Abstract
Objective:
Safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde, C10H14O) is an active ingredient in the saffron, which is used in traditional medicine, and also, the biological activity of saffron in anti-cancer is in development. It has been reported to have anti-oxidant effects, but its anti-tumor effects remain uncertain. The aim of this study was to evaluate effects of safranal on anti-tumor on neuroblastoma cells.
Materials and Methods:
Neuroblastoma cells were cultured and exposed to safranal (0, 10, 15, 20, 50 μg/ml). Cell proliferation was examined using the 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Apoptotic cells, cell cycle distribution, and sub-G1 fraction were analyzed using flow cytometric analysis after propidium iodide staining.
Results:
Safranal inhibited the growth of malignant cells in a dose-and time-dependent manner. The IC (50) values against the neuroblastoma cell line were determined as 11.1 and 23.3 μg/ml after 24 and 48 h, respectively. Safranal induced a sub-G1 peak in the flow cytometry histogram of treated cells compared to control cells indicating that apoptotic cell death is involved in safranal toxicity.
Conclusions:
Our pre-clinical study demonstrated a neuroblastoma cell line to be highly sensitive to safranal-mediated growth inhibition and apoptotic cell death. Although the molecular mechanisms of safranal action are not yet clearly understood, it appears to have potential as a therapeutic agent.
Keywords: Apoptosis, cytotoxcicity, neuroblastoma, propidium iodide, safranal
INTRODUCTION
Neuroblastoma is the most common extracranial solid cancer in childhood and the most common cancer in infancy, with an annual incidence of about 650 cases per year in the US,[1] and 100 cases per year in the UK.[2] Close to 50% of neuroblastoma cases occur in children younger than 2 years old.[3] It is a neuroendocrine tumor, arising from any neural crest element of the sympathetic nervous system. It most frequently originates in one of the adrenal glands, but can also develop in nerve tissues in the neck, chest, abdomen, or pelvis. Neuroblastoma is one of the few human malignancies known to demonstrate spontaneous regression from an undifferentiated state to a completely benign cellular appearance.[4]
The need for anti-tumor drugs with high efficacy and low toxicity has led to studies evaluating putative anti-neoplastic factors in fruits, vegetables, herbs, and spices. Saffron, obtained from the dried red-dark stigmas of Crocus sativus L., an important spice rich in carotenoids, is commonly consumed in different parts of the world, and there are ancient reports of saffron being used to treat various diseases, particularly cancer, by the Indian, Greek, and Chinese cultures.[5,6,7] A number of studies have reported an anti-tumor effect associated with saffron treatment in multiple cell culture systems and animal models. During the last decade, a number of studies in animal model systems have shown an anti-tumor effect of saffron.[8,9] It was reported first in 1990s and confirmed in recent years that saffron extract inhibited growth of malignant cells in vitro and also in vivo.[5,10] Inhibition of DNA, RNA, and protein synthesis was shown in 3 human malignant cells exposed to saffron constituents,[10] and the mechanism of action was reported through suppression of the activity of DNA-dependent RNA polymerase II.[11,12]
Different hypotheses for anti-tumor effects of saffron and its ingredients have been proposed, including inhibition of nucleic acid and free radical chain reactions and interaction of carotenoids with topoisomerase II.[13,14] Despite these studies, the role of its main ingredients is not clarified yet. Comprehensive chemical analysis of saffron extracts has shown that one of active constituent of saffron is safranal.[15]
The flower stigma are composed volatile oils, but the most important being safranal, which gives saffron its distinct hay-like flavor. Other volatile oils in saffron are cineole, phenethenol, pinene, borneol, geraniol, limonene, p-cymene, linalool, terpinen-4-oil, etc.[16] Safranal showed anti-oxidant activity and reduced oxidative damages in different organs such as skeletal muscle,[17] kidney,[18] and hippocampus.[19]
Apoptosis, or programmed cell death, is an active gene directed form of cell death that is different from cell necrosis with respect to its morphology, biochemistry, pharmacology, and biological significance. Many types of mammalian cells undergo apoptosis during normal development or in response to a variety of stimuli, including DNA damage, growth factor deprivation, and abnormal expression of oncogene or tumor suppressor genes. Apoptosis is a widely accepted important mechanism that contributes to cell growth reduction. Crocus sativus has been shown to induce apoptosis in different cancerous cell types.[20]
The present study was undertaken to explore whether safranal induces apoptosis in neuroblastoma cell line, which exhibits aggressive clinical behavior. We studied the effect of safranal on the growth in culture of neuroblastoma cell line (N2A).
