Abstract
Background:
Detection of latent tuberculosis infection (LTBI) in transplant candidates is very important. The tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) are standard immunologic tools for LTBI detection. The aim of this study was to compare the TST results and T-SPOT®.TB test (a type of IGRAs) in kidney transplant candidates for the screening of LTBI and follow the patients with positive test for an activation of tuberculosis (TB) after transplantation and using anti-TB prophylaxis.
Materials and Methods:
This study was a prospective study and carried out in 44 renal transplant candidates from March 2010 to February 2011 in the teaching hospitals of Isfahan University of Medical Sciences, Iran. TST and T-SPOT®.TB test were performed and their results evaluated. Patients with a positive skin test and/or T-SPOT®.TB test were started on anti-TB prophylaxis and followed after transplantation for an activation of their LTBI for 1 year.
Results:
Overall, 8 (18.2%) patients were positive for TST and 6 (13.6%) patients for T-SPOT®.TB test. The agreement between TST and T-SPOT®.TB test was moderate (κ = 0.49, 95% confidence interval 0.145-0.839). The overall agreement between TST and T-SPOT®.TB test was 86%. No relation was found between the underlying diseases and TST or T-SPOT®.TB test positivity. Although isoniazid prophylaxis was used for patients with positive TST and/or T-SPOT®.TB test, one patient had reactivation of TB.
Conclusion:
In kidney transplant candidates both TST and T-SPOT®.TB test were comparable for the diagnosis of LTBI with reasonable agreement between the tests. However, further studies are needed to determine the ability of T-SPOT®.TB test to detect LTBI and to evaluate the need for prophylaxis in these patients.
Keywords: Kidney transplant, latent tuberculosis, T-SPOT®.TB test, tuberculin skin test
INTRODUCTION
According to World Health Organization reports on 2010, 9 million new and relapse tuberculosis (TB) cases has been estimated around the world and 17,000 patients with TB in Iran.[1] Due to the prevalence of TB in our area, detection of latent tuberculosis infection (LTBI) in limited groups of patients such as a candidate for organ transplants or other immunosuppressed individuals is very important and useful. The incidence of LTBI among kidney transplant recipients is estimated at 20-70 times higher than in the general population.[2] Therefore, current guidelines recommend a generalized screening for evidence of latent infection prior to and after transplantation, to start target appropriate preventative prophylaxis.[3] The tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) are standard immunologic tools for LTBI detection.[4] Until 2001, the TST was the only method and gold standard for diagnosing LTBI,[5] but after that developing IGRAs change the way. These tests measure the interferon (IFN)-gamma released by T-lymphocytes in response to Mycobacterium tuberculosis-specific antigens including early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10).[6,7] These blood tests offer several potential advantages over TSTs, including lack of booster effect, once patient encounter for testing, uniform standards for positivity and lack of cross-reactivity with the Mycobacterium bovis-bacillus Calmette-Guerin (BCG) and most non-tuberculosis mycobacteria.[8] Published data on the performance of the IGRAs in kidney transplant candidates are limited.[3,9] Moreover, in our country, there is little information about the usefulness of the IGRAs in detection of LTBI in these patients.[10] One of the aims of the present work was a comparison of the IGRA (T-SPOT®.TB) and TST test in detection of LTBI in kidney transplant candidates and evaluating the agreement between the two tests.
Chemoprophylaxis of immuncompormised patients with positive TST and\or IGRAs in our area according to the high prevalence of TB would be able to prevent active TB. The currently recommended prophylaxis of latent TB in our country is based on the monotherapy with isoniazid (isonicotinylhydrazine) for at least 9 months.[11] Therefore, in this study the patients with positive tests, received isoniazid prophylaxis and had been followed up for active TB for 1 year.
