Abstract
Background:
Nosocomial infection caused by Acinetobacter baumannii has emerged as a serious problem world-wide. Finding the suitable drug is an important priority. The aim of this study was to determine colistin (polymyxin E) resistance in clinical isolates of A. baumannii from intensive care units (ICUs) of Al Zahra Hospital.
Materials and Methods:
Sixty isolates of A. baumannii from patients hospitalized in ICU (Al Zahra Hospital, Isfahan University of Medical Sciences [IUMS]) were studied. All isolates of A. baumannii were tested for colistin susceptibility by Eopsilometer test (E-test).
Results:
Of the 60 isolates 57, (95%) were multidrug resistant (MDR) and 76.6% (46/60) were highly resistant. The rate of colistin resistant with the E-test method was 11.6% (7/60).
Conclusion:
As the frequency of resistance to colistin is low, it can be used as an easily available drug for treatment of MDR A. baumannii strains, which are susceptible to colistin.
Keywords: Acinetobacter baumannii, colistin resistance, multidrug resistant organism
INTRODUCTION
Nosocomial infections or hospital-acquired infections are contaminations obtained during hospital care and aren’t present at the entrance or incubation period of infection on admission.[1] Nosocomial infection caused by Acinetobacter baumannii has emerged as a serious problem world-wide.[2] Resistance of clinical isolates of A. baumannii to different antibiotics causes many difficulties in the treatment of these infections, especially in patients of intensive-care units.[3] This omnipresent bacteria frequently colonizes on the skin and in the respiratory tract of hospitalized Patients and lead to bacteremia, meningitis, in burn wounds infection, pneumonia, and urinary tract infections.[4]
Acinetobacter spp. has the ability to survive on dry surfaces for prolonged time and obtaining multiple antibiotic resistances.[3,4] In two recent decades unfortunately multidrug resistant (MDR) A. baumannii clinical isolates has increased world-wide especially in some Asian countries and it is classified as a difficult nosocomial infection to treat and control.[5,6] MDR A. baumannii is determined as resistance to more than three classes of antibiotics.[7,8]
Risk factors that lead to infection with MDR are prolonged hospitalization, hospitalization in an intensive care unit (ICU), exposure to equipment contaminated with these bacterial species, broad-spectrum antibiotic therapy, concomitant serious medical and surgical illnesses and catheterization.[7] Scanty data are available on the prevalence of MDR A. baumannii in Iran.[8] In a previous study that was performed in medical and surgical ICUs of Masih Daneshvari Hospital in Tehran, Iran the most frequently isolated organism was A. baumannii (22.4%).[9] Acinetobacter spp. account for 2-10% of all nosocomial Gram-negative bacterial infections in the United States and Europe.[5] Rates of mortality from A. baumannii infections have been reported between 23% and 73%, respectively.[10]
Tigecycline as an approved new therapeutic option has potential toxicities similar to tetracycline and in many countries have limited its clinical applications.[6] The old antibiotic, colistin (polymyxin E) is a narrow spectrum bactericidal antibiotic that is a recent trend towards its application in the treatment of infections.[6,11] The in vitro and in vivo studies of colistin determined that it would be an effective antimicrobial agent against A. baumannii; however, some colistin resistant strains have been reported (e.g., 30.6% in tertiary care hospitals in Korea).[2,5] The Eopsilometer test (E-test) has been reported to be a simple and accurate method for determining the antimicrobial susceptibilities of fastidious bacteria. MDR A. baumannii clinical isolates has increased world-wide recently and it could be sever life-threatening bacterium in ICU hospitalized patients. The objective of the present study was detection of colistin resistance in clinical isolates of A. baumannii to find the best therapeutic modality toward this bacterium.
MATERIALS AND METHODS
This is a cross-sectional study performed in medical and surgical ICUs of Al Zahra Hospital, under approvement of Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. The study was carried out on 60 strains of A. baumannii isolated from the bacterial samples obtained from different organs of patients (with more than 48 h of hospitalization) followed in the 5 ICUs of Al Zahra Hospital, IUMS between March 2011 and June 2012. Study was approved by the Ethics Committee of Isfahan University of Medical sciences, Iran (Research project number: 291082).