MATERIALS AND METHODS
Cell culture
Experiments were carried out using mouse neuroblastoma N2A cell line (tumor-like neuroblasts), which were kindly provided by Dr. M. Mojarad. The cells were cultured either in 96-well tissue (TC) plate (NUNC, Wiesbaden, Germany) or in 25-cm2 TC flasks (NUNC, Wiesbaden, Germany). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum (Gibco–Invitrogen), 100 IU·ml-1 penicillin (Gibco–Invitrogen), and 100 μg·ml-1 streptomycin (Gibco–Invitrogen). The cells were incubated in a humidified 5% CO2 and 95% air atmosphere at 37°C in CO2 incubator MCO-17AI (Sanyo Electric Co., Ltd., Japan). The culture medium was replaced 3 times a week. At the time of the experiment, confluent cells were trypsinized and plated in 96-well plates or into tissue culture dishes (40 mm in diameter; TPP). N2A cells were plated at a density of 31, 000 cells/cm2. Experiments were initiated 72 h after plating.
In vitro evaluation of safranal-induced cytotoxicity
Safranal (was purchased from Sigma-Aldrich (St. Louis, MO)) at different concentrations (10, 15, 20, and 50 μg/ml) were used to examine its cytotoxic effects. Eight replicates were used for each dose per 96-well plate in 7 independent experiments. Cells were exposed safranal for 24,48, and 72 h.
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT; Sigma, St. Louis, U.S.A.). MTT was dissolved at a concentration of 5 mg·ml-1 in sterile phosphate-buffered saline (PBS) at room temperature, and the solution was further sterilized by passing through a 0.2 μm filter and stored at 4°C in the dark. The final concentration of MTT added to each well was 1 mg·ml-1. After 2 h of incubation at 37°C, a same volume of lysis buffer was added. Lysis buffer was prepared as follows: 20% (w/v) sodium dodecil sulfate was dissolved at 37°C in a solution of 50% (v/v) of N, N-dimethyl formamide and reagent grade water, pH 4.7. After an overnight incubation at room temperature, optical densities were measured at 560 nm. Cell viability was normalized to data measured under control conditions, without safranal. Safranal oxidation in the medium was monitored by method based on the formation of quinones, which absorb light at 560 nm. The time-course of safranal-induced cytotoxicity was studied by treating cells with 10, 15, 20, and 50 μg/ml of this compound. A total of 9 tissue culture dishes (40 mm in diameter) containing N2A cells were exposed to safranal. The dishes were divided into 3 separate groups (n = 3 for each group), which were subjected for 24, 48, and 72 h of exposition, respectively. Three control dishes for each time were kept under the same conditions without being exposed to safranal. Cell viability was measured as described above.
Measurement of apoptosis
Apoptotic cells were detected using PI staining of treated cells followed by flow cytometry to detect the so-called sub-G1 peak.[21] Briefly, N2A cells were cultured overnight in a 24-well plate and treated with various concentrations of safranal (10, 15, 20, and 50 μg/ml) for 48 h. Floating and adherent cells were then harvested and incubated at 4 °C overnight in the dark with 750 mL of a hypotonic buffer (50 mg/mL PI in 0.1% sodium citrate + 0.1% Triton X-100) before flow cytometric analysis using flow cytometer (Münster, Germany) was conducted. Ten thousand events were acquired.
