Abstract
Background:
Adaptive immune response is an important factor in the healing process and development of protection in cutaneous leishmaniasis (CL). Little information is available in human CL about the importance of the balance between effector and regulatory immune responses. Therefore, the aim of this study was to asses messenger ribonucleic acid (mRNA) expression of interleukin-10 (IL-10), IL-4, transforming growth factor-β1 (TGF-β1), interferon-g (IFN-γ), and forkhead box P3 (Foxp3) (as a marker of regulatory T cells) in acute and chronic CL lesions caused by Leishmania major compared with normal skin samples.
Materials and Methods:
Thirty biopsies were obtained from CL patients with acute lesions (AL, n = 13), chronic lesions (CH, n = 11) and healthy volunteers (n = 6). Relative expressions of target genes were determined by means of reverse transcription real time polymerase chain reaction and were compared with the controls.
Results:
Expression of Foxp3, IL-4, and IFN-γ were significantly more in CH than AL group of patients (Foxp3: Median 0.48, inter-quartile range 0.32-0.76 [arbitrary units] for AL, and 0.97 (0.75-1.30) for CH, P = 0.006; IFN-γ: 45.98 (33.39-173.48) for AL, and 200.53 (97.49-361.76) for CH, P = 0.023; IL-4: 0.49 (0.34-2.16) for AL, and 2.14 (1.30-7.11) for CH, P = 0.021). Expression of TGF-β was not significantly different between groups.
Conclusion:
The results indicate that IL-4 secretion at the site of L. major infection rather than low IFN-γ production might have a role in prolongation of disease. Despite a moderate increase of Foxp3 expression in chronic lesions, function of Tregs in persistent infection is not clear.
Keywords: Cutaneous leishmaniasis, cytokines, gene expression, Leishmania major, regulatory T cells
INTRODUCTION
Cutaneous leishmaniasis (CL) is a vector born protozoan infection caused by different species of Leishmania, which upon healing leaves unpleasant permanent scar(s). CL is a common skin disease in some tropical countries, and also is an emerging infection in developed countries due to population movements, wars, and travels.[1] Current available chemotherapy used for CL is not fully effective, especially in case of chronic form of the disease.[2] Surrogate marker(s) of healing and protection in CL is not yet clearly defined, and evaluation of immune response during lesion development and healing might provide useful information for vaccine development and treatment.
T cell subsets such as T helper (Th) Th1, Th2, regulatory T cells (Tregs), and secreted cytokines are known important factors in leishmaniasis.[3] In the murine model of leishmaniasis induction of Th1 type of response accompanies with secretion of high level of interferon-g (IFN-γ) and leads to control of infection, but development of Th2 response in BALB/c mice is associated with disease progression and dissemination of the parasite.[4] Several studies in old world CL have shown dominant peripheral Th1 response in healing form and Th2 response in the non-healing form of the disease,[5,6] however in situ measurement showed that in old world CL high expression of interleukin-10 (IL-10) and IFN-γ is associated with unfavorable evolution of the lesions.[7] Furthermore, a mixture of Th1/Th2 cells and cytokines are reported from both acute and chronic lesions of the new world CL.[8]
Recent studies in murine models of CL indicate that the balance between effector and regulatory immune responses is a crucial factor for the disease development and control.[9,10] Immune regulatory factors such as IL-10, transforming growth factor-β1 (TGF-β1), and Tregs, as well as Th2 activation at the site of infection suppress or counter-balance Th1 response and cause chronic Leishmania infection.[9,10] Several studies on human CL have been carried out, but the exact role of Th2 and immune regulatory factors in chronic CL is not clarified yet; particularly for Leishmania major infection little information is available. This partly might be due to the fact that in most of the studies, peripheral responses are evaluated which might not be a true reflection of the site of infection. In this study, gene expression of IL-10, TGF-β1, IL-4, IFN-γ, and forkhead box P3 (Foxp3) (as a marker of Tregs) was evaluated in patients with acute or chronic lesions of CL caused by L. major, and were compared with healthy skin.
MATERIALS AND METHODS
The protocol was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran (project number: 187096). The procedure was described to every potential candidate, and those who were willing to donate samples were included. All of the participants signed an informed consent.
