blind analysis of 114 patient samples. Our method identified a total of 14 genotypes, including 19 wild-type, 84 heterozygous, i.e. TATA-28/N(12), CD17/N(7), CDs41–42/N(34), CDs71–72/N(3), IVS-2–654/N(28), seven compound heterozygous, i.e. TATA-28/CD17(1), TATA-28/CDs41–42(1), TATA-28/ IVS-2–654(1), CD17/IVS-2–654(2), CDs41–42/IVS-2–654(1), CDs41–42/CDs71–72(1) and four homozygous, i.e. CDs41–42/CDs41–42(3), IVS-2–654/IVS-2–654(1). The genotypes scored by both individuals were completely concordant, and all 114 samples were correctly genotyped.
DISCUSSION
Displacing probes were initially developed based on the spontaneous displacement hybridization between a double-stranded oligonucleotide and a single-stranded oligonucleotide target. Their specificity has been demonstrated by the single-nucleotide variation discrimination at room temperature, where the sequential displacement is the dominant pathway in the kinetics of displacement hybridization. In the case of PCR, however, the dissociation pathway prevails since the annealing step is always after the denaturation step. Thus, the annealing step will involve both self-annealing of the probes themselves and cross-annealing between the probes and the template DNA. In the presence of matched DNA templates, the cross-annealing will dominate over self-annealing since the positive strands prefer to bind to the targets that are longer than the negative strands. On the other hand, if there is a mismatch between the probe and the target, the cross-annealing would involve two mismatched hybridizations, i.e. hybridizations of the positive and the negative strands with each of their mismatched targets, both of which are thermodynamically unfavorable compared with the self-annealing of the probes themselves. In other words, the positive and the negative strands are synergistic in allele discrimination. Accordingly, probes with smaller length difference between the positive and the negative strands will be more effective in allele discrimination.
The synergistic specificity-enhancing effect inherent in displacing probes allows allele discrimination within a wide temperature range, a characteristic that is indispensable for genotyping of various mutations under non-stringent conditions. Such a synergistic effect, however, cannot be found among the existing single-stranded probes, which may partially explain the limitations of the single-stranded probe-based real-time PCR genotyping. A prerequisite for real-time PCR probes is that the probes must be bound to the template at the annealing temperature. In the case of 5′-nuclease chemistry, the qualified probes need to be of 25–40 nucleotides in order to form sufficiently stable hybrids. While probes of such size are generally suitable for ordinary quantification of, for example, viral load determination or gene expression monitoring, they are too long for most SNP detections. Usually, the Tm difference between a perfectly matched duplex of 25 bp long and a similar duplex with a single mismatch is only ∼3°C. Without careful optimization of probe sequence and PCR conditions, it would be difficult, if not impossible, to detect SNPs with an acceptable signal-to-noise ratio for routine assays (16). So far, many modifications have been made to improve the allele-discriminating ability of the single-stranded probes. For example, an additional MGB (minor groove binding) group is attached to TaqMan probes (16,17), or a hairpin structure of a molecular beacon has been designed (18,19), or shorter probes with much lower Tm have been designed (20), to enhance the allele-discriminating ability. In most cases, these approaches have been proved to be effective, though some drawbacks, e.g. increased difficulties in probe design, or extra costs for probe synthesis and labeling, or rather stringent annealing temperatures, are often associated with these modifications. Instead of real-time PCR genotyping, post-PCR melting analysis-based genotyping using FRET probes makes mutation-induced Tm change easily resolvable (8,21). However, the number of mutations that can be analyzed within a single reaction is restricted by the number of melting peaks that can be distinguished over the range of probe melting temperatures. Also, there is a risk of ‘temperature cross talk’ between different sensor/anchor probes that may lead to erroneous interpretation in multiplex genotyping. Moreover, the dependence of the quality of melting analysis in LightCycler technology on both melting protocol and annealing conditions makes the process complicated and sometimes even impossible for simultaneous multiplex genotyping. Thus, the displacing probes, with their intrinsic, synergistic specificity-enhancing characteristics, provide good alternatives for simple yet reliable homogenous real-time genotyping chemistry.