Statistical analysis
One-way analysis of variance (ANOVA) and Bonferroni's post-hoc were used for data analysis. All results were expressed as mean ± SEM. P < 0.05 was considered statistically significant. The IC50 was obtained by non-linear regression using the Graphpad program (Intuitive Software for Science, San Diego, CA, USA).
RESULTS
Effects of safranal on cell proliferation and viability of tumor cells
To study the effects of safranal treatment on tumor cells, we treated an aggressive metastatic neuroblastoma cell line with increasing concentrations of safranal over a maximum of 72 h. As indicated by MTT assay, safranal exerted time and concentration-dependent cytotoxic effects on the cell line tested (IC50 = 11.1, 23.2 μg/ml after 48 and 72 h respectively). Figure 1 shows that exposure of N2A cells for 48 and 72 h with safranal inhibits significantly proliferation of neuroblastoma cells. Exposure of the N2A cell line for 24 h decreased the number of viable cells, but it was not significant in any doses of safranal. On the other hand, treatment of the N2A cells for 48 and 72 h with different concentrations of safranal resulted in marked reduction of number of viable cells (P < 0.001). There is statically significant cell death difference between neuroblastoma cells after 24 h and 48 h (P < 0.001). This toxicity was associated with morphological changes including reduction of cell volume and rounding of the cells.
Figure 1.
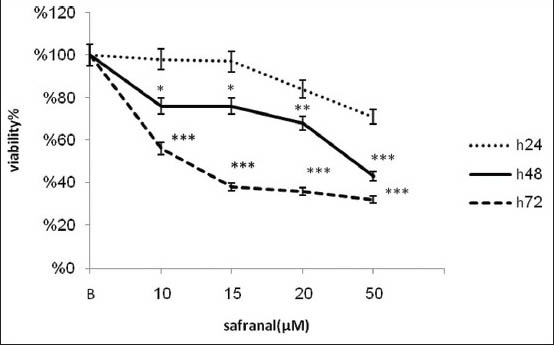
Cytotoxic effect of safranal on neuroblastoma cells. Cells were incubated with different concentrations of safranal for 24, 48 and 72 h when the cell viability was determined by the MTT assay. The cytotoxicity was calculated as percentage of inhibition compared with control
Role of apoptosis in neuroblastoma cells treated with safranal
Safranal cytotoxicity was then evaluated by flow cytometric analysis using propidium iodide (PI) to assess cell membrane integrity and DNA fragmentation. Apoptosis following treatment with safranal (0, 10, 15, 20, 50 μg/ml)
was measured with PI staining and flow cytometry, aiming to detect the sub-G1 peak resulting from DNA fragmentation. Flow cytometry histogram of the positive control in which cells were cultured in serum-free medium and of safranal-treated cells were studied. Flow cytometry histogram of the safranal-treated cells exhibited a sub-G1 peak in N2A cells, which indicates the involvement of an apoptotic process in safranal-induced cell death [Figure 2]. As shown in Figure 3, safranal induced variable loss of cell membrane integrity at different concentrations in the cell line.
Figure 2.
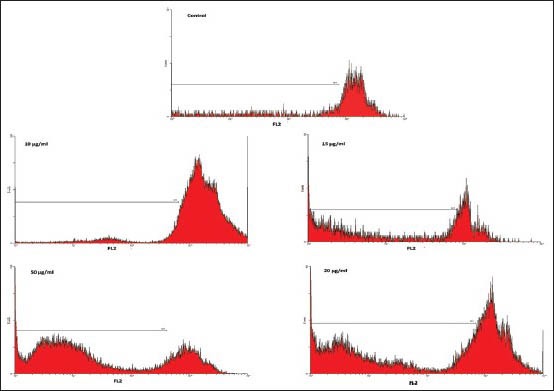
Flow cytometry histograms of apoptosis assays by PI method in N2A cells. Flow cytometry histograms of PI-stained N2A cells treated with different concentration of safranal for 48 h. Sub-G1 peak as an indicative of apoptotic cells, was induced in safranal-treated but not in control cells. Flow cytometry histogram of positive control in which cells were cultured in serum free medium and safranal-treated cells. Safranal -treated cells exhibited a sub-G1 peak in N2Aells that indicates the involvement of an apoptotic process in safranal-induced cell death
Figure 3.