Biopsies
A total of 30 skin punch biopsies of 3.5 mm were taken and used in this study: normal skin samples from six female volunteers who underwent cosmetic surgeries (mammoplasty or abdominoplasty) and 24 biopsy specimens from volunteer patients with CL lesions caused by L. major referred to Centre for Research in Skin Diseases and Leishmaniasis, Isfahan University of Medical Sciences. Diagnosis of CL was based on visualization of amastigotes in direct smear of the lesions or positive polymerase chain reaction (PCR) using amplification of 18s ribosomal ribonucleic acid (RNA) of the parasite. Parasite species were identified to be L. major by means of high-resolution melting analysis of 7SL RNA as described elsewhere.[11] Skin samples were obtained as explained earlier.[12] Samples were collected from two groups of CL patients: patients with lesion onset of <6 months (n = 13) which is defined as acute lesions (AL) and patients with lesion duration of more than 6 months (n = 11) defined as chronic lesions (CH).[13] Subjects with a history of chronic cutaneous diseases or internal diseases were excluded from the study. Samples were immediately transferred into RNAlater RNA Stabilization Reagent (Qiagen) and stored at −20°C until use.
RNA extraction and real time PCR
RNA extraction was done as previously described.[12] Skin biopsies were disrupted and homogenized using bead beating method. Briefly, Lysing Matrix D tubes (MP-biomedical, Irvine, CA) containing 1.4 mm ceramic beads were loaded with skin biopsies and buffer RLT (Qiagen). A Ribolyser reciprocal shaker (Hybaid, United Kingdom) was used to process the samples. Total RNA was extracted and reverse transcribed into complementary deoxyribonucleic acid (cDNA) using RNeasy Mini Kit (Qiagen) and QuantiTect Reverse Transcription Kit (Qiagen) respectively according to manufacturer's instructions. Quantity and quality of extracted RNA were examined by means of spectrophotometer and agarose gel electrophoresis. Real time PCR was performed on cDNA templates using QuantiFast SYBR Green PCR Kit (Qiagen) on a Rotor-Gene 6000 system (Corbett). As a positive control, tonsil specimens obtained from tonsillectomy were used parallel with the skin biopsies. PCR reactions were done in triplicate, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as housekeeping gene. Published primers for GAPDH, Foxp3, IL-10, IL-4, IFN-γ,[14] and TGF-β1[15] were used in this study. The messenger RNA expression was quantified by means of 2−ΔCT equation. Relative expression of target genes to GAPDH were calculated and expressed as arbitrary units.
Statistical analysis
Data were analyzed using SPSS-PC version 16.0 (SPSS Inc., Chicago, IL, USA). Non-parametric tests were used to compare the data. Kruskal–Wallis test detected the significance of differences between the study groups (healthy skin, AL, and CH). Mann-Whitney U-test was used for pair-wise comparison of levels of target gene expression between groups. P < 0.05 was considered as significant.
RESULTS
Expression of Foxp3 was significantly higher in CH (median 0.97, inter-quartile range [IQR] 0.75-1.30 [arbitrary units]) than in AL (median 0.48, IQR: 0.32-0.76, P = 0.006). However, Foxp3 expressions in CL lesions were significantly (P value of 0.003 and 0.048 for AL and CH respectively) lower than in healthy skin (median 1.99, IQR: 1.47-3.19).
Levels of IL-10 and TGF-β1 gene expression were not significantly different in AL and CH. TGF-β1 was highly expressed in healthy skin (median 113.59, IQR: 84.46-167.44), but no significant difference was seen with the CL lesions (AL: Median 73.16, IQR: 57.44-87.16; CH: Median 42.12, IQR: 33.39-171.36). IL-10 was higher significantly in diseased skin (AL: Median 11.61, IQR: 8.09-18.22, P = 0.012; CH: Median 7.45, IQR: 5.26-10.68, P = 0.037) than in healthy skin (median 3.25, IQR 1.15-6.01).