All probable Acinetobacter isolates, which were non-hemolytic oxidase-negative, non-lactose fermentative (10%), and Gram-negative coccobacilli were identified as A. baumannii by using the conventional biochemical tests and growth potential at 37°C and 44°C. Biochemical tests used for detection of A. baumannii included growth on MacConkey medium (produce mucoid and pale pink colonies), oxidation of glucose, hydrolysis of esculin, decarboxylation of lysine, hydrolysis of arginine, and reduction of nitrate.[8]
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests was carried out on all isolates of Acinetobacter spp. using the Kirby-Bauer disk diffusion agar method according to Clinical Laboratory Standard Institute guidelines.[12] 10 antimicrobial agents were tested: Imipene, meropenem, ciprofloxacin, gentamicin, amikacin, cefepime, ceftazidime, piperacillin/tazobactam and ampicillin/sulbactam. All isolates of A. baumannii were tested for colistin susceptibility by E-test according to the manufacturer's guidelines (Liofilchem, Italy). Suspension of each isolate in Mueller-Hinton broth, adjusted to the density of a 0.5 McFarland standard, was swabbed in three directions to ensure uniform growth onto Mueller-Hinton agar plates. An E-test colistin strip (ranging from 0.016 to 256 mg/ml) was used for each plate when the agar surface was completely dry, and the plates were incubated at 35°C for 16-20 h. The minimum inhibitory concentration (MIC) was read at the point of complete inhibition of all growth, including hazes. The interpretive criteria used were those established in Clinical and Laboratory Standards Institute standard Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as control strains.[12] Obtained data analyzed using SPSS version 18 software (SPSS Inc., Chicago, IL, USA). Chi-square test was applied to assess patient's characteristics and infection with A. baumannii. The significance level threshold was a P ≤ 0.05.
RESULTS
A total of 60 isolates of A. baumannii of ICUs were studied. (Respiratory specimens, n = 23; urine specimens, n = 16; blood specimens, n = 11; tracheal aspirates specimens, n = 5; and cerebrospinal fluids specimens, n = 5). The mean age of A. baumannii infected ICU patient's was 43.1 ± 28.6 years. 26 (43.4%) of them were female and 34 (56.6%) were male. About 78% of patients had a previous history of antibiotic exposure. The average duration of hospitalization was 42.3 ± 39.5 days that was significantly associated with A. baumannii infection (P = 0.003). We couldn’t find any significance association between age, gender and previous history of antibiotic exposure among infected patients (P > 0.05).
The antibiotic resistances of these strains are shown in Figure 1. In disk diffusion method gentamicin and ciprofloxacin showed the most (100%) and imipenem the least resistant (83%) antimicrobial agents against A. baumannii strains.
Figure 1.
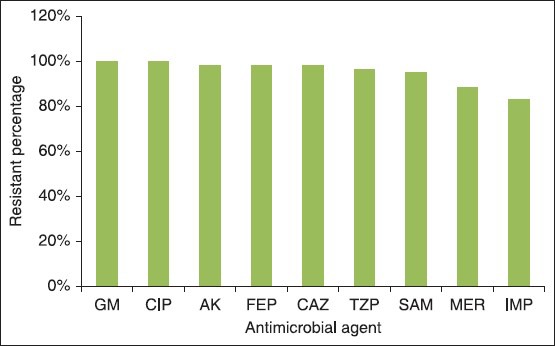
Antimicrobial resistance rates among Acinetobacte baumannii isolates. GM = Gentamicin, CIP = Ciprofloxacin, AK = Amikacin, FEP = Cefepime, CAZ = Ceftazidime, TZP = Piperacillin/tazobactam, SAM = Ampicillin/sulbactam, MER = Meropenem, IMP = Imipene
Of the 60 isolates 57, (95%) were MDR. 76.6% (46/60) were resistant to imipenem, amikacin, and ampicillin-sulbactam (highly resistant).[7] The rate of colistin resistant with the E-test method in all studied strains was determined 11.6%. The MIC concentration ≥4 μg/ml considered as resistance. In our study, only seven samples were assessed resistance [Table 1].
