Abstract
Background:
Microbes have been implicated in a wide variety of human diseases many of which are of life-threatening nature. New antimicrobials are urgently needed not only for combating these organisms but also to counter the menace of the harmful microbes developing resistance against drugs at alarming rates. Mangrove plants are rich sources of secondary metabolites having many beneficial biological activities including antimicrobial ones. True to this fact, this report describes identification, isolation and partial characterization of two novel antifungal compounds from Aegiceras corniculatum, a mangrove plant from Indian Sundarban estuary.
Materials and Methods:
Two compounds, named as Acornine 1 and Acornine 2, having antifungal activities were isolated from the bark of A. corniculatum, a mangrove plant, by using standard techniques. The compounds were characterized using routine microbiological and physicochemical methods.
Results:
Partial structural characterization of the two compounds indicated they are oleanane triterpenoids with linked sugar moieties. While both the compounds exhibited growth inhibition in tested Gram positive bacteria, Acornine 2 in particular demonstrated strong antifungal activities against several pathogenic fungi tested. Results also indicated that various environmental factors may govern the secondary metabolite profiles of the same mangrove plants growing in different geographical areas.
Conclusion:
Tissue extracts of Aegiceras corniculatum, a mangrove plant from Indian Sundarban estuary, exhibited the presence of remarkable antifungal activities. The isolated compounds responsible for such activities, named as Acornine 1 and Acornine 2, appear to have potential in food processing and health care industry. They need to be studied further.
Keywords: Acornine 1, Acornine 2, Aegiceras corniculatum, Antifungal, Mangrove, Oleanane triterpenoid glycosides
INTRODUCTION
The Sundarban, covering about one million hectare in the delta of the rivers Ganges, Brahmaputra and Meghna, is shared between India and Bangladesh. It is also the world's largest coastal wetland forest with a rich diversity of mangrove plants.[1] In this area the mangroves exist under very hostile and inhospitable conditions as the plants which grow there have to encounter constantly changing water pH, salinity and wind velocity, higher temperature and extreme humidity, tidal extremes, muddy anaerobic soil etc., No terrestrial plant can survive well under these adverse conditions.[2] To exist in such hostile living conditions with different biotic and abiotic stress factors the mangrove plants are known to synthesize a variety of phytochemicals that are known to have medicinal values.[3] The local population in and around Sundarban have been using the tissue extracts of various mangrove plants for ages as cures for various diseases. The black mangrove Aegiceras corniculatum (Linn.) Blanco, which has many ethnopharmaceutical uses by the local people such as in the treatments of rheumatism, arthritis, inflammation, asthma, diabetes etc.[4,5] has been selected for the present study. It belongs to the myrsinaceae family and is distributed in coastal and estuarine areas of India, including the Sundarban estuary. Probably because of the widespread ethnomedicinal uses, this plant has attracted interests of researchers from diverse areas. Roome et al.,[6] and Agoramoorthy et al.,[7] have studied the antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective actions of A. corniculatum stem extracts. Some researchers have analyzed the antimicrobial activity of A. corniculatum.[8,9,10,11] Giron et al.,[12] isolated two benzoquinone compounds, 2,5-dihydroxy-3tridecyl-1,4-benzoquinone and 2-hydroxy-5-methoxy-3-undecyl-1,4-benzoquinone from A. corniculatum which showed fungal inhibition. Wahidullah et al.,[13] isolated and patented corniculatonin, an antifungal compound, from this plant. In this study we report the isolation and characterizations of two new antifungal compounds named as Acornine 1 and Acornine 2 from Aegiceras corniculatum. The results of these studies indicate that they may have potential uses in the human health care and food processing industries.
MATERIALS AND METHODS
General experimental procedures
For the purity analysis of two extracted bands of Acornine 1 and Acornine 2 from preparative Thin layer chromatography (TLC) plates, analytical HPLC experiment on a C-18 column was carried out with solvent A-water/ac and solvent B-methanol. Liebermann-Burchard test for triterpenoids was carried out by dissolving 0.001 gm of the sample in chloroform and treating the solution with one drop of concentrated H2SO4 and one drop of acetic anhydride when a pink coloration is developed. Optical rotations were measured on a Bellingham + Stanley Ltd. ADP-410 polarimeter at 20°C. The Fourier transform infrared (FTIR) spectra were recorded on a Varian FTIR 640 spectrometer with 2 mg of the sample milled in 50 mg of potassium bromide (KBr) and then compressed into a thin pellet which was analyzed. The FTIR spectra were recorded within the range of 400-4000 cm-1. The DART-MS was recorded on a JEOL-AccuTOF JMS-T100LC mass spectrometer having a DART source. The samples were subjected as such in front of the DART source. Dry helium was used with 4.0 LPM flow rate for ionization at 350°C. The orifice 1 potential was set at 28V. Data acquisition was from m/z 50 to 1000 and print outs were average spectra of 6-8 scans. The 1H NMR spectra (for both Acornine 1 and 2) and 13C NMR spectra for Acornine 1 were recorded on a Bruker spectrometer (300 MHz) with Methanol-d4 (MeOD) as the solvent; the chemical shifts are reported in parts per million (d). 13C NMR analysis of Acornine 2 (in MeOD) was carried out in a Bruker Avance NMR equipment at 600 MHz with TCI cryoprobe and data analyzed using standard Bruker Pulse program.
