Abstract
Background:
Tanshinone IIA (TSIIA) on solid dispersions (SDs) has thermodynamical instability of amorphous drug. Ternary solid dispersions (tSDs) can extend the stability of the amorphous form of drug. Poloxamer 188 was used as a SD carrier. Nano-CaCO3 played an important role in adsorption of biomolecules and is being developed for a host of biotechnological applications.
Objective:
The aim of the present study was to investigate the dissolution behavior and accelerated stability of TSIIA on solid dispersions (SDs) by the use of ternary systems with nano-CaCO3 and poloxamer 188.
Materials and Methods:
The TSIIA tSDs were prepared by a spray-drying method. First, the effect of combination of poloxamer 188 and nano-CaCO3 on TSIIA dissolution was studied. Subsequently, a set of complementary techniques (DSC, XRPD, SEM and FTIR) was used to monitor the physical changes of TSIIA in the SDs. Finally, stability test was carried out under the conditions 40°C/75% RH for 6 months.
Results:
The characterization of tSDs by differential scanning calorimetry analysis (DSC) and X-ray powder diffraction (XRPD) showed that TSIIA was present in its amorphous form. Fourier transforms infrared spectroscopy (FTIR) suggested the presence of interactions between TSIIA and carriers in tSDs. Improvement in the dissolution rate was observed for all SDs. The stability study conducted on SDs with nano-CaCO3 showed stable drug content and dissolution behavior, over the period of 6 months as compared with freshly prepared SDs.
Conclusion:
SDs preparation with nano-CaCO3 and poloxamer 188 may be a promising approach to enhance the dissolution and stability of TSIIA.
Keywords: Combination carriers, in vitro dissolution, stability, ternary solid dispersions
INTRODUCTION
Tanshinone IIA (TSIIA), the major liposoluble bioactive ingredient extracted from the root of Salvia miltiorrhiza Bunge, exhibits a variety of cardiovascular activities including vasorelaxation and a cardio-protective effect.[1,2,3] However, TSIIA shows poor solubility in water[4] and insufficient dissolution rate,[5,6,7] which can give rise to incomplete and/or unpredictable bioavailability. Currently, sodium TSIIA sulfonate, a water soluble derivative, has been used in clinical practice to treat patients with cardiac metabolic disorders.[8] However, because ionic compounds cannot penetrate the blood-brain barrier, sodium TSIIA sulfonate is not effective for cerebrovascular disease.[9]
Among the numerous techniques purpose for improving the dissolution properties of poorly water-soluble drugs and hence, possibly, their bioavailability, solid dispersions (SDs) have attracted considerable interest and have been successfully applied.[10,11,12,13,14] Dissolution rates can be improved by particle size reduction, increased wettability through mixing with highly soluble carriers, and maintenance of the drug in the amorphous form.[15,16,17] Although the application of SDs has been reported regularly in the pharmaceutical literature, only a few commercial products rely on the SDs strategy. One of the main reasons for this discrepancy is the possible thermodynamical instability of amorphous drug[18,19] that have the tendency to change to a more stable state under recrystallization on storage,[20,21,22] which inevitably results in decreased solubility and the dissolution rate. In particular, recent investigations have shown that formulation of ternary solid dispersions (tSDs) by using suitable carrier combinations or adding an appropriate third component can extend the stability of the amorphous form of drug with respect to the corresponding binary systems.