IFN-γ and IL-4 were also more expressed in CH than in AL (IFN-γ: Median 45.98, IQR: 33.39-173.48 for AL, and median 200.53, IQR: 97.49-361.76 for CH, P = 0.023; IL-4: Median 0.49, IQR: 0.34-2.16 for AL, and median 2.14, IQR: 1.30-7.11 for CH, P = 0.021). IFN-γ was significantly more expressed in CL lesions than in healthy skin (healthy skin: Median 0.58, IQR: 0.18-2.34, P < 0.001 for both AL and CH). IL-4 was more expressed in CH than in healthy skin (healthy skin: Median 0.41, IQR: 0.34-0.73, P = 0.001), and its expression was the same in AL compared with healthy skin.
Age and gender of volunteers and characteristics of the lesions are summarized in Table 1, and the results are summarized in Figure 1.
Table 1.
Basic information of the patients referring to the Centre for Research in Skin Diseases and Leishmaniasis and healthy volunteers who donated biopsy samples
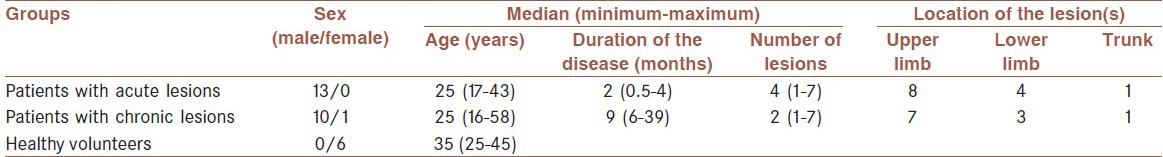
Figure 1.
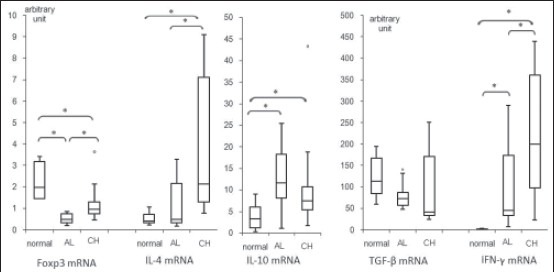
Cytokine gene expression in six normal skin biopsies, 13 acute lesions and 11 chronic lesions of cutaneous leishmaniasis patients. Expressions of target genes are normalized to glyceraldehyde-3-phosphate dehydrogenase gene and are expressed in arbitrary units. Box plots show median value and inter quartile range of relative gene expressions and bars display min and max values. Outliers are displayed by small circles (*P < 0.05)
DISCUSSION
The results of the current study showed that Foxp3 expression level in lesions of patients with CL is significantly lower than in healthy skin. This is in agreement with the fact that epidermal Langerhan cells limit activation of Tregs, but induce proliferation and activation of effector T cells during skin infections.[16] In a model of L. major infection in C57BL/6 mice, Belkaid et al. showed that CD4+ CD25+ Tregs accumulate rapidly at the site of parasite inoculation and then massive parasite expansion occurs,[10] but at the acute phase of infection the number of Tregs sharply decreases with the concurrence of effecter responses.[17]
In this study, Foxp3 gene expression was also higher in CH than in AL group, yet lower than in healthy skin. Previously, it was shown by the same group that Tregs are more frequent in AL than in chronic ones due to L. major infection.[12] Bourreau et al. also showed that CD4+ CD25+ cells isolated from lesions due to L. guyanensis infection were functionally Tregs and the level of Foxp3 expression was higher in chronic CL than in acute CL.[18] However, recently, it has been shown that despite increased Tregs in PBMCs as well as higher in situ Foxp3 expression in chronic American leishmaniasis, these cells are functionally unable to inhibit specific Th1 cells.[19] Thus, without functional assay of cutaneous Tregs in L. major infection, it is not possible to identify their role in chronic infections.