Plant materials
The mangrove plants used in this study were collected from Sundarban estuary in West Bengal state, India. These plant materials were identified by Dr. K. Naskar and Prof. K. Bhattacharya. The dried specimens of these plants were deposited in the Herbarium of Botany department (Voucher no. VB/CBT/AR/005), Visva-Bharati University, India. Fresh bark of A. corniculatum was washed thoroughly with tap water to get rid of all foreign deposits and air dried under shade. Any area of the plant tissue that could not be cleaned totally free of foreign deposits were discarded. Dried tissues were then crushed into fine powder with the help of a mechanical grinder and stored at room temperature until used.
Extraction and isolation of Acornine 1 and Acornine 2
Powdered bark (1.5 kg) of A. corniculatum was used for the extraction of Acornine 1 and Acornine 2. The bark powder was extracted sequentially with 2 L each of hexane, benzene and chloroform in a 3 L soxhlet apparatus for 40 h each. The temperature for extractions was 65°C for hexane, 80°C for benzene and 60°C for chloroform. After the final extraction with chloroform in the soxhlet, the residual material was collected from the soxhlet apparatus and extracted further with 2 L of methanol at room temperature for 10 days after which the suspension was filtered. The filtrate containing the active substances was concentrated by air drying to a volume of about 150 mL. For the next round of purification, preparative TLC method was used. 75 g silica Gel G (Merck), as adsorbent, was mixed with 150 mL of distilled water to make a thick slurry. The slurry was immediately poured into a spreader and plates for TLC were prepared by spreading the slurry on 56 × 16.7 cm size glass plates with the thickness of the layer fixed at 2 mm. Plates were allowed to air dry overnight and the adsorbent layer on glass plate was fixed by drying it further at 75°C for 1 h just before use. Using a micropipette, about 700 μL of bark methanol extract of A. corniculatum was applied gradually at the point of application on the preparative TLC plates. The plates were then air-dried again and run in BAW (n-butanol-acetic acid-water: 4: 1.15: 5) running solvent in a closed glass chamber already saturated with BAW. The positions of the two separated bands with Rf 0.36 (representing Acornine 1) and Rf 0.32 (representing Acornine 2) on the chromatograms were localized after spraying carefully only a part of the TLC bed on one side with H2SO4/C2H5OH solution and heating it at 75°C for 5 min. The desired two bands from unstained portions of the plates were then scraped off and the substances inside were eluted from the silica with methanol which was next evaporated to dryness to obtain the pure compound in a powdery form. The pure materials were stored at 4°C till use. This process of preparative TLC was repeated several times to accumulate enough of the two compounds for subsequent analyses.
Structure elucidation
Partial structure elucidation of Acornine 1 and Acornine 2 was achieved using standard spectroscopic techniques: FTIR, 1H and 13C NMR, DART-MS (data not shown). The backbone structures were confirmed by comparison of the spectroscopy data with that in the literature.[13]
Test organisms and other microbiological methods
The samples were screened against non-pathogenic fungi S. cerevisiae and T. clypeatus and Gram-positive bacteria Bacillus subtilis and Bacillus coagulans as model organisms for routine tests. For testing against pathogenic fungi the purified compounds were screened against Candida albicans, Trichophyton mentagrophytes and Trichophyton rubrum. The composition and preparation semi-solid (YPD-agar or Sabouard dextrose agar) or liquid (YPD or Sabouard dextrose broth) media, maintenance, propagation and growth of microorganisms etc., all were as described.[14] Stock cultures of the microorganisms were maintained at 4°C on slants of YPD-agar. For experimental purpose, fungi were grown in Sabouard dextrose broth or YPD-broth. Active and healthy cultures of microorganisms for experiments were prepared by transferring single colony from the stock culture to 1.5 mL of Sabouard dextrose broth or YPD-broth and incubated at 180 rpm for 24 h at 30°C. These healthy mini cultures were used routinely as inoculums, after diluting them suitably in 0.9% saline, for the disc diffusion assays (Bauer et al.,[15] and determination of MIC.[16] The basidiomycetes fungus T. clypeatus was maintained and grown in the laboratory as previously described.[17,18]
Antifungal activity assay
The antifungal activity of different samples were determined by disc diffusion method[14] using YPD-agar and PD-agar plates. The assay plates were prepared by pouring 30 mL of sterile agar-media into sterile 90 mm petridish and allowed to solidify. The inoculums prepared as described above were then spread uniformly on agar plates using a sterile cotton swab dipped into the experimental microbial suspension. Then paper discs (made from Whatman No. 1 filter paper) of 6 mm diameter were placed on the agar surface and 8 μL extract of concentration 250 mg/mL dissolved in dimethyl sulphoxide (DMSO) was spotted on each disc (2 mg/disc). The plates were then incubated at 30°C up to 36 h. Diameter of the inhibition zone around each disc was measured in mm. 3 μL of antibiotics (10 mg/mL) such as commercial fluconazole, an antifungal, were used as standards and DMSO as a negative control to determine the sensitivity of the microorganisms tested.
