Abstract
Background:
Application of ultrasound (US) to biotechnology is relatively new but several processes that take place in the presence of cells or enzymes are activated by ultrasonic waves. Genista tinctoria L. (Fabaceae) is rich on various kind of flavonoids, including isoflavones with valuable estrogenic activity.
Objective:
This study verified use of low-energy US elicitor to enhance secondary metabolite production in plant cell cultures.
Materials and Methods:
Suspension cultures of G. tinctoria cells was exposed to low-power US (with fixed frequency 35 kHz and power level 0.1 mW/cm3) for period 1-5 min.
Results:
The US exposure significantly stimulated genistin content (0.8 mg/g DW) after 3 min of US treatment (sampled after 72 h). The highest daidzein level (1.4 mg/g DW) was reached after US irradiation for 5 min and 168 h sampling.
Conclusion:
The achieved results suggest that US can act as a potent abiotic elicitor to induce the defense responses of plant cells and to stimulate secondary metabolite production in plant cell cultures.
Keywords: Daidzein, Genista tinctoria, genistin, suspension, ultrasound
INTRODUCTION
In the last few years obtaining raw plant materials has become increasingly more and more difficult due to a decrease in plant sources, changes in the environment, or other factors.
In vitro cultures have been seen as an alternative source of biologically active compounds.[1] Therefore, various methods have been tested to enhance and to initiate secondary metabolite biosynthesis and production of important metabolites in in vitro plant cells.
The treatment of plant cells with biotic or abiotic elicitors has been one of the most effective approaches to improve the yields of secondary metabolites in plant cell cultures.[2] The strategy is based on the fact that the accumulation of most secondary metabolites in plants is part of defense response to pathogenes (bacteria, viruses) and environmental stimuli. The elicitor can be regarded as a stress factor involved in the reaction: Plant-microorganism, plant-pesticide, plant heavy metal, plant-UV irradiation, etc., Due to chemical defensive reactions, signal substances (elicitor) increase the activity of certain enzymatic systems for a short period and these systems catalyze the formation of stress substances similar to the particular secondary metabolites.[3]
The elicitors tested in former studies to increase secondary metabolite accumulation in plant cell cultures were mostly chemical agents-heavy metals, carbohydrate fractions of fungal and plant cell walls.[2] Few reports on the use of physical or mechanical stimuli to enhance the production of important substances were reported. Mechanical stress also has been found deleterious effect to the growth and viability of many plant cells.[4]
Application of ultrasound (US) to biotechnology is relatively new but several processes that take place in the presence of cells or enzymes are activated by ultrasonic waves. High intensity ultrasonic waves damage the cells and denaturize enzymes. Low intensity ultrasonic waves can modify cellular metabolism or improve the mass transfer of reagents and products through the boundary layer or through the cellular wall and membrane. In the case of enzymes the increase in mass transfer rate of the reagents to the active site seems to be the most important factor. Immobilized enzymes are more resistant to thermal deactivation produced by US than native enzymes.[5]
Low-intensity US dramatically enhanced the content of secondary metabolites in plant cell cultures, e.g. ginsenoside saponins of Panax ginseng[6] and shikonins of Lithospermum erythrorhizon cells.[7]
Ananthakrishnan at all as the first announced stimulation of in vitro regeneration by ultrasonic treatment. US stimulated also massive explant growth in Cucurbita pepo L.[8]
The purpose of this study was to verify the elicitor effects of ultrasonic waves on the content of secondary metabolites. The experiments were carried out in suspension cultures of Genista tinctoria exposed to low-intensity US.
The genus Genista L.(Fabaceae) is rich in isoflavones particularly substituted isoflavones such as 5-methylgenistein and O-glucosylated isoflavones, which are considered to the most important phytoestrogens.[9] Daidzein, genistein and isoprunetin are the most representative substances for genus. Many Genista species show interesting biological properties such as hypoglycemic, antiinflammatory, antiulcer, spasmolytic, antioxidant, estrogenic and cytotoxic activity against different human cancer cell lines.[10]
MATERIALS AND METHODS
Plant material
Suspension cultures of G. tinctoria used in this work were continued on Schenk and Hildebrant medium/SH/[11] supplemented with 2,4-dichlorphenoxyacetic acid at a concentration of 0.5 mg/L and kinetin at a concentration of 0.1 mg/L in Erlenmeyer flasks. The cultures were shaken constantly on the shaker at 110-120 rpm. These cultures were incubated in growth room at 26 ± 1°C under 16 h light and 8 h dark. The suspension cultures from the 29th to 34th passages were used for elicitation.