Obviously, drug stability of SDs largely depends on the properties of carriers. However, the amount of carriers that can be used to keep the stability of drug is still rather limited. Therefore, more complex carriers remain to be explored. Poloxamer 188, as 80% of its weight is polyoxyethylene (PEO) groups with a lower melting point (52°C),[23,24] was used as a SD carrier of poorly water-soluble drugs with two roles, one as a polymeric carrier and the other as surface active agent. The polymeric carrier with surface active properties significantly enhances dissolution of poor water soluble drugs.[25,26,27] However, high amount of hydrophilic polymer in SDs may also increase the availability of moisture, which may promote drug migration and crystallization. Similarly, tackiness and stickiness are imparted by poloxamer 188 causes processing problems. However, the previous report that stable free flowing SDs of glibenclamide were obtained using polyglycolized glycerides carriers with the aid of silicon dioxide.[28]
Nano-CaCO3 had received much more attention because of its satisfying biocompatibility, non-toxicity, small size and high specific surface area.[29,30] Moreover, it also represented the highest output and probably the lowest cost of commercial nanoparticles in the world because of their widespread applications. Nano-CaCO3 played an important role in the adsorption of biomolecules due to their large specific surface area and high surface energy. Additionally, it is being developed for a host of biotechnological applications such as cancer therapy and drug delivery.[31,32]
In this context, we report here, for the first time, nano-CaCO3 was combined with surfactant poloxamer 188 for the preparation of TSIIA SDs. Firstly, the effect of combination of poloxamer 188 and nano-CaCO3 on TSIIA dissolution was studied. Subsequently, a set of complementary techniques (DSC, XRPD, SEM and FTIR) was used to monitor the physical changes of TSIIA in the SDs. Finally, stability test was carried out under the conditions of 40°C/75% RH for 6 months.
MATERIALS AND METHODS
Materials
TSIIA standard was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). TSIIA was supplied by the Nanjing Zelang Medical Technology Co. Ltd. (Nanjing, China) and purity was greater than 98%. Nano-CaCO3 with average particle size of 60 nm was supplied by Shanxi Ruicheng Huaxin Nano Material Co. Ltd. (Shanxi, China). All reagents were of analytical grade except methanol of chromatographic grade.
Preparation of solid dispersions and physical mixtures
Binary dispersions (bSDs) of TSIIA and nano-CaCO3 or poloxamer 188 and TSIIA/nano-CaCO3/poloxamer 188 ternary dispersions (tSDs) were prepared using a spray dryer (SD-06 Labplant, Labplant UK Limited, North Yorkshire, Britain) [Table 1]. A fixed set of adjustable parameters of the equipment (the inlet and outlet temperatures of the drying chamber were maintained at 75 and 38°C, respectively, feeding rate: 8 mL/min) were used throughout. The resultant powders were stored in a desiccator for further investigation. PMs was prepared by blending the components in a mortar [Table 1].
Table 1.
Composition of the binary/ternary solid dispersions
In vitro dissolution study
HPLC analysis of TSIIA
The concentration of TSIIA in the dissolution medium was determined by high pressure liquid chromatography (HPLC, Agilent 1200) equipped with a Diamonsil™ RP-C18 column (250 × 4.6 mm, 5 μm). The mobile phase of methanol and water (85:15, v: v) was used at a flow rate of 1.0 mL/min. The UV detector was set at 270 nm to analyze the column effluent and the column temperature was 30°C. The entire solution was filtered through a 0.45 μm membrane filter (Millipore Corp.) and degassed prior to use. The injection volume was 10 μL. The recovery rates for TSIIA were in the range of 99-102%, and the RSD were less than 2%. Intra-day and inter-day precisions for TSIIA were below 2%.
In vitro dissolution studies
The pharmaceutical performance of pure TSIIA, its SDs and PMs were evaluated using in vitro dissolution studies. The tests were carried out according to the USP 24 method 2 (paddle method) in a Beiyang SR8 and dissolution apparatus (D-800LS Precise Dissolution Apparatus, Tianjin University Co., Ltd., Tianjin, China). The dissolution test was performed using 900 ml of distilled water contained 0.5% sodium dodecyl sulfate at 37 ± 0.5°C and 50 rpm for 2 h. Samples equivalent to 5 mg of TSIIA were taken for dissolution studies. Five-milliliter samples were taken at 5, 10, 15, 30, 45, 60, 120 and 180 min and immediately replaced with fresh dissolution medium at the same temperature. These samples were filtered with a membrane filter (pore size 0.45 μm). The first 2 mm was discarded and remainder was analyzed by HPLC for TSIIA as described above.
Characterization of TSIIA solid dispersion
Differential scanning calorimetry
Thermal analysis was performed with differential scanning calorimeter (204A/G Phoenix® instrument, Netzsch, Germany). The samples were heated under nitrogen atmosphere on an aluminum pan at a heating rate of 10°C/min over the temperature range of 25 and 350°C. All the DSC measurements were conducted in a nitrogen atmosphere, and the flow rate was 50 mL/min.