Minimum inhibitory concentration (MIC) was determined using Inhibitory Concentration in Diffusion (ICD) method.[16] In this method the discs of sterile Whatman no. 1 filter papers (6 mm diameter) were placed on the surface of agar plates containing the spread-out microbial culture with the help of forceps; they were then spotted with serially diluted concentrations of Acornine 1 and Acornine 2. For making these dilutions a stock solution each of Acornine 1 and 2 was prepared and then serially diluted (1:1) in DMSO to obtain different concentrations. The lowest concentration of a particular extract that inhibited the growth of a test microorganism was noted as the MIC value of that extract for that strain. MIC tests for antifungal activities using pathogenic fungi were carried out by Genohelix Corporation, Bangalore, India using the disc diffusion assays.
Thin layer chromatography and Bioautography
Silica GF254 plates (Merck), 20 × 20 cm2 and 0.2 mm thick, were used for analytical TLC. Methanol extracts of bark at a concentration of 100 mg/mL (5 μL) were applied on TLC plates. The chromatograms (in triplicate) were run in BAW as running solvents (composition described earlier). At the end of the run, the TLC plates were either (i) sprayed with C2H5OH-H2SO4 solution 95:5, v/v) and heated at 100°C for 5 min for the purpose of visualizing separated phytochemical components or (ii) subjected to bioautography (detailed below) for the purpose of identifying components having antifungal activities.
Bioautography is a useful technique to determine bioactive compound with antimicrobial activity from plant extract on TLC plate. For the purpose of detection of antifungal components present in the methanolic bark extracts of A. corniculatum, bioautography technique of Nostro et al.,[19] was used with some modifications, after first separating these phytochemical components on TLC plates as described above. At the end of TLC run, YPD-agar containing 1% inoculums of S. cerevisiae were poured on these TLC plates. After solidification of the YPD-agar medium on the TLC plates, they were incubated for 24 h at 30°C. Subsequently, the bioautogram was sprayed with a 1% aqueous solution of 3-(4-5-Dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and incubated at 30°C for 1-2 h. Zones in which fungal growth was inhibited (due to the presence of a phytochemical component having antifungal activities on the TLC plate) failed to take the stain and indicated the presence of active compounds in that area of TLC plate. TLC-Bioautography technique showed how many active compounds are present in the experimental sample.
RESULTS AND DISCUSSION
Earlier we have reported a detailed characterization of antimicrobial activities found in the different tissues of this plant elsewhere.[11] Following this lead, we characterized the antifungal activities in the bark extracts of A. corniculatum further. Figure 1 shows that the bark extract (methanol) of A. corniculatum, run in a TLC plate with BAW as the running solvent system, exhibit the presence of two dominant bands at Rf of 0.36 and 0.32 [Figure 1. Panel (i), Lane a]; both these two bands show strong fungicidal activities when the same TLC plate is subjected to bioautography using S. cerevisiae as test organism [Figure 1. Panel (i), Lane b]. We have named the compounds representing these two bands as Acornine 1 (Rf 0.36 in BAW solvent system) and Acornine 2 (Rf 0.32 in BAW solvent system) for easy reference. We have taken advantage of the clear difference in the mobility of Acornine 1 and Acornine 2 [Figure 1. Panel (i), Lane a] in BAW solvent system for their eventual purification. Following preliminary and routine extractions of powdered bark of A. corniculatum using hexane, benzene and chloroform in a soxhlet apparatus and extraction in methanol, we used long-bed preparative TLC to separate Acornine 1 and Acornine 2 from each other. Then the bands were scraped off the TLC plate and the Acornine 1 and 2 were individually extracted with methanol and concentrated for further use [Figure 1. Panel (ii), Lanes b and c respectively]. Materials in the extracted bands, when tested for their fungicidal activities by bioautography technique using S. cerevisiae, were found to have retained the activity quite well [Figure 1. Panel (ii), Lanes e and f]. In addition, we tested the purified Acornines on T. clypeatus, (a basidiomycetes fungus), and Bacillus subtilis as well as Bacillus coagulans, both Gram-positive bacteria using TLC-bioautography method; both the Acornines exhibited growth inhibitory activity against the fungus T. Clypeatus and the Gram-positive bacteria tested [Figure 1. Panel (iii), Lanes b, c and d respectively]. Acornines did not exhibit any detectable activity against Gram-negative bacteria (data not shown). Both the purified compounds were found to be white amorphous materials.