In our study, there was no significant difference in the levels of IL-10 and TGF-β1 expression between the AL and CH groups. Numerous studies on the new world CL indicated that the level of IL-10 and TGF-β expression in CL lesions depends greatly on the parasite species and clinical forms of the disease. As an example in L. mexicana or L. braziliensis infection both IL-10 and TGF-β were more expressed in chronic lesions,[20,21] but in L. guyanensis infection IL-10 expression was not different between acute and chronic lesions.[18]
It was previously reported that cells positive for IL-10 and TGF-β were more frequent in chronic CL than in acute CL due to L. major infection.[22] Although, quantification of cytokines is more precise than their gene expression measurements, but in the mentioned study only intracellular fractions of IL-10 and TGF-β1 were measured. A study which was performed on human L. major infection showed that IL-10 gene expression was positively associated with unfavorable evolution of the lesion after 2 weeks,[7] but in another study IL-10 expression level was the same in CL lesions at the diagnosis time and during the healing phase of the lesions.[23] More studies with larger sample sizes are needed to understand the role of IL-10 and TGF-β1 in human CL. A variety of inflammatory cells can secret IL-10 and TGF-β, but the source of these cytokines could not be identified in this study, which is a limitation for data interpretation.
IFN-γ was highly expressed in CL lesions, and its expression was considerably higher in CH than in AL. In situ Th1 hyper activation might be the cause of high inflammation and tissue damage seen in chronic L. major infection. Interestingly, IL-4 expression was only higher in CH, comparable with several in situ cytokine measurements in different CL types, which revealed low IL-4 gene expressions in acute and localized CL.[21,24] So far IL-4 has not been evaluated in CL lesions due to L. major with durations of more than 6 months. It is shown that stimulated PBMCs from patients with non-healing form of lesions due to L. major (lesion duration of more than 1 year) produce a higher level of IL-4, but lower level of IFN-γ compared with patients with healing form of the disease.[6] This indicate that IL-4 secretion occurs in localized as well as systemic immune responses to chronic L. major infection, however IFN-γ secreting cells possibly accumulate in the lesions at the late phase of the disease, and they are few in PBMCs of these patients.
Level of different cytokines and Foxp3 gene expression varied greatly between individual patients as well as healthy volunteers. The same variation was also seen in other in situ expression studies,[7,18,25] which might be due to differences in contacts with environmental stimuli and specialized cutaneous immune responses.
According to results of the current study, IL-4, IFN-γ, and Foxp3 were more expressed in CH than in AL lesions of L. major infection, but the role of these factors in chronicity of the disease is not clear. As previously stated by Louzir et al., high IFN-γ expression in chronic lesions indicates that insufficient Th1 response is not the cause of long lasting form, and possibly immune inhibitory mechanisms such as IL-4 secretion induce this condition. Although the gene expression of IL-10 and TGF-β1 was not significantly different between AL and CH groups, but the role of IL-10 and TGF-β1 in chronic CL cannot be ruled out. Further investigations on mechanism(s) of Treg suppression and Th2 activation may provide new targets for vaccine development, therapy, and management of non-healing CL which is refractory to available treatments.
AUTHORS’ CONTRIBUTION
All authors have contributed in designing and conducting the study. SGH, LR, SHZ and KK, collected the data and SGH, SHJ, SHH, AKh did the analysis. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and are accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the patients participated in this project. Also they acknowledge Professor Mohsen Janghorbani for his contribution in preparation of the manuscript.