Elicitor
An ultrasonic bath with fixed frequency 35 kHz and power level 0.1 mW/cm3 was used to insonate the 21 day old Genista cells. For exposure, the flasks were sinked into the ultrasonic bath to a depth at which the liquid in the flasks was about 10 cm bellow the liquid in the bath. The ultrasonic bath temperature was maintained at 25 ± 0.5°C during exposition. The time of US exposition was 1, 2, 3, 4 and 5 min. The US exposed suspension cultures were taken after 6, 12, 24, 48, 72 and 168 h after ultrasonic treatment and some of them immediately after this exposition. The suspension cells were separated from the liquid nutrient medium by filtration through Whatman filter paper (No. 1-6) under vacuum. The cells were dried and the content of isoflavonoids was determined. Simultaneously, the controls (without US exposition) were run. All tests were triplicated; each data point reported is the mean of three replicate measurements.
Analysis of isoflavonoids
The content of isoflavonoids in G. tinctoria suspension cultures was determined by High Performance Liquid Chromatography (HPLC) on a UNICAM CRYSTAL 200 Liquid Chromatograph, using LiChrospher RP-18 (250 mm × 4 mm) column.
0.100 g (dry mass) of suspension cells was extracted twice (in a water bath under reflux cooler) with 10 mL of 80% (v/v) methanol for 30 min. The extract was filtered via Teflon filter (diameter 22 μm) and 2 mL of this filtrate was analyzed by HPLC metod. HPLC conditions were prepared in our laboratory as follows. The mobil phase consisted of methanolic solution of 0.15% phosphoric acid p.a. (w/v). Isoflavonoids were eluted with linear gradient from 30% methanol to 80% methanol in 9 min, following by isocratic elution with 80% methanol for 15 min. The flow rate was 1.1 mL/min. Substances were detected by absorption at 260 nm and their identification was carried out by comparison of retention times and absorption spectrum with standards. As the standards were used: Genistin, p.a.; daidzein, p.a.; genistein, p.a.; formononetin, p.a. and biochanin A, p.a., Figures 1 and 2.
Figure 1.
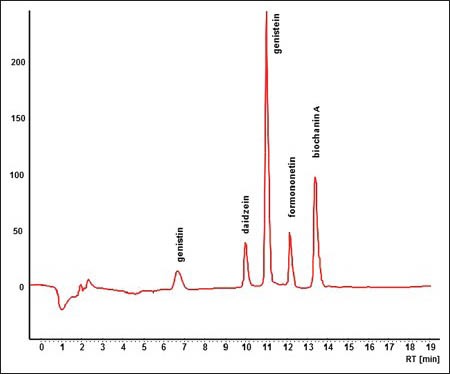
High performance liquid chromatography record of standard analysis
Figure 2.
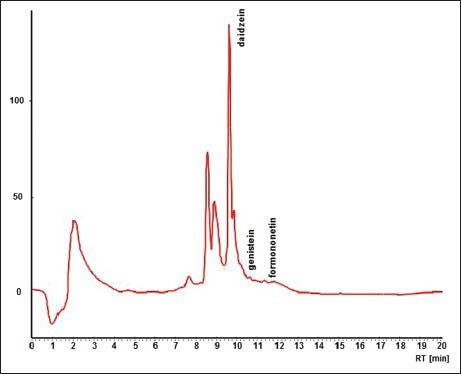
High performance liquid chromatography record of suspension culture analysis
Statistical analyses
To determine whether there was difference between values of samples the T-test was applied. Values of P ≤ 0.05 were considered as significantly different. The differences between means were determined using Tukey's multiple comparison test.
RESULTS
The abiotic elicitation is one of the methods for increasing the secondary metabolites production in plant cell tissue cultures.
The production of the separated isoflavonoids in G. tinctoria suspension cells was changed in the dependence on time of ultrasonic exposition.
Figure 3 shows daidzein content (mg/g DW) in G. tinctoria suspension culture after US treatment. US exposition of G. tinctoria suspension culture enhanced the daidzein level in this culture. Suspension culture without US exposition (controls) produced only daidzein (0.7 mg/gDW). The highest daidzein production (1.4 mg/g DW) was reached after ultrasonic treatment for 5 min and 168 h sampling [Figure 3]. The daidzein production was about 56% higher in comparison with the control [Figure 3].
Figure 3.