Figure 1.
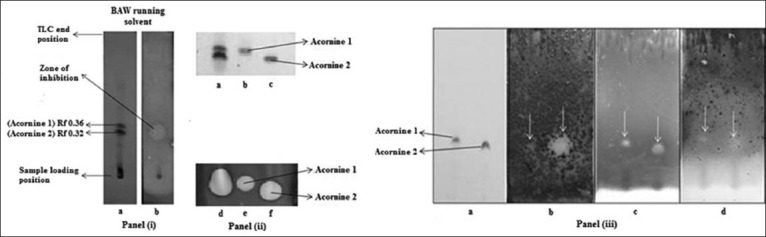
Antifungal activities of A. corniculatum. Panel (i) Lane a -TLC showing Acornine 1 and Acornine 2 in bark extract at Rf 0.36 and 0.32; Lane b - Growth inhibition of S. cerevisiae by Rf 0.36 and 0.32 bands. Panel (ii) -TLC (lanes a, b and c) and bioautography (lanes d, e and f) of bark extract, purified Acornine 1 and Acornine 2 respectively. Panel (iii) a: TLC of purified Acornine 1 and Acornine 2; Panels (iii) b, c and d - bioautography of Acornine 1 and Acornine 2 with T. clypeatus, B. subtilis and B. coagulans respectively. White arrows in Panel (iii) indicate positions of growth inhibitions
HPLC analysis was used next for the purpose of checking the purity and suitability of the isolated Acornine 1 and Acornine 2 for subsequent analyses. Figure 2 shows the retention times of the two compounds. The larger peaks (at 12.29 min for Acornine 1 and 13.34 min for Acornine 2; Figure 2 (a) and Figure 2 (b) respectively) are that of the active compounds, each more than 95% pure, whereas very small peaks (at 2.36 min for Acornine 1 and at 2.72 min for Acornine 2) may indicate a negligible amount of contaminating phytochemicals in the otherwise pure samples. Both Acornine 1 and Acornine 2 give pinkish colouration in Liebermann-Burchard test suggesting their triterpenic nature.[20]
Figure 2.
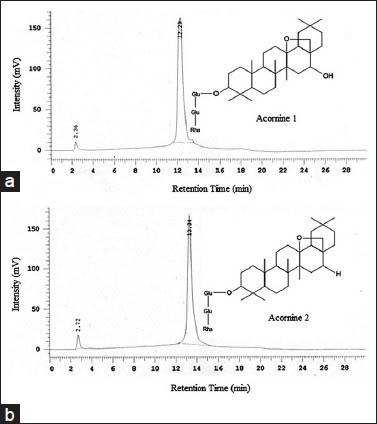
Analytical HPLC (c18, 5 μM, 250 × 4.6 mm, UV detector) chromatograms of Acornine 1 (a) and Acornine 2 (b) isolated from the bark of A. corniculatum. Spectral data were recorded at 254 nm. Purity of both compounds was determined using the area and area% of the peaks, automatically calculated by the HPLC system software. Both Acornine 1 and 2 are in sufficient state of purity (more than 98% and 95% respectively) for structure determination
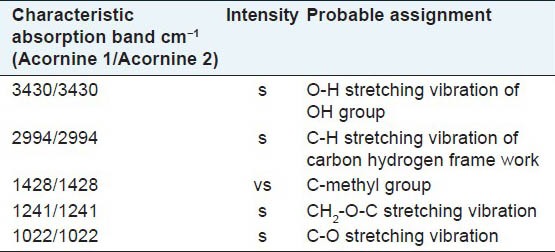
Having purified the compounds Acornine 1 and Acornine 2 from the bark of Aegiceras corniculatum, they were tested on some pathogenic fungi having detrimental effects on human health by determining their MIC values. MIC is the minimum concentration of the antimicrobial agent at which the growth of the microorganisms gets inhibited. The MIC values (μg/disc) of the Acornine 2 against the experimental organisms obtained are represented in Table 1. It shows that Acornine 2 is active against Candida albicans, Trichophyton mentagrophytes and Trichophyton rubrum. The same fungi were not responsive to Acornine 1 treatments. However, there are still possibilities for Acornine 1 to be effective against some other fungi which we have not tested. Infrared (IR) spectroscopy is an excellent method for the identification of organic functional groups. It is often used to confirm the presence (or absence) of a specific functional group in a compound as well as the compound's purity. The FTIR spectra of Acornine 1 and Acornine 2 are presented in Table 2; they are almost identical to each other. It is evident that Acornine 1 and Acornine 2 in their IR spectra show a strong broad band at 3430 cm-1 characteristic of the stretching vibration of a free hydroxyl group. The appearance of strong bands at 1241 and 1022 cm-1 for stretching vibration of C-O bonding is suggestive of the presence of CH2-O-C linkage in the compound. The strong absorption at 2994 cm-1 is assignable to C-H stretching vibration of carbon-hydrogen frame work while the absorption at 1428 cm-1 may be ascribed to C-H stretching vibration of C-methyl groups in Acornine 1.