MATERIALS AND METHODS
Patient population and study design
This prospective study was conducted in the teaching hospitals of Isfahan University of Medical Sciences, Iran, from March 2010 to February 2011 and was approved by the Ethics Committee of this University (Research project number: 185185). A total of 44 adult patients candidate for receiving a kidney transplant were included in the study. These patients were evaluated for ruling out active pulmonary and extra-pulmonary TB. Individuals with a history of prior TB or isoniazid prophylactic treatment were excluded. Furthermore, if patients refused to continue prophylactic treatment and following up and/or symptoms of isoniazid-induced hepatitis or drug reaction were occurred, they were excluded. Demographic and clinical details were obtained from each patient by a detailed questionnaire. Patients underwent TST and T-SPOT®.TB testing before scheduled transplant surgery (blood samples were collected before TST) and follow up for activating LTBI after surgery.
T-SPOT®.TB testing
T-SPOT®.TB assay (Oxford Immunotec, Oxford, UK) was performed according to the manufacturers’ recommendation and defined as positive, negative or indeterminate based on manufacturers’ recommended criteria. Briefly, before the TST, 8 ml peripheral venous blood was collected and processed within 4 h. The peripheral blood mononuclear cells (PBMCs) were isolated by standard ficoll-hypaque density-gradient centrifugation. The PBMCs were counted and adjusted to a cell number of 2.5 × 105 PBMCs/1 ml. Four wells of the 96-well Microtitre plates (nil control, positive control, panel A and panel B), precoated with monoclonal antibody to gamma IFN, were seeded with 100 μl of 2.5 × 105 PBMCs/well. Two wells contained different peptide antigens (ESAT-6 [panel A] and CFP-10 [panel B]), the nil control well contained the cell in medium alone, and the positive control well contained the cell that was stimulated with phytohemagglutinin. After the appropriate incubation time (16-20 h) at in a humidified incubator at 37°C and 5% CO2, the plates were washed with phosphate-buffered saline (PBS) four times. An appropriate volume of conjugate working solution was prepared (1:200 dilution in PBS) for the secondary incubation (60 min at 2-8°C) after which the wells was washed again (×4), as suggested above. Results are presented as the number of spot-forming cells and the reaction was observed visually. We used criteria for positive, negative, and indeterminate outcomes that were recommended by the manufacturer.
TST
TST was performed using the 5 IU purified protein derivative (PPD) (Pasteur Institute, Tehran, Iran) injection into the volar aspect of the forearm intradermally by trained personnel. A positive test was defined by the size of induration (not the erythema) induced by PPD 48-72 h after the injection. If induration size was ≥10 mm, test was considered positive as recommended by local guidelines (Ministry of Health and Medical Education).[11]
Patients follow up for activating LTBI
For all individuals with a positive skin test and/or T-SPOT®.TB test isoniazid prophylaxis was started and continued for 9 months, according to national guidelines and recent studies.[11,12,13] After transplantation, patients were followed up for 1 year. If suspected sign and symptoms of TB were observed in these patients, they were evaluated for pulmonary and extra-pulmonary TB based on symptoms by specialists.
Statistical analysis
The results were analyzed using the SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Numerical variables are reported as mean ± standard division and categorical variables as number and percentage. The categorical variables were compared by Fisher's exact tests or Pearson's Chi-square test, as appropriate. The kappa test was used to analyze agreement between the two tests where κ > 0.75 represented excellent agreement, 0.4-0.75 represented fair to good agreement, and <0.4 represented poor agreement. All tests of significance were two-tailed; P ≤ 0.05 were considered to be significant.