Table 1.
The determined MIC concentration for colistin in the isolated A. baumannii strains of the Al Zahra Hospital ICU

DISCUSSION
A. baumannii frequently causes endemic nosocomial infections.[6] Increased use of incorrect antimicrobials has led to the appearance of MDR species, which are highly difficult to treat.[13] MDR rates in our country are reported much higher than in the USA, Canada and Germany.[8] Recently colistin comes back into use as an effective antibiotic, especially for the treatment of nosocomial serious infections with multiple antibiotic resistances.[11] The results of various studies have shown that colistin treatment in patients with MDR A. baumanii caused a good clinical outcome and less mortality.[1] Therefore, this increased usage may lead to colistin resistance and selection of appropriate antibiotic therapy is essential.[11,14] Because of the poor diffusion of colistin to agar there is no accepted zone diameter in different guidelines for the disk diffusion method however the MIC susceptibility breakpoints are similar.[15] In the present study, about 11.6% of the specimens were resistant to colistin. The number of reports of colistin resistant Acinetobacter spp. have increased year by year all over the world such as reports from Spain (from 19.1% in 2005 to 40.7% in 2009)[2,16] and Korea (from 9.1% in 2002 to 30.6% in 2007).[5,17] Lower rates of resistance were detected in South America and USA (7.1% and 2.1%, respectively).[17] This resistance develops through mutation or adaptation mechanisms. Inappropriate previous usage of colistin might be a risk factor for a higher rate of resistance in Asia and Europe.[5,6,17] Treatment with colistin decreases patient mortality and it is cost-effective and our results showed that only 5% of A. baumannii isolates were susceptible to other antibiotics and many of these isolates have demonstrated MDR [Figure 1]. These findings were consistent with other studies conducted in Iran and some European countries and Egypt.[8,18] Other studies have shown that barrier precautions, environmental decontamination and staff knowledge established in the ICU caused significant reduction in colonization and infection with MDR - A. baumannii.[19] Furthermore hand washing and other infection control exercises such as closed suctioning system has also been recommended.[1] Performing combined infection control including regular closure of all ICUs for decontamination and strict compliance with cross-transmission prevention protocols, changing from general to isolation rooms may lead to the decrease of nosocomial A. baumannii infections especially in mechanically ventilated patients.[20] Whilst the rate of antimicrobial resistance in developing countries (e.g., Iran) is significantly higher than more developed countries (e.g., USA).[8] This may be related to improper infection control programs and extended empiric use of inappropriate antimicrobials in developing countries.[8] Therefore, urgent infection control strategies must be performed in our hospitals and ICUs. Because of probably increasing rate of colistin resistance in Acinetobacter spp. we must seriously consider execution of the strategies promoted by the Centers for Disease Control and Prevention to avoid antimicrobial resistance in health care settings.
CONCLUSION
Due to the high rate of antibiotic resistance in our samples, strategies for the prevention and control of MDR infections are required. We recommend the use of colistin as an available cost-effective drug that decreases patient mortality only for treatment of MDR A. baumannii strains, which are susceptible to colistin.
AUTHORS’ CONTRIBUTION
All authors have contributed in designing and conducting the study. BV, PS, MY, and MKh collected the data and HF, BA, FKh, and BV did the analysis. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and are accountable for all aspects of the work.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.Ducel G, Fabry J, Nicolle L, Girard R, Perraud M, Prüss A, et al. Prevention of hospital-acquired infections. [Last accessed on 2013 Dec 11]. Available from: http://www.who.int/emc .