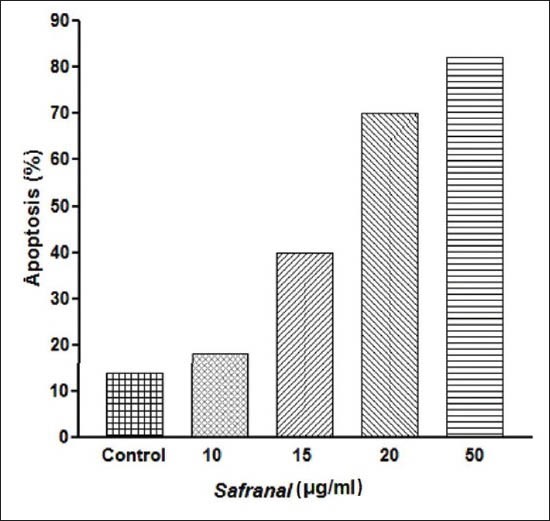
Role of apoptosis in safranal-induced toxicity in the N2A cells. Cells were treated with 0, 10, 15, 20 and 50 μg/ml of safranal for 48 h
DISCUSSION
Agents capable of inducing apoptosis, inhibiting cell proliferation, or modulating signal transduction are currently used for the treatment of cancer. A combination of multiple chemo-preventive agents or agents with multiple targets is considered to be more effective than a single agent.[22] This is an important facet of biomedical research, providing a practical approach for identifying potentially useful inhibitors of tumor development.
Natural products have long been used to prevent and treat diseases including cancers and might be good candidates for the development of anti-cancer drugs.[23] Crocus sativus has been shown to induce apoptosis in human cancer cell lines.[24,20] Crocus sativus has been used to treat several medical conditions, such as gastrointestinal disorders, urological infections, as well as in treating malignancies.[25,26,27] Crocus sativus contains components like safranal, alpha crocin or crocin is the major component. Additionally, Crocus sativus also contains amino acids, flavonoids, and other chemical compounds.[27,28] Among these, safranal is the most important since it is the major component in Crocus sativus and has shown significant biological activities.[26] Safranal is an organic compound isolated from saffron. It is the constituent primarily responsible for the aroma of saffron. It is believed that safranal is a degradation product of the carotenoid zeaxanthin via the intermediacy of picrocrocin.