RESULTS
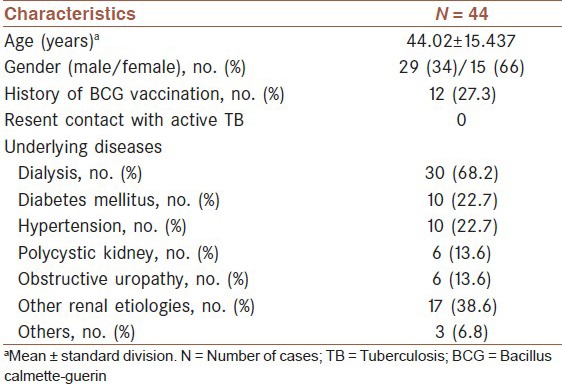
A total of 44 adult patients that candidate for receiving kidney transplant were enrolled in the study. Demographic and clinical characteristics of patients are shown in [Table 1].
Table 1.
Demographic and clinical characteristics of the study participants
Overall, 8 (18.2%) of cases were positive by TST and 6 (13.6%) subjects by T-SPOT®.TB test. The agreement between TST and T-SPOT®.TB test was moderate (κ = 0.49, 95% confidence interval [CI] 0.145-0.839). As shown in [Table 2], the concordance between T-SPOT®.TB test and TST was 86%. The induration size on TST was significantly associated with positivity on the T-SPOT®.TB test [P < 0.001, Figure 1]. None of the patients had recent contact with active TB case. The relation between history of BCG vaccination and underlying diseases and two tests are shown in [Table 3].
Table 2.
Agreement between TST and T-SPOT®.TB test
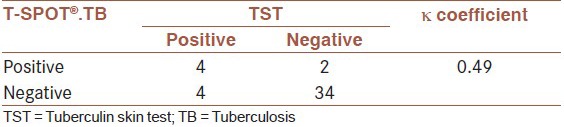
Figure 1.
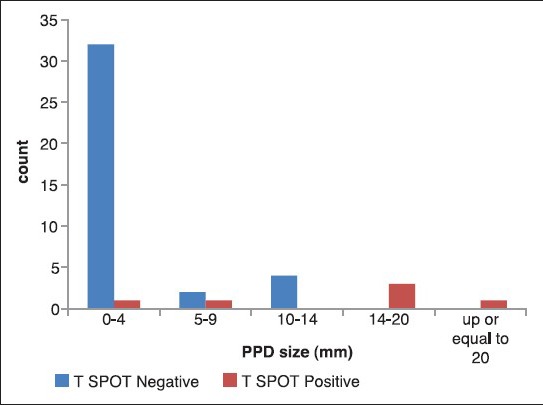
Comparison between the induration size on tuberculin skin test and T-SPOT®.TB test
Table 3.
The association between clinical items and TST or T-SPOT®.TB results
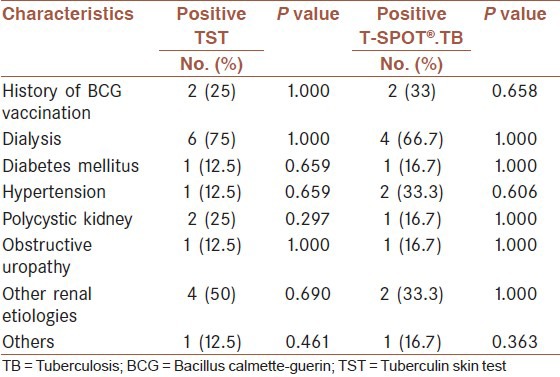
We found that 10 (22.7%) patients had positive skin test and/or T-SPOT®.TB test and they had indication to start isoniazid prophylaxis. Of these, four had positive results for two tests, four had positive TST, but negative T-SPOT®.TB test and two had negative TST but positive T-SPOT®.TB test. Although, all the patients with positive test had isoniazid prophylaxis, one patient was diagnosed as active TB after tuberculin prophylaxis. This patient has positive results in both tests. In addition, one patient with TST positive result was lost to follow-up after transplantation, because of his death that was caused by renal transplant rejection.
