Abstract
Background:
Lipid metabolism is one of the hepatitis C virus (HCV) life cycle steps. Statins can reduce cholesterol level and finally can decrease HCV replication. Thus, we assessed the effect of Statins in combination with standard antiviral treatment on hyperlipidemic genotype I HCV infected patients.
Materials and Methods:
This study was a prospective clinical trial. 40 patients were selected from those referred to educational and Therapeutic Centers of Isfahan University of Medical Sciences from 2009 to 2010 with confirmed HCV viremia. All patients received Peg-interferon-a2a and ribavirin. 20 hyperlipidemic Patients received 20 mg atorvastatin nightly for 3 months and placebo was prescribed for 20 normolipidemic HCV infected patients as a control group. Liver enzymes and complete blood count were checked monthly and thyroid stimulating hormone was checked every 3 months. We also performed quantitative HCV-ribonucleic acid (RNA) test in 12th week of therapy, at the end of treatment and 6 months after therapy for all samples.
Results:
We didn’t find any significant differences in the mean of HCV-RNA numbers between statin and placebo groups in 12th week of treatment, in the end of treatment and 6 months after treatment (P > 0.05).
Conclusion:
Atorvastatin has no effect on the mean of HCV viral load when we added it to standard treatment for hepatitis C infection. Further studies are necessary to examine the possible antiviral properties of statins and their potential role as adjuncts to standard HCV therapy.
Keywords: Hepatitis C, hyperlipidemia, statins
INTRODUCTION
Hepatitis C virus (HCV) is an important human pathogen, not only because of its high prevalence and worldwide burden, but also because of the potentially serious complications of persistent HCV infection. These complications include cirrhosis, hepatocellular carcinoma, and end-stage liver disease necessitating liver transplantation. The incidence rates for all of these complications are expected to rise in the near future.[1,2]
It is estimated that 130-170 million people have chronic HCV (about 3% of the world population).[3,4] More than 3,00,000 people die every year from hepatitis C related liver diseases.[5] There is no vaccination for HCV, so the treatment is very important.
The standard medication of HCV (pegylated interferon (IFN) alpha plus ribavirin [RBV]) in HCV genotype I infected patients have shown 40-50% sustained virological response (SVR),[3] so we need more effective treatment method.
Lipid metabolism is one of the HCV life cycle steps. HCV forms a replication complex in lipid raft membrane that is full of cholesterol and sphingolipids.[6,7] 3-hydroxy-3 methyl-glutaryl Coenzyme A inhibitors (statins) can reduce cholesterol level and finally can decrease HCV replication. Hence, statin therapy can be introduced as a complementary treatment.
In vitro examinations have found that some statins, especially fluvastatin and atorvastatin can inhibit HCV replication,[8] although statins should be used with caution in advanced end-stage liver disease because of decompensation risk.[9]
Recently, beneficial effect of statin use among patients with HCV-related liver disease has been suggested. In vitro studies show that high concentrations of statins disrupt HCV replication through depletion of isoprenoid geranylgeranyl pyrophosphate.[10,11] Statins may thus have antiviral effects through mechanisms not related to lipid metabolism.[12,13] The low-density lipoprotein (LDL) receptor and the high-density lipoprotein scavenger receptor B1 putatively facilitate HCV entry into hepatocytes. Complex host proteins are found to be closely associated with HCV nonstructural proteins. The process which links these host and HCV proteins is termed prenylation. Statin agents which block the formation of the lipid precursors for prenylation, could theoretically interfere with viral replication.[14,15,16]
Some human studies have done for assessing the effect of statins in hepatitis c treatment, but their results are different. O’Leary et al. have shown no reduction in HCV-ribonucleic acid (RNA) titers with 20 mg atorvastatin.[17] However, another study indicates non-dose-dependent discretion in HCV-RNA in %50 of patients,[18] the purpose of this study was to determine the effect of statin therapy on standard antiviral treatment response in hyperlipidemic genotype I HCV patients.