Table 1.
MIC (μg/disc) for antifungal activity of compound, Acornine 1 and Acornine 2 from A. corniculatum
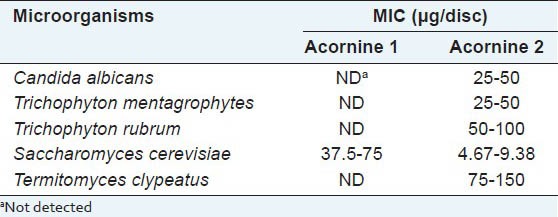
Table 2.
Infrared absorption spectrum of Acornine 1 and Acornine 2
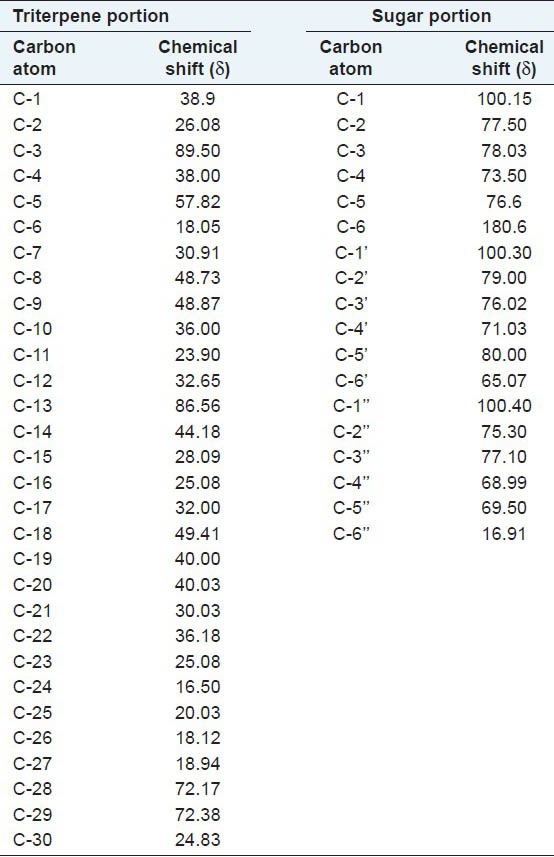
Study of 1H NMR spectra of Acornine 1 and Acornine 2 has been done with a view to secure important information about the proton environments of the molecules. Accordingly, the 1H NMR spectra were studied in deuterated methanol (MeOD) with a 300 MHz instrument. Tetramethyl silane (TMS) was employed as the internal standard. The chemical shift values along with their probable assignments are tabulated in Table 3. From the Table 3 it appears that the 1H NMR spectra of Acornine 1 and Acornine 2 are almost similar and furnish a lot of valuable information which may help to establish the structures of these compounds. Thus the proton signals at d 0.896, 0.921, 0.945, 1.22, 1.25, 1.28 and d 1.30 in the 1H NMR spectrum of Acornine 1 and the signals at d 0.868, 0.926, 0.947, 1.23, 1.25, 1.28 and d 1.30 in the 1H NMR spectrum of Acornine 2 indicate the presence of seven tertiary C-methyl groups in these two triterpene saponins. The doublet of three protons around d 1.65 (J = 10 Hz) in both the spectra is attributable to a secondary (CH-CH3) protons in these two compounds. The presence of an epoxy linkage (CH2-O-) in these triterpenoids is obvious from the appearance of a two proton singlet at d 3.30. The three doublet signals at d 3.98 (J = 7 Hz), 4.02 (J = 7 Hz) and d 4.07 (J = 7 Hz) in the 1H NMR spectra of both these compound reveal the presence of three anomeric protons of glycosidic moiety. The appearance of a singlet of one proton at d 4.87 suggests that presence of a carbinol methine of the glycosidic linkage (CH-OG). A series of short peaks appearing in the region d 1.70 to 1.91 in the spectra of Acornine 1 and Acornine 2 are considered to be the triterpenoid finger print. Thus, the essential structural features of Acornine 1 and Acornine 2 that are revealed from the 1H NMR spectra may be summarized as follows: The presence of (i) Seven tertiary C-methyl groups, (ii) One secondary C-methyl group (CH-CH3), (iii) Three anomeric protons of glycosidic linkage, iv) One carbinol methine as > CHOG and v) one epoxy linkage. The above structure of Acornine 2 is also in agreement with the high resolution 13C NMR spectral data described in Table 5. 13C NMR data of Acornine 1 is practically completely identical with that of Acornine 2 excepting the carbon signal at C16 (data not shown) and confirms the proposed structure of Acornine 1.