- 2.Arroyo LA, García-Curiel A, Pachón-Ibañez ME, Llanos AC, Ruiz M, Pachón J, et al. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:903–5. doi: 10.1128/JCM.43.2.903-905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manikal VM, Landman D, Saurina G, Oydna E, Lal H, Quale J. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: Citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis. 2000;31:101–6. doi: 10.1086/313902. [DOI] [PubMed] [Google Scholar]
- 4.Chien ST, Lin CH, Hsueh JC, Li PL, Hsu CH, Chang SH, et al. Mutation of gyrA and parC in clinical isolates of Acinetobacter baumannii and its relationship with antimicrobial drugs resistance in Taiwan. Ann Microbiol. 2009;59:369–72. [Google Scholar]
- 5.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60:1163–7. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 6.Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59:473–7. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 7.Dent LL, Marshall DR, Pratap S, Hulette RB. Multidrug resistant Acinetobacter baumannii: A descriptive study in a city hospital. BMC Infect Dis. 2010;10:196. doi: 10.1186/1471-2334-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadtaheri Z, Pourpaki M, Mohammadi F, Namdar R, Masjedi MR. Surveillance of antimicrobial susceptibility among bacterial isolates from intensive care unit patients of a tertiary-care university hospital in Iran:2006-2009. Chemotherapy. 2010;56:478–84. doi: 10.1159/000321032. [DOI] [PubMed] [Google Scholar]
- 9.Soroush S, Haghi-Ashtiani MT, Taheri-Kalani M, Emaneini M, Aligholi M, Sadeghifard N, et al. Antimicrobial resistance of nosocomial strain of Acinetobacter baumannii in Children's Medical Center of Tehran: A 6-year prospective study. Acta Med Iran. 2010;48:178–84. [PubMed] [Google Scholar]
- 10.Jain R, Danziger LH. Multidrug-resistant Acinetobacter infections: An emerging challenge to clinicians. Ann Pharmacother. 2004;38:1449–59. doi: 10.1345/aph.1D592. [DOI] [PubMed] [Google Scholar]
- 11.Sinirtaş M, Akalin H, Gedikoğlu S. Investigation of colistin sensitivity via three different methods in Acinetobacter baumannii isolates with multiple antibiotic resistance. Int J Infect Dis. 2009;13:e217–20. doi: 10.1016/j.ijid.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Cockerill FR. Twenty Second International Supplement M100-S22 USA: Clinical and Laboratory Standards Institute; 2012. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 13.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–9. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, et al. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit Care. 2003;7:R78–83. doi: 10.1186/cc2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The European Committee on Antimicrobial Suceptibility Testing (EUCAST) Clinical MIC breakpoints. [Last accessed on 2013 Jan 7]. Available from: http://www.srga.org/eucastwt/MICTAB/MIC miscellaneous.html .
- 16.Arroyo LA, Mateos I, González V, Aznar J. In vitro activities of tigecycline, minocycline, and colistin-tigecycline combination against multi- and pandrug-resistant clinical isolates of Acinetobacter baumannii group. Antimicrob Agents Chemother. 2009;53:1295–6. doi: 10.1128/AAC.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–15. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed SH, Fekry Abdelwahab S, Hasanen AM, Mohammed DS. Multidrug resistant Egyptian isolates of Acinetobacter baumannii. J Am Sci. 2011;7:1013–9. [Google Scholar]
- 19.Mastoraki A, Douka E, Kriaras I, Stravopodis G, Saroglou G, Geroulanos S. Preventing strategy of multidrug-resistant Acinetobacter baumanii susceptible only to colistin in cardiac surgical intensive care units. Eur J Cardiothorac Surg. 2008;33:1086–90. doi: 10.1016/j.ejcts.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Mulin B, Rouget C, Clément C, Bailly P, Julliot MC, Viel JF, et al. Association of private isolation rooms with ventilator-associated Acinetobacter baumanii pneumonia in a surgical intensive-care unit. Infect Control Hosp Epidemiol. 1997;18:499–503. doi: 10.1086/647655. [DOI] [PubMed] [Google Scholar]