DISCUSSION
Although IGRAs are increasingly recommended for diagnosis of TB,[14] but in our region IGRAs has been used in research settings only, and these tests are not routine. The renal transplant recipients are usually screened for LTBI by TST. In this study, the usefulness of TST and T-SPOT®.TB test to detect LTBI was tested in renal transplant candidates. In our study, 18.2% of renal transplant candidates were TST positive and 13.6% were T-SPOT®.TB test positive. Ahmadinejad et al. used TST and QuantiFERON® -TB (QFT) Gold for detection of LTBI in candidates of kidney transplantation. They found that 21.9% patients had positive TST and QFT Gold and agreement between QFT and TST was 75%. Furthermore, they did not find any association between two test and BCG vaccination.[10] In another study by Kim et al., 22% and 30% renal recipients had positive TST and T-SPOT®.TB test; respectively. They reported the agreement between two tests was fair.[9] In other studies, the different percentages of TST and IGRAs positivity were reported in immunosuppressed and hemodialysis patients. Seyhan et al. have reported 34% and 43% TST and QFT Gold positivity in hemodialysis patients; respectively, and agreement between two test was 65%.[15] Also Soysal et al. reported 39% and 61% TST and T-SPOT®.TB test positivity in this group of patients; respectively and agreement between two test was 60%.[16] Piana et al. found 17.4% and 44.2% TST and T-SPOT®.TB test positivity in immunosuppressed hematology patients; respectively and agreement between two tests was 67.8%.[17] The positive percentage of our results are less than other studies and agreement between two tests was moderate, although the induration size of TST significantly associated with positivity on T-SPOT®.TB test. Due to lack of access to complete clinical records of transplant recipient candidates and the absence of organ transplantation bank in our country, the patients may be immuncompormised before the assays because of their underlying disease or their treatment schedule. These reasons can be caused the T-SPOT®.TB test false negative results in our study.
There are few studies on isoniazid prophylaxis in transplant candidates. In our study, 10 patients had indication for isoniazid prophylaxis. None of them had previously received anti-TB drugs. Kim et al. found that 27 patients had indication for isoniazid prophylaxis and of these, only five patients received isoniazid. They did not mention how long patients were followed up and whether the isoniazid prophylaxis is effective or not.[9] In our study, after the prophylaxis, one patient was diagnosed with active TB. These findings show that isoniazid prophylaxis was not as effective as we thought and we should use a more effective regimen. Also, there is few data on the appropriate time to begin the prophylactic treatment. Some patients require immediate transplantation and they receive immunosuppressive drug before the prophylaxis is completed. Thus, additional studies are needed to determine the right time to begin the prophylaxis.
CONCLUSION
Because there is no diagnostic gold standard test for LTBI, the evaluation of sensitivity and specificity of IGRAs is difficult. Therefore, further studies are needed in various patients with different levels of the competency of the immune system. In addition, these tests should be evaluated in different geographic areas according to the incidence of TB, performance of BCG vaccination and the social and health level.
AUTHORS’ CONTRIBUTION
All authors have contributed in designing and conducting the study. MY, PS, MM, ShSh, HH, ShS, and ShT collected the data and RSh, ZF, and SR did the analysis. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and are accountable for all aspects of the work.