Scanning electron microscopy (SEM)
The surface morphology of TSIIA and tSDs were examined using a scanning electron microscope (S-3000N, Hitachi, Japan).
X-ray powder diffraction
XRPD patterns of samples were performed on a X-ray diffractometer (X-pro Pan analytical, Phillips, Mumbai, India). Data were collected using primary monochromated radiation (Cu Kα1, λ =1.5406 Å), over a 2θ range of 0-70° at a step size of 0.04 and a dwell time of 10 s per step.
Fourier transform infrared spectroscopy
FTIR measurements were carried out with an infrared spectrophotometer (Victor22 BRUKER) at room temperature. Samples of TSIIA, combined carriers, PMs and SDs were previously ground and mixed thoroughly with potassium bromide (0.5% (w/w) of the sample). The scanning range was 400 to 4000 cm-1 and the resolution was 1 cm-1.
Stability test
The accelerated stability study of prepared solid dispersion was conducted at 40°C/75% RH for 6 months according to related literature. The samples were evaluated for drug content and in vitro drug dissolution.
RESULTS AND DISCUSSION
In vitro dissolution studies
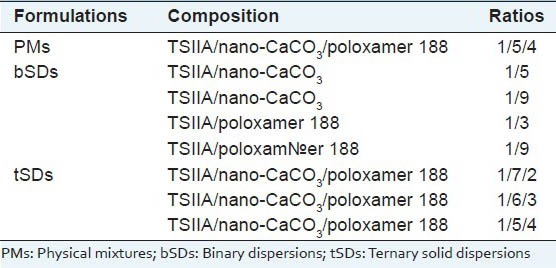
The dissolution behaviors of TSIIA from various tSDs compared with pure TSIIA and PMs were shown in Figure 1. Both pure TSIIA [Figure 1a] and PMs of TSIIA/nano-CaCO3/poloxamer 188 [Figure 1b] presented a poor dissolution rate of less than 30% after 120 min, which could be related to its crystalline form shown by DSC and XRPD. PMs including poloxamer 188 [Figure 1b] exhibited a slight improvement in the dissolution rate compared with pure TSIIA, which was most likely attributed to the hydrophilic and weak solubilizer effect of poloxamer 188. Also as shown in Figure 1, it was evident that bSDs of TSIIA/poloxamer 188 [Figure 1e and f] exhibited faster dissolution rates than TSIIA. At 60 min, the 1/3 and 1/9 bSDs with poloxamer 188, approximately 53.6% and 95.2% of the TSIIA were dissolved, respectively. It was demonstrated that the higher the carrier content, the faster the dissolution rate of TSIIA in SDs. Poloxamer 188 may generate a high surfactant concentration, which could enhance the solubility of dissolved drug and prevent agglomeration of drug into large globules or particles in the aqueous environment, and thus effectively inhibited crystallization during dissolution.[33,34] An improved dissolution rate of TSIIA also was observed in bSDs with nano-CaCO3 [Figure 1c and d]. At TSIIA/nano-CaCO3 ratio of 1/9, 65% of TSIIA was dissolved within 60 min [Figure 1d]. This improvement in the drug dissolution might be due to the significant reduction in particle size during the formation of SDs as well as the adsorption of TSIIA onto the large surface area of nano-CaCO3. As reported by Monkhouse and Lach,[35] adsorption onto insoluble, non-porous, high surface-area carriers was a well-known technique to enhance drug dissolution and was already described for silica-based excipients in the early 1970s.
Figure 1.
The dissolution profiles of (a) TSIIA, (b) TSIIA/nano-CaCO3/ poloxamer 188 (1/5/4, PMs) and bSDs at different ratios of (c) TSIIA/ nano-CaCO3 (1/5), (d) TSIIA/nano-CaCO3 (1/9), (e) TSIIA/poloxamer 188 (1/3), (f) TSIIA/poloxamer 188 (1/9), and tSDs at different TSIIA/ nano-CaCO3/poloxamer 188 ratios of (g) TSIIA/nano-CaCO3/poloxamer 188 (1/7/2), (h) TSIIA/nano-CaCO3/poloxamer 188 (1/6/3), (i) TSIIA/ nano-CaCO3/poloxamer 188 (1/5/4). Each point represents the mean ± SD (n = 3)
Judging from the dissolution results of tSDs, increase TSIIA dissolution from tSDs [Figure 1g–i] was also observed. The polymeric carrier with surface active properties and adsorption of TSIIA onto the large surface area of nano-CaCO3 might be responsible for the observed dissolution behavior of TSIIA. This improved drug dissolution could also be attributed to the presence of amorphous TSIIA, as confirmed by DSC and XRPD studies.