In this study, the cytotoxic and proapoptotic effects of safranal on neuroblastoma (N2A) cells were investigated. To the author's knowledge, this is the first report on safranal-induced cytotoxicity in the cancer cell line. The result of present study demonstrated that safranal is strong suppressor of neuroblastoma (N2A) cell proliferation. It is important to point out that growth inhibitor effects of safranal were observed at concentration that may be generated through dietary intake. We also found that safranal exerts activity against proliferation of N2A cells by arresting cells in the G1 phase of cell cycle and causing apoptosis. Our data confirmed that a constituent of saffron has cytotoxic activity against the N2A cell line, which is consistent with previous studies conducted on other main compounds of saffron.[7,24] In this study, we evaluated safranal as an anti-cancer agent on N2A cells, and the most important is that our findings revealed that apoptosis is involved in safranal-mediated cancer cell death. Based on the result of our previous research on saffron extract, the inhibition of tumor growth by saffron extract may be through apoptosis.[20,24]
A balance between cell proliferation and apoptosis controls normal organ development.[29,30,31] The induction of apoptosis in tumor cells is considered a valuable way to treat cancer.[24] A wide variety of natural substances has been recognized to have the ability to induce apoptosis in various tumor cells. It is thus considered important to screen apoptotic inducers from plants, either in the form of crude extracts or as components isolated from them.[30] Apoptotic cell death is known to be induced by many chemotherapeutic agents routinely used in cancer treatment regimens. The apoptotic process comprises a series of events including cell shrinkage, increased cytoplasmic density, chromatin condensation cell, nuclear shrinkage, membrane blebbing, and oligonucleosomal DNA fragmentation. Apoptosis is an important homeostatic mechanism that balances cell division and cell death and maintains the appropriate number of cell in the body. In the present study, safranal-induced apoptosis was also shown to be involved in the induction of cell death in neuroblastoma cells [Figure 2 and 3]. In our current study, apoptosis was determined using PI staining of DNA fragmentation by flow cytometry (sub-G1 peak). It has been reported that DNA fragmentation creates small fragments of DNA that can be eluted following incubation in a hypotonic phosphate-citrate buffer. When stained with a quantitative DNA-binding dye such as PI, cells that have lost DNA will take up less stain and will appear to the left of the G1 peak.[32,33]
Our current and previous studies have shown that saffron extract and its constituents, safranal, significantly inhibit the proliferation of cancer cells.[20,24] Taken together, they have shown that this inhibitory activity is partly due to safranal. This is in accordance with other studies, which have shown that anti-oxidative activity of saffron is related to safranal.[19,34,35]
It should be notified that cell death is not always accompanied by the typical features of either apoptosis or necrosis. Examples of cell death have been described, in which the pattern of morphological and/or biochemical changes neither resembled typical apoptosis nor necrosis, but often had features of both. In some cases, the integrity of the plasma membrane was preserved, but DNA degradation was random, without evidence of internucleosomal cleavage. In other situations, DNA degradation was typical of apoptosis, but nuclear fragmentation and other features of apoptosis were not apparent. Generally, while most hematopoietic lineage cell types are primed to apoptosis and their death has typical features of apoptosis, the death of epithelial type cells is more complex and sometimes difficult to classify. Furthermore, some drugs which cause apoptosis may also mystify the pattern of cell death due to the drug-induced secondary effects on the cell.[36] For example, when apoptosis is triggered by drugs affecting cell structure and function or by drugs affecting one or more pathways of the apoptotic cascade, particular features of apoptosis may not be apparent. Likewise, prolonged cell arrest in the cell cycle induced by some drugs leads to growth imbalance, which may significantly alter cell biochemistry and morphology.[37]
Previous studies involving saffron extracts and crocin, the other active compound of saffron, showed that these compounds influence on the proliferation of the cancer cells. Their data from this study suggests that crocin from Crocus sativus may be efficacious in treating pancreatic cancer.[34,38,39] In contrast, little is known about the activities of the other compound of saffron. The only growth inhibition assay that has been carried out with safranal was done on the breast cancer cells.[7] It is considered important to screen apoptotic inducers from plants, either in the form of crude extracts or as components isolated from them. In the present study, safranal-induced apoptosis was involved in the induction of cell death. Apoptotic cells exhibit several biochemical modifications such as protein cleavage, protein cross-linking, DNA fragmentation, and phagocytic recognition that together result in the distinctive structural pathology.[40,41]
According to Figure 1, a concentration-dependent induction of apoptosis was detected in the safranal-treated N2A cells. It has been reported that DNA fragmentation creates small fragments of DNA that can be eluted following incubation in a hypotonic phosphate–citrate buffer. When cells are stained with a quantitative DNA-binding dye such as PI, aiming to detect the sub-G1 peak resulting from DNA fragmentation, cells that have lost DNA will take up less stain and will appear to the left of the G1 peak.[33,36]
Safranal, a degradation product of the carotenoid, is an effective anti-convulsant shown to act as an agonist at GABAA receptors.[31,42,43] Safranal also exhibits high anti-oxidant and free radical scavenging activity.[31,44] It has also been shown to have anti-depressant properties.[10,45] We also, in current study, demonstrated that it has great potential as cancer chemo-preventive/chemotherapeutic agents, in particular, in neuroblastoma.