We also noted that the displacing probes with higher specificity have higher detection sensitivity as well. This coupled merit constitutes another characteristic of displacing probes, which not only makes them suitable for analyzing raw samples of various resources, but also greatly simplifies their design. The sensitivity of displacing probes in real-time PCR detection is determined by two factors: the signal-generating ability of the probes, and the potential interference of the probes with target amplification. The signal-generating ability is controlled by the hybridization efficiency between the probes and their corresponding targets. Any probe that can form a stable duplex with the target can produce a signal. Longer probes will not necessarily generate stronger signals than the shorter ones. Conversely, shorter probes will leave more easily from the target than longer probes when extension begins, bringing less interference into the primer extension. Taken together, displacing probes should be as short as possible in order to achieve higher sensitivity, so long as their Tm values are high enough to endure the annealing temperature. In practice, displacing probes as short as the primers are preferred. This design principle is distinct from that of single-stranded probes, which generally requires the probe’s Tm to be 7–10°C higher than the annealing temperature. In the latter case, effects of interference from the probe annealing on primer extension can be more significant in lowering the total amplification efficiency.
Another merit of the displacing probes, in addition to their simple design, is that each of their strands can be separately synthesized and purified with much higher yield than the dual- or ternary-labeled probes of the same length. With our technique, one 3′ Dabcyl CPG column of 50-nmole scale (3′ Succinate Linkage DABCYL Synthesis Column, 5′-DMT-mdC(TEG-DABCYL)-Succinate-CPG, 500 Å, Biosearch Technologies, Inc. Novato CA, USA) can be used for synthesis of ∼1–3 OD of a single-labeled oligonucleotide at PAGE or HPLC purity, whereas it is not enough for synthesis of a dual-labeled oligonucleotide at any meaningful scale. Similarly, one 3′ Dabcyl CPG column of 200-nmole scale can be used for synthesis of ∼10–15 OD of a single-labeled oligonucleotide at PAGE or HPLC purity, whereas it can be used for synthesis of only ∼2–3 OD for a dual-labeled oligonucleotide of the same purity. Although the combined length of the strands in displacing probes is actually longer than single-stranded probes, the cost to synthesize them is otherwise significantly less than that of dye modification. In addition, the use of the degenerated negative strand in SNP genotyping will further reduce the expense of probe synthesis and labeling, as well as the hands-on time in quality control.
The accurate genotyping of both HFE and HBB genes demonstrated the robustness and flexibility of the displacing probe-based real-time PCR method. Not only is this method specific enough to detect the difficult C282Y mutation, it can also simultaneously detect five mutations of various types. In the latter case, the probes designed had Tms ranging from 55.79 to 67.45°C (Table 1). However, they could work under the same conditions, thus the condition optimization was greatly simplified. The simplicity of the real-time PCR genotyping protocol is obvious since the generated threshold cycle values for each sample could be directly used for automatic genotype assignment.
Genotyping of both HFE (22–27) and HBB (28–30) have been extensively studied using FRET probe-based post-PCR melting analysis, yet only HFE has been genotyped directly by real-time PCR using 5′-nuclease probes (16,20,31,32). It was found that traditional TaqMan probes, if not modified with a proprietary MGB group, failed to accurately distinguish wild-type from mutant alleles (16,32). By using shorter 5′-nuclease probes with much lower annealing/extension temperatures, multiplex HFE genotyping could be successfully implemented (20). Nevertheless, the narrow temperature window for allele discrimination demands a delicate experimental optimization to minimize mismatch hybridization. Moreover, the much lower annealing/extension temperature used inevitably lowers the total amplification efficiency and the final detection sensitivity. Although FRET probe-based melting analysis has been well established for both HFE and HBB genotyping, some difficulties remain to be overcome. For example, in HFE multiplex genotyping, an unusual melting curve profile was found to prevent allelic discrimination (27). The difficulties become more complicated in simultaneous HBB genotyping that involves multiple genotypes. As was observed, when using detection probes complementary to the mutant sequence (30), the melting curves for some mutant alleles were not clearly distinguished from the wild-type allele (28). Conversely, when detection probes complementary to the wild-type sequence were used, some different mutations displayed identical melting curves. Furthermore, variance occurred in the annealing program among different mutations and this might exclude FRET probes from simultaneous multiplex genotyping even though an identical PCR program was used (29). It is noteworthy that all current genotyping strategies using FRET probes were focused only on those closely clustered mutations, enabling one pair of PCR primers to encompass all the mutation sites. In the present case of the five Chinese-featured mutations that are widely dispersed within a 1.5 kb region, separate PCR primer pairs will have to be used, making simultaneous genotyping more difficult for FRET probe-based melting analysis. Because it will not only need different anchor/sensor probe pairs and PCR primer pairs to be designed to work under the same conditions, but will also need the annealing program among mutations to stay the same before melting curve acquisition.