Table 3.
1H NMR proton signals of Acornine 1 and Acornine 2 in MeOD at 300 MHz
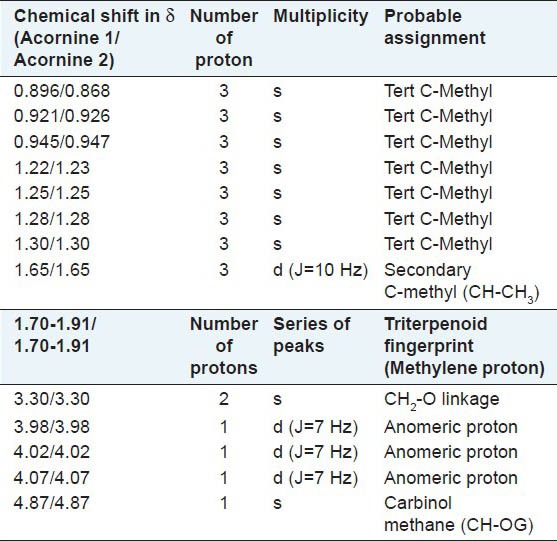
Table 5.
13C NMR (600 Mhz) data of Acornine 2
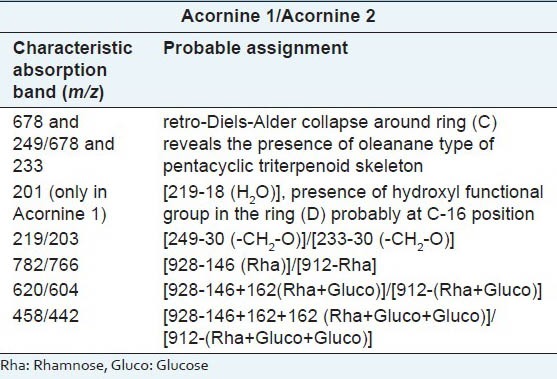
Acornine 1 and Acornine 2 were subjected to electron impact mass spectral studies next. Various mass peaks obtained from these two triterpenoidal saponins and the probable interpretations of the genesis of these mass peaks are elaborated as follows: The mass spectrum [Table 4] of Acornine 1 exhibits molecular ion peak at m/z 928 which is in complete harmony with the molecular composition, C48H80O17. The appearance of significant peaks in the mass spectrum at m/z 678 and at m/z 249 resulting from retro-Diels-Alder collapse around ring (C) under electron impact reveals the presence of oleanane type of pentacyclic triterpenoid skeleton in the compound.[21] However, the absence of any olefinic proton signal in its 1H NMR spectrum as well as the presence of an epoxide functionality in Acornine 1 suggests the presence of an epoxide group in the compound. This assumption is further supported by the appearance of ion peaks containing ring D and E at m/z 249 and 219 [249-30(-CH2-O)] in the mass spectrum. Further, the display of ion peak at m/z 201 (219-H2O) by the mass spectrum indicates the presence of a hydroxyl function in the ring (D) probably at C-16 position. It may be mentioned that C-16 H proton signal probably overlapped in the region around d 3.20. Once again, ion peaks at m/z 782, 620, 458 and at m/z 457 in the mass spectrum is suggestive of the presence of gluc-gluc-rha type of glycosidic linkage. The appearance of the peak at m/z 678 in the mass spectrum generated by retro-Diels-Alder cleavage represents the rings A/B. The mass value of this ion peak indicates the presence of the glycosidic linkage in the rings A/B portion and its location at C3 is highly probable from biogenetic background. The mass spectrum of Acornine 2 [Table 4] is almost identical in all respects with that of its congener Acornine 1 excepting in the mass values of molecular ion peaks which appears at 16 mass units less than that of Acornine 1 is at at m/z 912 as well as of other ion peaks. As expected it exhibits ion peaks at m/z 912 (M+), 678, 233, 203, 766, 604, 442 and m/z 441. From the analyses of different spectral characteristics described above the most probable structures of Acornine 1 and Acornine 2 may be elaborated as shown in Figure 2. The nomenclature of the isolated compounds may be designated as follows:
Table 4.