Administering individual pure compounds is advantageous, because it eliminates the inconsistencies involved in plant cultivation and extraction procedures and reduces the side-effects that may be attributed to undesirable chemicals within the plant.[46,47] The results reported here indicate that some of the activities of saffron extracts toward the cancer cells that we previously evaluated can be recapitulated with safranal. Safranal might be one of the potential compounds in the crude extract of saffron that could be effective in the prevention and/or treatment of cancer.
Taking together, this study showed that safranal inhibits the proliferation of malignant cell line with the involvement of apoptosis or programmed cell death. Safranal could also be considered as a promising chemotherapeutic agent in cancer treatment. However, exact molecular mechanism of safranal action is not yet clearly understood. It is ongoing work in our laboratory, and soon we will come up with systematic explanation of mechanism of safranal action.
ACKNOWLEDGEMENTS
The authors would like to thank Research Affairs of Neyshabur University of Medical Sciences for financially supporting this work. We also thank Dr H. Nasirli for her assistance in flow cytometry.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sun W, Modak S. Emerging treatment options for the treatment of neuroblastoma: Potential role of perifosine. Onco Targets Ther. 2012;5:21–9. doi: 10.2147/OTT.S14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan C, Sabai SM, Tin AS, Quah TC, Aung L. Neuroblastoma: Experience from National University Health System, Singapore (1987-2008) Singapore Med J. 2012;53:19–25. [PubMed] [Google Scholar]
- 3.Li K, Dong K, Gao J, Yao W, Xiao X, Zheng S. Neuroblastoma management in Chinese children. J Invest Surg. 2012;25:86–92. doi: 10.3109/08941939.2011.605203. [DOI] [PubMed] [Google Scholar]
- 4.Bénard J, Raguénez G, Kauffmann A. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: A molecular portrait of stage 4S. Mol Oncol. 2008;2:261–71. doi: 10.1016/j.molonc.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair SC, Panikkar B, Panikkar KR. Antitumor activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–14. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 6.Tarantilis PA, Morjani H, Polissiou M. Inhibition of growth and induction of differentiation of promyelocytic leukemia (HL-60) by carotenoids from C. Sativus L. Anticancer Res. 1994;14:1913–8. [PubMed] [Google Scholar]
- 7.Chrissanthi DG, Lamar FN, Iatrou G. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007;27:357–62. [PubMed] [Google Scholar]
- 8.Tseng TH, Chu CH, Huang JM. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–7. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- 9.Liao YH, Houghton PJ, Hoult JR. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J Nat Prod. 1999;62:1241–5. doi: 10.1021/np990092+. [DOI] [PubMed] [Google Scholar]
- 10.Abdullaev FI, Frenkel GD. Effect of saffron on cell colony formation and cellular nucleic acid and protein synthesis. Biofactors. 1992;3:201–4. [PubMed] [Google Scholar]
- 11.Abdullaev FI. Inhibitory effect of Crocetin on intracellular nucleic acid and protein synthesis in malignant cells. Toxicol Lett. 1994;70:243–51. doi: 10.1016/0378-4274(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 12.Nair SC, Kururumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: A review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 13.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;2(27):20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 14.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of Saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Bharti S, Golechha M, Kumari S, Siddiqui KM, Arya DS. Akt/ GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur J Nutr. 2012;51:719–27. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- 16.Hooshmandi Z, Rohani AH, Eidi A, Fatahi Z, Golmanesh L, Sahraei H. Reduction of metabolic and behavioral signs of acute stress in male Wistar rats by saffron water extract and its constituent safranal. Pharm Biol. 2011;49:947–54. doi: 10.3109/13880209.2011.558103. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–50. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharmacol Sci. 2005;8:387–93. [PubMed] [Google Scholar]