Scanning electron microscopy
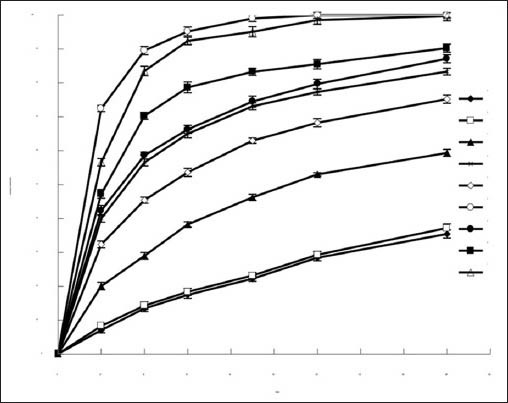
The scanning electron microscopy images of pure TSIIA and tSDs at TSIIA/nano-CaCO3/poloxamer 188 ratio of 1/5/4 were shown in Figure 2. Crystal morphology of pure TSIIA [Figure 2a] exhibited flat broken needles of different sizes, with well-developed edges. However, the tSDs [Figure 2b] appeared as irregular particles. The original morphology of TSIIA was not visibly observed in the photomicrographs, suggesting that TSIIA dispersed uniformly into the carrier. Therefore, it was possible that the reduced particle size and increased surface area might be responsible for the enhanced drug dissolution of the SDs.
Figure 2.
SEM photomicrographs of TSIIA (a) and tSDs at TSIIA/ nano-CaCO3/poloxamer 188 ratio of 1/5/4 (b)
Differential scanning calorimetry
The DSC thermograms of TSIIA, PMs of nano-CaCO3/poloxamer 188, PMs composed of TSIIA/nano-CaCO3/poloxamer188, and the corresponding tSDs were presented in Figure 4. The DSC curve of TSIIA [Figure 3a] exhibited a sharp melting peak with onset temperatures of 208.3°C, indicating its crystalline nature, and followed by an exothermic peak at 223.6°C, which may be attributed to the decomposition of TSIIA. Concerning PMs of carriers [Figure 3b], endothermic peak of poloxamer 188 at 55.8°C was observed. Similar results have been reported by Li et al.[17] and Zhao et al.[15] During scanning of TSIIA/nano-CaCO3/poloxamer PMs [Figure 3c], the drug endothermic peak was found at 208.3°C, indicating that the absence of interaction between TSIIA and carriers in PMs and TSIIA existed in a virgin form in the system. In DSC spectra of tSDs [Figure 3d], the characteristic peak of TSIIA had completely disappeared, suggesting that the drug may be present in tSDs as amorphous state, which was responsible for the enhancement of drug dissolution. Additionally, the decomposition exothermic peak also vanished, which suggested stabilization effect of TSIIA by combined carriers (nano-CaCO3/poloxamer 188) solid dispersion.
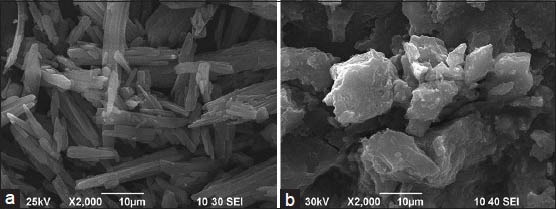
Figure 4.
The X-ray powder diffractograms: (a) TSIIA, (b) nano-CaCO3/ poloxamer 188 ratio of 5/4, (c) TSIIA/nano-CaCO3/poloxamer 1/5/4 PMs, and (d) tSDs at TSIIA/nano-CaCO3/poloxamer 188 ratio of 1/5/4
Figure 3.