Mass spectrum of Acornine 1 and Acornine 2
Oleanane 13,28 epoxy-16 hydroxyl-3-glycoside (Acornine 1)
Oleanane 13,28 epoxy-3-glycoside (Acornine 2).
Wahidullah et al.,[13] isolated and characterized an oleanane triterpenoid oligoglycoside having antifungal activity from this plant which they named as corniculatonin. The deduced structure for corniculatonin[13] has similarities with that of Acornine 1 and Acornine 2. Both corniculatonin and acornines have oleanane triterpenoid backbones. The points of major differences are however, the (i) existence of only three sugar units in cases of Acornine 1 and Acornine 2 as opposed to five in case of corniculatonin and (ii) absence of hydroxyl group (OH) in Acornine 2. In addition, Wahidullah et al.,[13] did not report any Gram-positive antibacterial activities for corniculatonin. Because we do not have corniculatonin we cannot determine the relative efficiency of this compound compared to the Acornines. Acornine 2 appears to be more efficient than Acornine 1 where antifungal activities are concerned. It is not clear at this time whether the absence of the −OH group in Acornine 2 is responsible for the observed differences in antifungal activities between themselves. Also, the backbone of the three compounds (corniculatonin and acornines) remaining similar to each other, yet the observation that Acornine 1 shows reduced activities compared to Corniculatonin and Acornine 2 may shed lights on the roles of side carbohydrate moieties in the observed antifungal activities and may help in the elucidation of their mechanism of action. Mention must be made here that Wahidullah et al.,[13] collected their plant samples from the west coast of India whereas our plant samples were collected from Sundarban estuarine areas in the east coast of India. Our findings suggest the presence in A. corniculatum, collected from Sundarban regions of West Bengal state of India, of two naturally occurring compounds having antifungal activities that are structurally similar to corniculatonin but not identical to it. When the bark extracts of our plant samples were run following the conditions described by Wahidullah et al.,[13] in a TLC experiment, we have not seen any component having the mobility characteristic even close to that of corniculatonin described by them (data not shown). Added to this, when one considers the structural similarities of corniculatonin and Acornines, it prompts us to opine that Acornine 1 and 2 may be different metabolic manifestations of corniculatonin expressed differently under different microenvironments. From that viewpoint, Acornine 1 and 2 may be regarded as novel compounds. We have named these two compounds as Acornine 1 (Oleanane 13,28 epoxy-16 hydroxyl-3-glycoside; Rf 0.36 in BAW solvent system) and Acornine 2 (Oleanane 13,28 epoxy-3-glycoside; Rf 0.32 in BAW solvent system) to distinguish them from corniculatonin described by Wahidullah et al.[13]. Acornine 2, in particular, was effective as antifungal against several pathogenic fungi tested and also against some Gram-positive bacteria.
CONCLUSION
We isolated two compounds having notable antifungal activities from the bark of Aegiceras corniculatum, a mangrove plant, found in the Sundarban estuary of India. We named the two compounds as Acornine 1 (Oleanane 13,28 epoxy-16 hydroxyl-3-glycoside) and Acornine 2 (Oleanane 13,28 epoxy-3-glycoside). Acornine 2 was effective as antifungal against several pathogenic fungi tested and also against some Gram-positive bacteria.
Wahidullah et al.,[13] collected plant samples from western coast of India whereas plant samples used in this study were collected from the Sundarban areas in the east coast of India. The differences in the microenvironments and biotic as well as abiotic stress factors in two geographically distinct coastal areas may be the reasons why the plant is synthesizing the compounds having antifungal activities in different ways. Differential gene expression under changed environments may have enabled the synthesis of slightly different compounds. Each of these compounds (corniculatonin and Acornines) may be endowed with better ability that is suited best in the particular environment in which the plant is growing for combating differential threats (biotic and abiotic) existing in two coastal areas of India having different climatic and stress conditions. Additionally, our finding strengthens the well-known belief that (i) environmental factors dictate the secondary metabolites profile of medicinal plants and (ii) the same plant species growing in different natural habitats around the world may have different metabolite profiles with respect to these compounds. Acornine 1 and Acornine 2 are previously unreported compounds having antifungal activities. Presence of both antifungal and Gram-positive antibacterial activities indicates necessity of further studies of these two compounds.
ACKNOWLEDGMENTS
This work has been supported in part by departmental funds from UGC and DBT. The authors are thankful to Sophisticated Analytical Instrument Facility, CDRI, Lucknow for HPLC, 1H NMR and DART-MS analysis. Thanks are also due to E. Padmanaban, IICB, Kolkata for 600 MHz 13C NMR run, Narindra K Singh, BHU for carrying out the FTIR analysis, Dr. K. Naskar for help with identification of plant samples and Aritra Simlai for critical reading of the manuscript.