In summary, we report in this paper that displacing probes could be successfully used in real-time PCR genotyping. The synergistic specificity-enhancing effect inherent in displacing probes allows allele discrimination of various mutations under non-stringent conditions. Especially the concordance of specificity and sensitivity makes these probes not only sensitive enough for analyzing raw samples of various sources, but also greatly simplifies their design. The proposed method is also cost effective compared with existing single-stranded probe-based genotyping. Thus, with its combined virtues of specificity, flexibility and simplicity, the displacing probe-based genotyping merits consideration in routine clinical testing and large-scale genetic screening.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Xilin Zhao, Fred Russell Kramer and Shengcai Lin for insightful comments on the manuscript, Guanshun Liao for technical assistance and Xiangmin Xu for providing the human DNA samples. This work was supported by National Science Foundation of China (grant No. 30170834) and Fujian Science Foundation (Key Program No. C0220002).
REFERENCES
- 1.Weiss K.M. (1998) In search of human variation. Genome Res., 8, 691–697. [DOI] [PubMed] [Google Scholar]
- 2.Mir K.U. and Southern,E.M. (2000) Sequence variation in genes and genomic DNA: methods for large-scale analysis. Annu. Rev. Genomics Hum. Genet., 1, 329–360. [DOI] [PubMed] [Google Scholar]
- 3.Kwok P.Y. (2000) High-throughput genotyping assay approaches. Pharmacogenomics, 1, 95–100. [DOI] [PubMed] [Google Scholar]
- 4.Foy C.A. and Parkes,H.C. (2001) Emerging homogeneous DNA-based technologies in the clinical laboratory. Clin. Chem., 47, 990–1000. [PubMed] [Google Scholar]
- 5.Germer S. and Higuchi,R. (2003) Homogeneous allele-specific PCR in SNP genotyping. Methods Mol. Biol., 212, 197–214. [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer B. and Kingsmore,S. (2001) Combining nucleic acid amplification and detection. Curr. Opin. Biotechnol., 12, 21–27. [DOI] [PubMed] [Google Scholar]
- 7.Livak K.J. (2003) SNP genotyping by the 5′-nuclease reaction. Methods Mol. Biol., 212, 129–147. [DOI] [PubMed] [Google Scholar]
- 8.Wittwer C.T., Herrmann,M.G., Gundry,C.N. and Elenitoba-Johnson,K.S. (2001) Real-time multiplex PCR assays. Methods, 25, 430–442. [DOI] [PubMed] [Google Scholar]
- 9.Marras S.A., Kramer,F.R. and Tyagi,S. (2003) Genotyping SNPs with molecular beacons. Methods Mol. Biol., 212, 111–128. [DOI] [PubMed] [Google Scholar]
- 10.Li Q., Luan,G., Guo,Q. and Liang,J. (2002) A new class of homogeneous nucleic acid probes based on specific displacement hybridization. Nucleic Acids Res., 30, E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi R., Krummel,B. and Saiki,R.K. (1988) A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res., 16, 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh K. and Bunce,M. (1999) Molecular typing for the MHC with PCR-SSP. Rev. Immunogenet., 1, 157–176. [PubMed] [Google Scholar]
- 13.Feder J.N., Gnirke,A., Thomas,W., Tsuchihashi,Z., Ruddy,D.A., Basava,A., Dormishian,F., Domingo,R., Jr, Ellis,M.C., Fullan,A. et al. (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genet., 13, 399–408. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y.Q. and Xu,X.M. (2003) The molecular basis and prenatal diagnosis of beta-thalassemia in China. Foreign Medical Sciences (Genetics), 18, 132–137. [Google Scholar]
- 15.Hardison R.C., Chui,D.H., Riemer,C., Giardine,B., Lehvaslaiho,H., Wajcman,H. and Miller,W. (2001) Databases of human hemoglobin variants and other resources at the globin gene server. Hemoglobin, 25, 183–193. [DOI] [PubMed] [Google Scholar]
- 16.deKok J.B., Wiegerinck,E.T., Giesendorf,B.A. and Swinkels,D.W. (2002) Rapid genotyping of single nucleotide polymorphisms using novel minor groove binding DNA oligonucleotides (MGB probes). Hum. Mutat., 19, 554–559. [DOI] [PubMed] [Google Scholar]