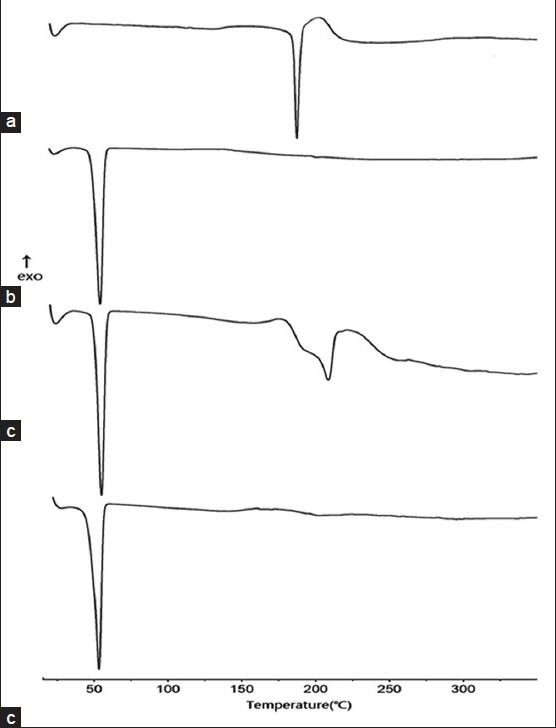
DSC curves of pure TSIIA (a), nano-CaCO3/poloxamer 188 ratio of 5/4 PMs (b), TSIIA/nano-CaCO3/poloxamer 1/5/4 PMs (c), and tSDs at TSIIA/nano-CaCO3/poloxamer 188 ratio of 1/5/4 (d)
X-ray powder diffraction
The X-ray powder diffraction of TSIIA, PMs of nano-CaCO3/poloxamer 188, PMs of TSIIA/nano-CaCO3/poloxamer and the corresponding tSDs were shown in Figure 4. TSIIA showed prominent diffraction peaks in the range of 5-30°, showing a typical crystalline pattern [Figure 4a]. Broad peaks at 18 and 66° were observed in the spectra of combined carriers [Figure 4b]. All major characteristic crystalline peaks of TSIIA were observed clearly in PMs of TSIIA/nano-CaCO3/poloxamer diffractograms [Figure 4c]. However, for the tSDs [Figure 4d], the discriminative peaks of TSIIA had obviously disappeared compared with the corresponding physical mixtures, indicating that TSIIA was no longer present in the crystalline state but was converted to amorphous form, which is consistent with the DSC results.
Fourier transform infrared
In order to further ascertain if TSIIA occurred a polymorphic change during the preparation of solid dispersion and to test the possibility of intermolecular interactions between TSIIA and carriers in the SDs, FTIR was carried out and the results were presented in Figure 5. FTIR spectrum of pure TSIIA [Figure 5a] showed characteristic carbonyl-stretching vibrating absorption of peaks at 1667 cm-1. Similar observations have been reported by Zhao et al.[15] The FTIR spectrum of carriers showed the peaks at 3487 cm-1, which corresponded to -OH stretching vibration mode. This could be attributed to the presence of hydroxyl groups and (or) adsorbed water on the surface of nano-CaCO3 particles.[28] The FTIR spectra of PMs [Figure 5c] were almost equivalent to the addition spectrum of TSIIA and carriers [Figure 5a and b]. This result suggested that there is only a physical effect and there was no chemical interaction between combined carriers and TSIIA in PMs. When scanned from 4000-400 cm-1 in the tSDs [Figure 5d], stretching vibrations of carbonyl group (1667 cm-1) in curcumin appeared at lower wave number 1649 cm-1, and the peak at 3478 cm-1 of- OH stretching vibration was weaken and simultaneously broadened, which suggested that TSIIA interacted with carriers, presumably by hydrogen bonds.
Figure 5.