Footnotes
Source of Support: This work has been supproted in parts by Departmental funds from UGC and DBT, Govt. of India
Conflict of Interest: None declared.
REFERENCES
- 1.Tomlinson PB. Cambridge: Cambridge University Press; 1986. The Botany of mangroves; p. 414. [Google Scholar]
- 2.Kathiresan K, Bingham BL. Biology of mangroves and mangrove ecosystems. Adv Mar Biol. 2001;40:81–251. [Google Scholar]
- 3.Vannucci M, editor. Tokyo: U.N. University Press; 2004. Mangrove management and conservation–present and future. [Google Scholar]
- 4.Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of Mangrove Plants. Wetlands Ecol Manage. 2002;10:421–52. [Google Scholar]
- 5.Gurudeeban S, Satyavani K, Ramanathan T, Balasubramanian T. Antidiabetic effect of a black mangrove species Aegiceras corniculatum in alloxan-induced diabetic rats. J Adv Pharm Technol Res. 2012;3:52–6. doi: 10.4103/2231-4040.93560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roome T, Dar A, Ali S, Naqvi S, Choudhary MI. A study on antioxidant, free radical scavenging, antiinflammatory and hepatoprotective actions of Aegiceras corniculatum (stem) extracts. J Ethnopharmacol. 2008;118:514–21. doi: 10.1016/j.jep.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Agoramoorthy G, Chen FA, Venkatesalu V, Kuo DH, Shea PC. Evaluation of antioxidant polyphenols from selected mangrove plants of India. Asian J Chem. 2008;20:1311–22. [Google Scholar]
- 8.Chandrasekaran M, Venkatesalu V, Anantharaj M, Rajendran S, Prabhakar K. Studies on the antibacterial activity of a mangrove Aegiceras corniculatum. J Annamalai Univ Sci. 2004:169–74. [Google Scholar]
- 9.Vadlapudi VR, Bobbarala V. In vitro antimicrobial activity of two mangrove plants Aegiceras corniculatum and Hibiscus tiliaceous. Biosci Biotechnol Res Asia. 2009;6:321–24. [Google Scholar]
- 10.Uddin SJ, Rouf R, Shilpi JA, Alamgir M, Nahar L, Sarker SD. Screening of some Bangladeshi medicinal plants for in vitro antibacterial activity. Orient Pharm Exp Med. 2008;8:316–21. [Google Scholar]
- 11.Gupta VK, Roy A. Comparative study of antimicrobial activities of some mangrove plants from Sundarban estuarine regions of India. J Med Plants Res. 2012;6:5480–8. [Google Scholar]
- 12.Giron OC, Sumera FC, Miles DH, Cajipe GJ, Chavez VB, Gomez ED, et al. A chemical toxicant from a mangrove plants Aegiceras corniculatum Blanko (Aegicerataceae) Philipp J Sci. 1988;117:39–53. [Google Scholar]
- 13.Wahidullah S, Bhosak SH, D’Souza ML. Composition containing novel compound Corniculatonin having antifungi properties and a process for preparing the same. Council of Scientific and Research, India. 2004 US patent no. 6777004 B1. [Google Scholar]
- 14.Sambrook J, Russel DW. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning, a laboratory manual; pp. A2–12. [Google Scholar]
- 15.Bauer AW, Kirby WM, Sherries T. Antibiotic susceptibility testing by a standard single disc method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 16.Guerin-Faublee V, Muller ML, Vigneulle M, Flandrois JP. Application of a modified disc diffusion technique to antimicrobial susceptibility testing of Vibrio anguillarum and Aeromonas salmonicida clinical isolates. Vet Microbiol. 1996;51:137–49. doi: 10.1016/0378-1135(96)00034-x. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, Sengupta S. Studies on biochemistry of higher fungi. II. Submerged growth of a few mushrooms in synthetic media. J Food Sci Technol. 1978;15:237–42. [Google Scholar]
- 18.Ghosh AK, Banerjee PC, Sengupta S. Purification and properties of xylan hydrolase from mushroom Termitomyces clypeatus. Biochim Biophys Acta. 1980;612:143–52. doi: 10.1016/0005-2744(80)90287-9. [DOI] [PubMed] [Google Scholar]
- 19.Nostro A, Germano MP, Angelo VD, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Let Appl Microbiol. 2000;30:379–84. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 20.Sofowara A. Canada: John Wiley and Sons; 2000. Medicinal plants and traditional medicine in Africa. [Google Scholar]
- 21.Budzikiewicz H, Djerassi C, Williams DH. Vol. 2. San Francisco, London, Amsterdam: Holden-Day Inc; 1964. Structure elucidation of natural products by mass spectrometry. [Google Scholar]