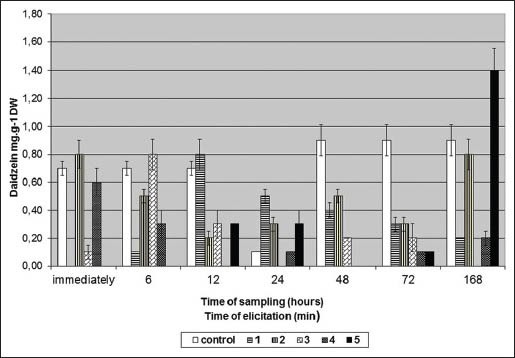
Daidzein content (mg/g DW) in Genista tinctoria suspension culture after ultrasound exposure
One minute of US treatment (sampled after 24 h) stimulated also daidzein production. On the other hand 4 min of US exposition had any or negative effect on daidzein production. Four minutes of US exposition (sampled after 12 and 48 h) decreased daidzein content about 100% [Figure 3].
Figure 4 shows genistin content (mg/gDW) in G. tinctoria suspension culture after US treatment.
Figure 4.
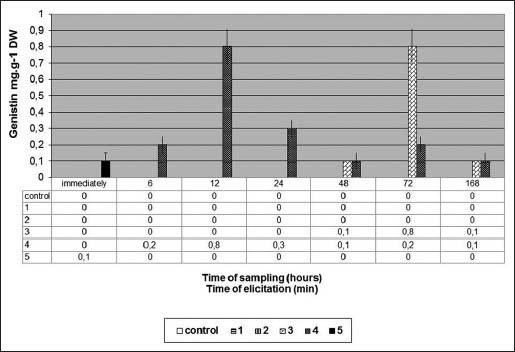
Genistin content (mg/g DW) in Genista tinctoria suspension culture after ultrasound exposure
When US exposition 3 and 4 min was used G. tincotria suspension culture started to produce isoflavone genistin. The maximal genistin content (0.8 mg/g DW) was reached after 3 min of US exposition (sampled after 72 h). The same genistin level after 4 min of US exposition (sampled after 12 h) was observed. Ultrasonic treatment for 1, 2 and did not any effect at genistin production [Figure 4].
US treatment of G. tinctoria suspension culture had no effect on other isoflavonoids (genistein, formononetin, biochanin A) production. Neither controls nor US treated G. tinctoria suspension cultures produced these isoflavonoids.
The releasing of isoflavonoids into nutrient medium after US exposition of G. tinctoria suspension culture was also a part of this study. The isoflavonoids genistin, genistein, formononetin and biochanin A were not eliminated into nutrient medium after US treatment. Daidzein was detected only in few samples and in trace amount (0.0256 mg/g DW) after 1 min of US exposition and 12 h sampling and (0.0368 mg/g DW) after 1 min of US exposition and 48 h sampling.
DISCUSSION
The achieved results confirm also various effects of US on plant cultures and secondary metabolite production. The ultrasonic treatment (38.5 kHZ) for 2 min on P. ginseng suspension culture increased polyphenol oxidase, peroxidase an phenylalanine amonia lyase and the production of polyphenols and phenolic compounds.[12]
UV and US treatment of peanuts resulted in increased α-resveratrol, piceid and total stilbenes but decreased overall acceptance compared to UV treatment. The optimum US process produced 2.64-4.40 μg/g resveratrol and 4.50-6.50 μg/g total stilbenes which were more than will be achieved by optimum UV process.[13]
US treatment stimulated also the synthesis of saponins of ginseng cells, without causing any net loss of the biomass yield of ginseng cell cultures.[14]
Sonification (3.5-55.6 mW/cm3 at 40 kHz fixed frequency) for 2 min induced a rapid and dose-dependent NO production in the Taxus cell culture.[15]
The bioeffects of US on cells in liquid media are mostly attributed to the mechanical stress arising from US-induced fluid motion and hydrodynamic events (acoustic cavitation and cavitation-induced microstreaming.[16] Some studies have shown that physical and mechanical stresses such as UV light[17], hydrostatic pressure[18] induce oxidative burst and other elicitor responses of plant and tissues and cells. Mechanical stress may trigger the defense responses and secondary metabolite production of plant cells induced by US. Very important characteristic of US-induced events is their rapidity, which could be detected. Post-elicitation lag of the oxidative burst was 1-3 min in soybean cell cultures induced by osmotic shock,[19] 4-8 min in soybean cell cultures treated by glycoprotein fraction of fungus[20] and 10 min by glucan elicitor,[21] Our experiments show the highest daidzein production (1.4 mg/g DW) after ultrasonic treatment for 5 min and 168 h sampling. Four minutes US exposition had any or negative effect on daidzein production. The maximal genistin content (0.8 mg/g DW) was reached after 3 min of US exposition (sampled after 72 h).
CONCLUSION
The achieved results suggest that US can act as a potent abiotic elicitor to induce the defense responses of plant cells and to stimulate secondary metabolite production in plant cell cultures.