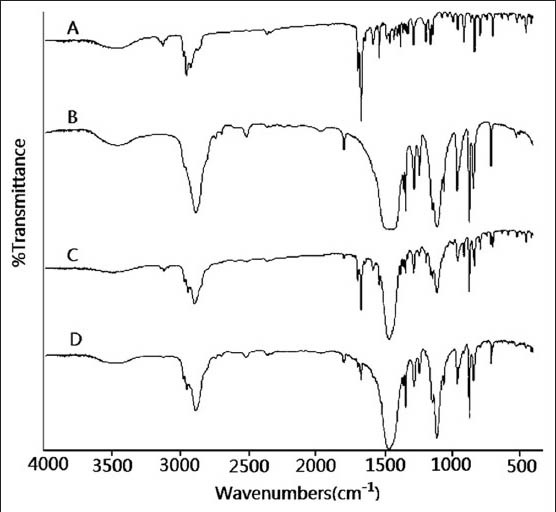
IR spectrums (a) TSIIA, (b) nano-CaCO3/poloxamer 188 ratio of 5/4, (c) TSIIA/nano-CaCO3/poloxamer 1/5/4 PMs, and (d) tSD at TSIIA/nano-CaCO3/poloxamer 188 ratio of 1/5/4
Stability test
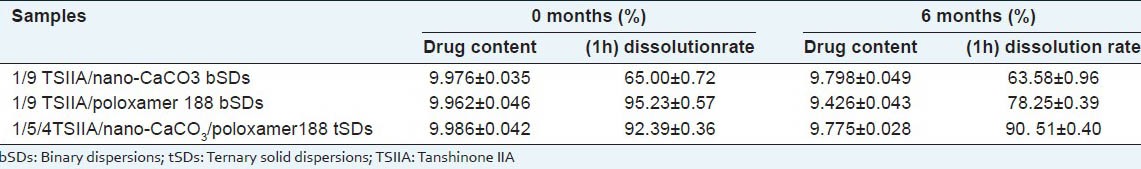
As we all know, moisture and other factors like molecular mobility may increase drug migration and promote drug crystallization in solid dispersions,[36] resulting in poor stability, decreased solubility and dissolution rate. Thus, in the current study, the stability of the SDs was estimated by tests on drug content and in vitro dissolution under the storage conditions of 75% RH and 40°C for 6 months. The results are listed in Table 2. Drug content in both bSDs and tSDs stored for 6 months was unchanged remarkably compared with freshly prepared samples. After 6 months of storage, the dissolution (60 min) of bSDs with poloxamer 188 decreased by about 17%. On the other hand, SDs consists of nano-CaCO3 which showed similar dissolution profiles compared with the freshly prepared samples within 60 min. These phenomena indicated that the nano-CaCO3 had a strongly stabilizing effect on the meta-stable drug in SDs and inhibited recrystallization of TSIIA. The improved stability of SDs probably be due to the interactions between drug and carriers, as well as the dispersion and adsorption on the surface of nano-CaCO3, which could slow down molecular mobility of amorphous drug.[37] The fabrication of nano-CaCO3 is simple, scalable, cost-effective, and controllable. Thus, we believe that nano-CaCO3 will have potential application in the filed of SDs as a specific dispersion carrier.
Table 2.
Stability test of TSIIA solid dispersions (n=6, ±s)
CONCLUSIONS
In this study, tSDs of TSIIA (poorly water-soluble drug) were successfully prepared by spray drying technique using poloxamer 188 with the aid of nano-CaCO3 as dispersing carrier. All SDs exhibited improvement in drug dissolution. As compared to the use of poloxamer 188 alone, very slight decrease in dissolution was observed in SDs with nano-CaCO3 during stability study, which suggested nano-CaCO3 had strongly stabilizing effect on the amorphous TSIIA in SDs. Thus, the experimental results demonstrated the high potential of spray drying technique for obtaining stable free flowing SDs of TSIIA using poloxamer 188 with the aid of nano-CaCO3 as dispersing carriers.