- 19.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–9. [PubMed] [Google Scholar]
- 20.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.). in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–14. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 22.Guzman M. Cannabinoids: Potential anticancer agents. Nat Rev Cancer. 2003;3:745–55. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 23.Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr Sci. 2001;80:1387–96. [Google Scholar]
- 24.Abdullaev FI, Riverón-Negrete L, Caballero-Ortega H, Manuel Hernández J, Pérez-López I, Pereda-Miranda R, et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) Toxicol In Vitro. 2003;17:731–6. doi: 10.1016/s0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 25.Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–14. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 26.Rios JL, Recio MC, Giner RM. Updates review of saffron and its active compounds. Phytother Res. 1996;10:189–93. [Google Scholar]
- 27.Nair SC, Varghese CD, Pannikar KR, Kurumboor SK, Parathod RK. Effects of saffron on vitamin A levels and its antitumor activity on the growth of solid tumors in mice. Int J Pharmacogn. 1994;32:105–14. [Google Scholar]
- 28.Winterhalter P, Straubinger M. Saffron-renewed interest in an ancient spice. Food Rev Int. 2000;16:39–59. [Google Scholar]
- 29.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 30.Belmokhtar CA, Hillion J, Egal-Bendirdjian ES´. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–62. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 31.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;9:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAILinduced apoptosis ofmelanoma.“. Cancer Res. 1999;59:2747–53. [PubMed] [Google Scholar]
- 33.Brohem CA, Sawada TC, Massaro RR, Almeida RL, Rivelli DP, Ropke CD, et al. Apoptosis induction by 4-nerolidylcatechol in melanoma cell lines. Toxicol In vitro. 2009;23:111–9. doi: 10.1016/j.tiv.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–6. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Kanakis CD, Tarantilis PA, Tajmir-Riahi HA, Polissiou MG. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: Stability and antioxidative properties. J Agric Food Chem. 2007;5:970–7. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 36.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 37.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in Cell Necrobiology: Analysis of Apoptosis and Accidental Cell Death (Necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 38.Sun J, Xu XM, Ni CZ, Zhang H, Li XY, Zhang CL, et al. Crocin inhibits proliferation and nucleic acid synthesis and induces apoptosis in the human tongue squamous cell carcinoma cell line Tca8113. Asian Pac J Cancer Prev. 2011;12:2679–83. [PubMed] [Google Scholar]
- 39.Bakshi H, Sam S, Rozati R, Sultan P, Islam T, Rathore B, et al. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev. 2010;11:675–9. [PubMed] [Google Scholar]
- 40.Dam AD, Mitchell AS, Rush JW, Quadrilatero J. Elevated skeletal muscle apoptotic signaling following glutathione depletion. Apoptosis. 2012;17:48–60. doi: 10.1007/s10495-011-0654-5. [DOI] [PubMed] [Google Scholar]
- 41.Talib WH, Zarga MH, Mahasneh AM. Antiproliferative, Antimicrobial and Apoptosis Inducing Effects of Compounds Isolated from Inula viscosa. Molecules. 2012;17:3291–303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–62. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–4. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Magesh V, Singh JP, Selvendiran K, Ekambaram G. Antitumor effect of Crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Mol Cell Biochem. 2006;287:127–35. doi: 10.1007/s11010-005-9088-0. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic. 2004;650:435–45. [Google Scholar]
- 46.Nelson PS, Montgomery B. Unconventional therapy for prostate cancer: Good, bad or questionable? Nat Rev Cancer. 2003;3:845–58. doi: 10.1038/nrc1210. [DOI] [PubMed] [Google Scholar]
- 47.Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics. 2011;66:1073–9. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]