ACKNOWLEDGMENTS
We would like to thank the vice-chancellor of research Isfahan University of Medical Sciences and Tropical and Infectious Diseases Research Centre for the financial support for this research and also the staff of Kidney Transplant Ward in Alzahra University Hospital for their great support especially Ms. Fadai the Head of Department.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.Geneva: WHO Press; 2012. [Last cited on 2014 Jan 26]. World Health Statistics. Available from: http://www.who.int/gho/publications/world_health_statistics/2012/en/index.html . [Google Scholar]
- 2.Arias Guillén M, Palomar R, Arias M. Advances in the diagnosis of latent tuberculosis infection in patients receiving renal replacement therapy. Nefrologia. 2011;31:137–41. doi: 10.3265/Nefrologia.pre2011.Feb.10823. [DOI] [PubMed] [Google Scholar]
- 3.Sester U, Junker H, Hodapp T, Schütz A, Thiele B, Meyerhans A, et al. Improved efficiency in detecting cellular immunity towards M. tuberculosis in patients receiving immunosuppressive drug therapy. Nephrol Dial Transplant. 2006;21:3258–68. doi: 10.1093/ndt/gfl416. [DOI] [PubMed] [Google Scholar]
- 4.Druszczyńska M, Kowalewicz-Kulbat M, Fol M, Włodarczyk M, Rudnicka W. Latent M. tuberculosis infection – Pathogenesis, diagnosis, treatment and prevention strategies. Pol J Microbiol. 2012;61:3–10. [PubMed] [Google Scholar]
- 5.Gideon HP, Flynn JL. Latent tuberculosis: What the host “sees”? Immunol Res. 2011;50:202–12. doi: 10.1007/s12026-011-8229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos JM, Robledano C, Masiá M, Belda S, Padilla S, Rodríguez JC, et al. Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: A comparison of QuantiFERON-TB Gold In Tube, T-SPOT. TB and tuberculin skin test. BMC Infect Dis. 2012;12:169. doi: 10.1186/1471-2334-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalvani A, Pareek M. Interferon gamma release assays: Principles and practice. Enferm Infecc Microbiol Clin. 2010;28:245–52. doi: 10.1016/j.eimc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Cruz AT, Geltemeyer AM, Starke JR, Flores JA, Graviss EA, Smith KC. Comparing the tuberculin skin test and T-SPOT. TB blood test in children. Pediatrics. 2011;127:e31–8. doi: 10.1542/peds.2010-1725. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Lee SO, Park IA, Park SJ, Choi SH, Kim YS, et al. Diagnostic usefulness of a T cell-based assay for latent tuberculosis infection in kidney transplant candidates before transplantation. Transpl Infect Dis. 2010;12:113–9. doi: 10.1111/j.1399-3062.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadinejad Z, Azmoudeh Ardalan F, Safy S, Nikbakht G. Diagnosis of latent tuberculosis infection in candidates for kidney transplantation (comparison of two tests) Acta Med Iran. 2012;50:305–10. [PubMed] [Google Scholar]
- 11.Nasehi M, Mirhaghani L. Tehran, Iran: Andishmand Press; 2009. [Last cited on 2014 Jan 26]. National guideline for prevention of TB 2009. Available from: http://www.cdc.hbi.ir/Tutorial_Documents_Inside.aspx . [Google Scholar]
- 12.Aguado JM, Torre-Cisneros J, Fortún J, Benito N, Meije Y, Doblas A, et al. Tuberculosis in solid-organ transplant recipients: Consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276–84. doi: 10.1086/597590. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581–7. doi: 10.1086/427692. [DOI] [PubMed] [Google Scholar]
- 14.Denkinger CM, Dheda K, Pai M. Guidelines on interferon-γ release assays for tuberculosis infection: Concordance, discordance or confusion? Clin Microbiol Infect. 2011;17:806–14. doi: 10.1111/j.1469-0691.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 15.Seyhan EC, Sökücü S, Altin S, Günlüoğlu G, Trablus S, Yilmaz D, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis. 2010;12:98–105. doi: 10.1111/j.1399-3062.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 16.Soysal A, Toprak D, Koc M, Arikan H, Akoglu E, Bakir M. Diagnosing latent tuberculosis infection in haemodialysis patients: T-cell based assay (T-SPOT. TB) or tuberculin skin test? Nephrol Dial Transplant. 2012;27:1645–50. doi: 10.1093/ndt/gfr516. [DOI] [PubMed] [Google Scholar]
- 17.Piana F, Codecasa LR, Cavallerio P, Ferrarese M, Migliori GB, Barbarano L, et al. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur Respir J. 2006;28:31–4. doi: 10.1183/09031936.06.00110205. [DOI] [PubMed] [Google Scholar]