ACKOWLEDGEMENT
The authors report no conflicts of interest in this work. This work was supported by Open fund of Key Laboratory of New Drug Delivery System of Chinese Meteria Medica (2011NDDCM01001).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wang P, Wu X, Bao Y, Fang J, Zhou S, Gao J, et al. Tanshinone IIA prevents cardiac remodeling through attenuating NAD (P) H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie. 2011;66:517–24. [PubMed] [Google Scholar]
- 2.Zhang W, Li J, Liu J, Wu Z, Xu Y, Wang J. Tanshinone IIA-loaded reconstituted high density lipoproteins: Atherosclerotic plaque targeting mechanism in a foam cell model and pharmacokinetics in rabbits. Pharmazie. 2012;67:324–30. [PubMed] [Google Scholar]
- 3.Xu W, Yang J, Wu LM. Cardioprotective effects of tanshinone IIA on myocardial ischemia injury in rats. Pharmazie. 2009;64:332–6. [PubMed] [Google Scholar]
- 4.Li CD, Liu JP, Zeng ZZ, Zheng Y ×. Study on the solubility and permeability of tanshinone IIA and on the excipients increasing the solubility and permeability. Lishizhen Med Mater Med Res. 2008;19:1724–6. [Google Scholar]
- 5.Yu XY, Lin SG, Zhou ZW, Chen X, Liang J, Liu PQ, et al. Role of P-glycoprotein in the intestinal absorption of Tanshinone IIA, a major active ingredient in the root of salvia miltiorrhiza bunge. Curr Drug Metab. 2007;8:325–40. doi: 10.2174/138920007780655450. [DOI] [PubMed] [Google Scholar]
- 6.Mao SJ, Hou SX, Liang Z, Bi YQ, Wu Y, Li H, et al. Ion-pair reversed-phase HPLC: Assay validation of sodium Tanshinone IIA sulfonate in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:163–8. doi: 10.1016/j.jchromb.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Jiang X, Xu W, Li C. Complexation of tanshinone IIA with 2- hydroxypropyl-beta-cyclodextrin: Effect on aqueous solubility, dissolution rate, and intestinal absorption behavior in rats. Int J Pharm. 2007;341:58–67. doi: 10.1016/j.ijpharm.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Jiang B, Zhang L, Wang Y, Li M, Wu W, Guan S, et al. Tanshinone IIA sodium sulfonate protects against cardiotoxicity induced by doxorubicin in vitro and in vivo. Food Chem Toxicol. 2009;47:1538–44. doi: 10.1016/j.fct.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Lam BY, Lo AC, Sun X, Luo HW, Chung SK, Sucher NJ. Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine. 2003;10:286–91. doi: 10.1078/094471103322004776. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Li G, Huang JG, Wang RH, Liu H, Tang R. Comparison of self-microemulsifying drug delivery system versus solid dispersion technology used in the improvement of dissolution rate and bioavailability of vinpocetine. Acta Pharm Sin. 2009;44:658–66. [PubMed] [Google Scholar]
- 11.Sancheti PP, Karekar P, Vyas VM, Shah M, Pore YV. Preparation and physicochemical characterization of surfactant based solid dispersions of ezetimibe. Pharmazie. 2009;64:227–31. [PubMed] [Google Scholar]
- 12.Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5:49–56. [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Pei L, Tong HH, Zheng Y. Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with pluronic F68 to improve dissolution and oral bioavailability. AAPS PharmSciTech. 2011;12:104–13. doi: 10.1208/s12249-010-9560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guedes FL, de Oliveira BG, Hernandes MZ, De Simone CA, Veiga FJ, de Lima Mdo C, et al. Solid dispersions of imidazolidinedione by PEG and PVP polymers with potential antischistosomal activities. AAPS PharmSciTech. 2011;12:401–10. doi: 10.1208/s12249-010-9556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Liu X, Gan L, Zhou C, Mo J. Preparation and physicochemical characterizations of tanshinone IIA solid dispersion. Arch Pharm Res. 2011;34:949–59. doi: 10.1007/s12272-011-0612-3. [DOI] [PubMed] [Google Scholar]
- 16.Bley H, Fussnegger B, Bodmeier R. Characterization and stability of solid dispersions based on PEG/polymer blends. Int J Pharm. 2010;390:165–73. doi: 10.1016/j.ijpharm.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Liu P, Liu JP, Zhang WL, Yang JK, Fan YQ. Novel Tanshinone II A ternary solid dispersion pellets prepared by a single-step technique In vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80:426–32. doi: 10.1016/j.ejpb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Al-Obaidi H, Brocchini S, Buckton G. Anomalous properties of spray dried solid dispersions. J Pharm Sci. 2009;98:4724–37. doi: 10.1002/jps.21782. [DOI] [PubMed] [Google Scholar]