- 17.Kutyavin I.V., Afonina,I.A., Mills,A., Gorn,V.V., Lukhtanov,E.A., Belousov,E.S., Singer,M.J., Walburger,D.K., Lokhov,S.G., Gall,A.A. et al. (2000) 3′-minor groove binder–DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res., 28, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi S., Marras,S.A. and Kramer,F.R. (2000) Wavelength-shifting molecular beacons. Nat. Biotechnol., 18, 1191–1196. [DOI] [PubMed] [Google Scholar]
- 19.Kostrikis L.G., Tyagi,S., Mhlanga,M.M., Ho,D.D. and Kramer,F.R. (1998) Spectral genotyping of human alleles. Science, 279, 1228–1229. [DOI] [PubMed] [Google Scholar]
- 20.Ugozzoli L.A., Chinn,D. and Hamby,K. (2002) Fluorescent multicolor multiplex homogeneous assay for the simultaneous analysis of the two most common hemochromatosis mutations. Anal. Biochem., 307, 47–53. [DOI] [PubMed] [Google Scholar]
- 21.Elenitoba-Johnson K.S., Bohling,S.D., Wittwer,C.T. and King,T.C. (2001) Multiplex PCR by multicolor fluorimetry and fluorescence melting curve analysis. Nat. Med., 7, 249–253. [DOI] [PubMed] [Google Scholar]
- 22.Bernard P.S., Ajioka,R.S., Kushner,J.P. and Wittwer,C.T. (1998) Homogeneous multiplex genotyping of hemochromatosis mutations with fluorescent hybridization probes. Am. J. Pathol., 153, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollhalder M., Mura,C., Landt,O. and Maly,F.E. (1999) LightCycler PCR assay for simultaneous detection of the H63D and S65C mutations in the HFE hemochromatosis gene based on opposite melting temperature shifts. Clin. Chem., 45, 2275–2278. [PubMed] [Google Scholar]
- 24.Mangasser-Stephan K., Tag,C., Reiser,A. and Gressner,A.M. (1999) Rapid genotyping of hemochromatosis gene mutations on the LightCycler with fluorescent hybridization probes. Clin. Chem., 45, 1875–1878. [PubMed] [Google Scholar]
- 25.Neoh S.H., Brisco,M.J., Firgaira,F.A., Trainor,K.J., Turner,D.R. and Morley,A.A. (1999) Rapid detection of the factor V Leiden (1691 G→A) and haemochromatosis (845 G→A) mutation by fluorescence resonance energy transfer (FRET) and real time PCR. J. Clin. Pathol., 52, 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks S.B., Popovich,B.W. and Press,R.D. (2001) Real-time polymerase chain reaction with fluorescent hybridization probes for the detection of prevalent mutations causing common thrombophilic and iron overload phenotypes. Am. J. Clin. Pathol., 115, 439–447. [DOI] [PubMed] [Google Scholar]
- 27.Tag C.G., Gressner,A.M. and Weiskirchen,R. (2001) An unusual melting curve profile in LightCycler multiplex genotyping of the hemochromatosis H63D/C282Y gene mutations. Clin. Biochem., 34, 511–515. [DOI] [PubMed] [Google Scholar]
- 28.Vrettou C., Traeger-Synodinos,J., Tzetis,M., Malamis,G. and Kanavakis,E. (2003) Rapid screening of multiple beta-globin gene mutations by real-time PCR on the LightCycler: application to carrier screening and prenatal diagnosis of thalassemia syndromes. Clin. Chem., 49, 769–776. [DOI] [PubMed] [Google Scholar]
- 29.Moreno I., Bolufer,P., Perez,M.L., Barragan,E. and Sanz,M.A. (2002) Rapid detection of the major Mediterranean beta-thalassaemia mutations by real-time polymerase chain reaction using fluorophore-labelled hybridization probes. Br. J. Haematol., 119, 554–557. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann M.G., Dobrowolski,S.F. and Wittwer,C.T. (2000) Rapid beta-globin genotyping by multiplexing probe melting temperature and color. Clin. Chem., 46, 425–428. [PubMed] [Google Scholar]
- 31.Restagno G., Gomez,A.M., Sbaiz,L., De Gobbi,M., Roetto,A., Bertino,E., Fabris,C., Fiorucci,G.C., Fortina,P. and Camaschella,C. (2000) A pilot C282Y hemochromatosis screening in Italian newborns by TaqMan technology. Genet. Test., 4, 177–181. [DOI] [PubMed] [Google Scholar]
- 32.Walburger D.K., Afonina,I.A. and Wydro,R. (2001) An improved real time PCR method for simultaneous detection of C282Y and H63D mutations in the HFE gene associated with hereditary hemochromatosis. Mutat. Res., 432, 69–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.