Footnotes
Source of Support: The study was supported financially by Isfahan University of Medical Sciences (grant number 187096)
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Dowlati Y. Treatment of cutaneous leishmaniasis (Old World) Clin Dermatol. 1996;14:513–7. doi: 10.1016/0738-081x(96)00047-8. [DOI] [PubMed] [Google Scholar]
- 3.Mansueto P, Vitale G, Di Lorenzo G, Rini GB, Mansueto S, Cillari E. Immunopathology of leishmaniasis: An update. Int J Immunopathol Pharmacol. 2007;20:435–45. doi: 10.1177/039463200702000302. [DOI] [PubMed] [Google Scholar]
- 4.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 5.Jafari-Shakib R, Ajdary S, Amiri ZM, Mohammadi AM, Nourijelyani K, Mortazavi H, et al. CD26 expression on CD4+T cells in patients with cutaneous leishmaniasis. Clin Exp Immunol. 2008;153:31–6. doi: 10.1111/j.1365-2249.2008.03666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habibi GR, Khamesipour A, McMaster WR, Mahboudi F. Cytokine gene expression in healing and non-healing cases of cutaneous leishmaniasis in response to in vitro stimulation with recombinant gp63 using semi-quantitative RT-PCR. Scand J Immunol. 2001;54:414–20. doi: 10.1046/j.1365-3083.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 7.Louzir H, Melby PC, Ben Salah A, Marrakchi H, Aoun K, Ben Ismail R, et al. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis. 1998;177:1687–95. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 8.Soong L, Henard CA, Melby PC. Immunopathogenesis of non-healing American cutaneous leishmaniasis and progressive visceral leishmaniasis. Semin Immunopathol. 2012;34:735–51. doi: 10.1007/s00281-012-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacchini-Cottier F, Weinkopff T, Launois P. Does T helper differentiation correlate with resistance or susceptibility to infection with L. major? Some insights from the murine model. Front Immunol. 2012;3:32. doi: 10.3389/fimmu.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 11.Nasereddin A, Jaffe CL. Rapid diagnosis of Old World leishmaniasis by high-resolution melting analysis of the 7SL RNA gene. J Clin Microbiol. 2010;48:2240–2. doi: 10.1128/JCM.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoseini SG, Javanmard SH, Zarkesh SH, Khamesipour A, Rafiei L, Karbalaie K, et al. Regulatory T-cell profile in early and late lesions of cutaneous leishmaniasis due to Leishmania major. J Res Med Sci. 2012;17:513–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Vega-López F, Hay RJ. Rook's Textbook of Dermatology. New Jersey: Wiley-Blackwell; 2010. Parasitic worms and protozoa; pp. 1669–713. [Google Scholar]
- 14.Miyagawa Y, Kiyokawa N, Ochiai N, Imadome K, Horiuchi Y, Onda K, et al. Ex vivo expanded cord blood CD4 T lymphocytes exhibit a distinct expression profile of cytokine-related genes from those of peripheral blood origin. Immunology. 2009;128:405–19. doi: 10.1111/j.1365-2567.2009.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwacharo J, Dunachie SJ, Kai O, Hill AV, Bejon P, Fletcher HA. Quantitative PCR evaluation of cellular immune responses in Kenyan children vaccinated with a candidate malaria vaccine. PLoS One. 2009;4:e8434. doi: 10.1371/journal.pone.0008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–84. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–55. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 18.Bourreau E, Ronet C, Darcissac E, Lise MC, Sainte Marie D, Clity E, et al. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect Immun. 2009;77:1465–74. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Pinto D, Navas A, Blanco VM, Ramírez L, Garcerant D, Cruz A, et al. Regulatory T cells in the pathogenesis and healing of chronic human dermal leishmaniasis caused by Leishmania (Viannia) species. PLoS Negl Trop Dis. 2012;6:e1627. doi: 10.1371/journal.pntd.0001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz NL, Zerpa O, Ponce LV, Convit J, Rondon AJ, Tapia FJ. Intermediate or chronic cutaneous leishmaniasis: Leukocyte immunophenotypes and cytokine characterisation of the lesion. Exp Dermatol. 2002;11:34–41. doi: 10.1034/j.1600-0625.2002.110104.x. [DOI] [PubMed] [Google Scholar]
- 21.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–42. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hejazi Sh, Hoseini S, Javanmard Sh, Zarkesh Sh, Khamesipour A. Interleukin-10 and transforming growth factor-β in early and late lesions of patients with Leishmania major induced cutaneous leishmaniasis. Iran J Parasitol. 2012;7:16–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Gaafar A, Veress B, Permin H, Kharazmi A, Theander TG, el Hassan AM. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin Immunol. 1999;91:314–20. doi: 10.1006/clim.1999.4705. [DOI] [PubMed] [Google Scholar]
- 24.Bourreau E, Prévot G, Gardon J, Pradinaud R, Launois P. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J Infect Dis. 2001;184:1628–30. doi: 10.1086/324665. [DOI] [PubMed] [Google Scholar]
- 25.Machado PR, Rosa ME, Costa D, Mignac M, Silva JS, Schriefer A, et al. Reappraisal of the immunopathogenesis of disseminated leishmaniasis: In situ and systemic immune response. Trans R Soc Trop Med Hyg. 2011;105:438–44. doi: 10.1016/j.trstmh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