- 19.Al-Obaidi H, Buckton G. Evaluation of griseofulvin binary and ternary solid dispersions with HPMCAS. AAPS PharmSciTech. 2009;10:1172–7. doi: 10.1208/s12249-009-9319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalaiselvan R, Mohanta GP, Manna PK, Manavalan R. Inhibition of albendazole crystallization in poly (vinylpyrrolidone) solid molecular dispersions. Pharmazie. 2006;61:618–24. [PubMed] [Google Scholar]
- 21.Al-Obaidi H, Ke P, Brocchini S, Buckton G. Characterization and stability of ternary solid dispersions with PVP and PHPMA. Int J Pharm. 2011;419:20–7. doi: 10.1016/j.ijpharm.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Weuts I, Kempen D, Decorte A, Verreck G, Peeters J, Brewster M, et al. Physical stability of the amorphous state of loperamide and two fragment molecules in solid dispersions with the polymers PVP-K30 and PVP-VA64. Eur J Pharm Sci. 2005;25:313–20. doi: 10.1016/j.ejps.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Ali W, Williams AC, Rawlinson CF. Stochiometrically governed molecular interactions in drug: Poloxamer solid dispersions. Int J Pharm. 2010;391:162–8. doi: 10.1016/j.ijpharm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Passerini N, Albertini B, González-Rodríguez ML, Cavallari C, Rodriguez L. Preparation and characterisation of ibuprofen-poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci. 2002;15:71–8. doi: 10.1016/s0928-0987(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 25.Serajuddin AT. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–66. doi: 10.1021/js980403l. [DOI] [PubMed] [Google Scholar]
- 26.Seo A, Holm P, Kristensen HG, Schaefer T. The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer. Int J Pharm. 2003;259:161–71. doi: 10.1016/s0378-5173(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 27.Kolašinac N, Kachrimanis K, Homšek I, Grujić B, Durić Z, Ibrić S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int J Pharm. 2012;436:161–70. doi: 10.1016/j.ijpharm.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci. 2005;26:219–30. doi: 10.1016/j.ejps.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Shan D, Zhu M, Xue H, Cosnier S. Development of amperometric biosensor for glucose based on a novel attractive enzyme immobilization matrix: Calcium carbonate nanoparticles. Biosens Bioelectron. 2007;22:1612–7. doi: 10.1016/j.bios.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Ueno Y, Futagawa H, Takagi Y, Ueno A, Mizushima Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J Control Release. 2005;103:93–8. doi: 10.1016/j.jconrel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Higaki M, Kameyama M, Udagawa M, Ueno Y, Yamaguchi Y, Igarashi R, et al. Transdermal delivery of CaCO3-nanoparticles containing insulin. Diabetes Technol Ther. 2006;8:369–74. doi: 10.1089/dia.2006.8.369. [DOI] [PubMed] [Google Scholar]
- 32.Li ZZ, Wen LX, Shao L, Chen JF. Fabrication of porous hollow silica nanoparticles and their applications in drug release control. J Control Release. 2004;98:245–54. doi: 10.1016/j.jconrel.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Vippagunta SR, Maul KA, Tallavajhala S, Grant DJ. Solid state characterization of nifedipine solid dispersions. Int J Pharm. 2002;236:111–23. doi: 10.1016/s0378-5173(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Hu N. Comparative bioelectrochemical study of core-shell nanocluster films with ordinary layer-by-layer films containing heme proteins and CaCO3 nanoparticles. J Phys Chem B. 2005;109:10464–73. doi: 10.1021/jp0505227. [DOI] [PubMed] [Google Scholar]
- 35.Kawabata Y, Yamamoto K, Debari K, Onoue S, Yamada S. Novel crystalline solid dispersion of tranilast with high photostability and improved oral bioavailability. Eur J Pharm Sci. 2010;39:256–62. doi: 10.1016/j.ejps.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Monkhouse DC, Lach JL. Use of adsorbents in enhancement of drug dissolution. II. J Pharm Sci. 1976;61:1435–41. doi: 10.1002/jps.2600610918. [DOI] [PubMed] [Google Scholar]
- 37.Tiwari R, Tiwari G, Srivastava B, Rai AK. Solid dispersions: An overview to modify bioavailability of poorly water soluble drugs. Int J PharmTech Res. 2009;1:1338–49. [Google Scholar]