ACKNOWLEDGMENT
The work was performed with financial support of Research Project SVV 267 004.
Footnotes
Source of Support: The work was performed with financial support of Research Project SVV 265 004
Conflict of Interest: None declared.
REFERENCES
- 1.Verpoorte R, Van der Heijden R, Ten Hoopen HJ, Memelink J. Metabolic engineering of plant secondary metabolite pathways for the production of fine chemicals. Biotechnol Lett. 1999;21:467–479. [Google Scholar]
- 2.Dörnenburg H, Knorr D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microbiol Technol. 1995;17:674–684. [Google Scholar]
- 3.Tůmová L, Psotová R. UV-radiation and the flavonoid content in callus culture of Ononis arvensis L. Herba Pol. 1998;44:22–6. [Google Scholar]
- 4.Zhong JJ. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv Biochem Engin/Biotechnol. 2001;72:1–26. doi: 10.1007/3-540-45302-4_1. [DOI] [PubMed] [Google Scholar]
- 5.Sinistera JV. Application of ultrasound to biotechnology an overview. Ultrasonics. 1992;30:180–5. doi: 10.1016/0041-624x(92)90070-3. [DOI] [PubMed] [Google Scholar]
- 6.Lin LD, Wu JY, Ho KP, Qi SY. Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound Med Biol. 2001;27:1147–52. doi: 10.1016/s0301-5629(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Wu J. Enhacement of shikonin production in single-and two-phase suspension culture of Lithospermum erythrorhizon cells using low-energy ultrasound. Biotechnol Bioeng. 2002;78:81–8. doi: 10.1002/bit.10180. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan G, Xia XD, Amutha S, Singer S, Muruganantham M, Yablonsky S, et al. Ultrasonic treatment stimulates multiple shoot regeneration and explant enlargement in recalcitrant squash cotyledon explants in vitro. Plant Cell Rep. 2007;26:267–76. doi: 10.1007/s00299-006-0235-1. [DOI] [PubMed] [Google Scholar]
- 9.Halabalaki M, Alexi X, Aligianni N, Lambrinidis G, Pratsinis H, Florentin I, et al. Estrogenic activity of isoflavonoids from Onobrychis ebenoides. Planta Med. 2006;72:488–93. doi: 10.1055/s-2005-916261. [DOI] [PubMed] [Google Scholar]
- 10.Rigano D, Cardile V, Formisano C, Maldini MT, Piacente S, Bevilacqua J, et al. Genista sessilifolia DC and Genista tinctoria L. inhibit UV light and nitric oxide-induced DNA damage and human melanoma cell growth. Chem Biol Interact. 2009;180:211–219. doi: 10.1016/j.cbi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Shenk R.U, Hildebrandt A.C. Medium and techniques for induction of growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. [Google Scholar]
- 12.Wu J, Lin L. Elicitor-like effects of low-energy ultrasound on plant (Panax ginseng) cells: Induction of plant defense responses and secondary metabolite production. Appl Microbiol Biotechnol. 2002;59:51–7. doi: 10.1007/s00253-002-0971-2. [DOI] [PubMed] [Google Scholar]
- 13.Sales JM, Resurreccion AV. Maximising resveratrol and piceid content in UV and ultrasound treated peanuts. Food Chem. 2009;117:674–80. [Google Scholar]
- 14.Wu JY, Lin LD. Ultrasound-induced stress responses of Panax ginseng cells: Enzymatic browning and phenolics production. Biotechnol Progress. 2002;8:862–6. doi: 10.1021/bp0255210. [DOI] [PubMed] [Google Scholar]
- 15.Wang JW, Zheng LP, Wu JY, Tan RX. Involvement of nitric oxide in oxidative burst, fenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide-Biology and Chemistry. 2006;15:351–8. doi: 10.1016/j.niox.2006.04.261. [DOI] [PubMed] [Google Scholar]
- 16.Miller MW, Miller D, Brayman AA. A review of in vitro bioeffects of internal ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–54. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 17.Tůmová L, Tůma J. The effect of UV light on isoflavonoid production in Genista tinctoria culture in vitro. Acta Physiol Plant. 2011;33:635–40. [Google Scholar]
- 18.Dörnenburg H, Knorr D. Evaluation of elicitor and high pressure-induced enzymatic browning utilizing potato (Solanum tuberosum) suspension cultures as a model system for plant tissues. J Agric Food Chem. 1997;45:4173–7. [Google Scholar]
- 19.Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–66. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultures plant cells. Plant Physiol. 1989;90:109–16. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrales the plant hypersensitive disease resistance response. Cell. 2002;79:583–93. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]