MATERIALS AND METHODS
Selection of patient groups
Our study was a prospective clinical trial which was done on genotype I HCV patients who had been referred to educational and therapeutic centers of Isfahan University of Medical Sciences between years 2009 and 2010. All patients were tested to confirm HCV viremia by Cobas Amplicor HCV Monitor test, version 2 (Roche Diagnostics, Mannheim, Germany). We selected our patients using simple random sampling. Subjects in the statin group were hyperlipidemic patients according to WHO definition for hyperlipidemia which is LDL cholesterol level more than 160 mg/dl, 130 mg/dl for patient with one risk factor and 100 mg/dl for patients with diabetes mellitus, 2 or more than risk factors. Patients in the placebo group were selected from HCV patients without hyperlipidemia. This study protocol was approved by Institutional Review Board and Ethics Committee of Isfahan University of Medical Sciences (research project number: 309079).
Patients were included in the study if they were naïve for HCV treatment, more than 18 years old and patients who did not have any clinical sign for decompensate Cirrhosis. All cases who were affected by other genotypes of HCV and chronic liver disease such as hepatitis B virus, HIV, Wilson disease, hemochromathosis, autoimmune liver disease, primary biliary cirrhosis, secondary biliary cirrhosis, obstructive liver disease, viral infections that affect liver function, and who had the history of alcohol drinking and drugs related steatosis and hepatotoxicity were excluded from our study. Patients were excluded from the study if the level of their liver enzymes (alanine transaminase and aspartate aminotransferase) had risen >3 times during statin treatment. Patients were examined clinically before antiviral treatment. Fasting blood sugar, plasma lipids, liver enzymes tests and liver and abdomen sonography (Hitachi, Tokyo Japan) were done 2 times (with 1 month apart) before treatment for all of them. We also checked their blood pressure, waist size, and body mass index (BMI) according to related guideline.[19] All patients weekly received 180 μg Peg-IFN-a2a and 1000, 1200 and 1400 mg RBV for weights <75 kg, 75-100 kg, and more than 100 kg, respectively (Pegasys Co.). Statin group received 20 mg atorvastatin (Farabi Co., Tehran, Iran) nightly for 3 months and placebo was prescribed for the placebo group the same as atorvastatin in the statin group. Liver enzymes and complete blood count were checked monthly and thyroid stimulating hormone checked every 3 months. We also did quantitative HCV-RNA test to reveal patients with undetectable test in 12th week of therapy (early virological response [EVR]), after therapy (end of treatment virological response) and 6 months after therapy SVR for all samples.
We analyzed our data using SPSS version 16 (Inc., Chicago, IL, USA) and considered P < 0.05 as valuable, Chi-square was applied for comparison of categorical parameters and Student's t-test for comparing the continuous parameters.
RESULT
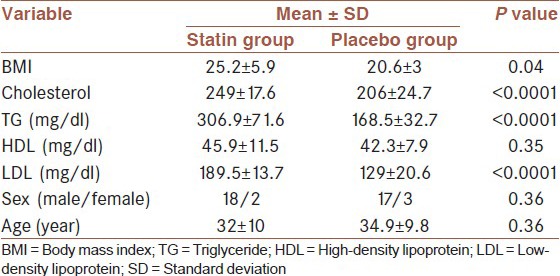
Our study included 40 HCV positive patients (20 hyperlipidemic and 20 normolipidemic patients). Our results showed that there are significant differences in the mean of BMI, triglyceride, LDL, and cholesterol between statin and placebo groups [Table 1].
Table 1.
demographic and lipid character in statin and placebo group
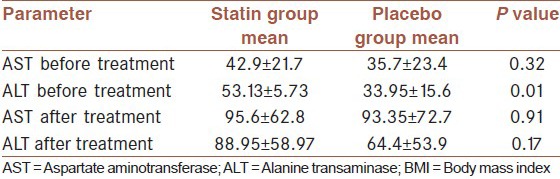
The evaluation of viral load before statin therapy did not show a significant difference between statin and placebo, also, we didn't find any significant differences in the mean of HCV-RNA numbers in statin and placebo groups in 12th week of Statins therapy (P > 0.05), at the end of treatment (P > 0.05) and 6 months after treatment (P > 0.05) [Table 2]. Our findings also implied that EVR in statin and placebo were 75 and 70% and SVR was 95% in both groups. ALT level was higher in statin group before treatment [Table 3]. Although both AST and ALT level rose after treatment, we did not find any difference between AST and ALT level after treatment.
Table 2.
Comparison between viral load before treatment and after start of treatment between statin and placebo groups
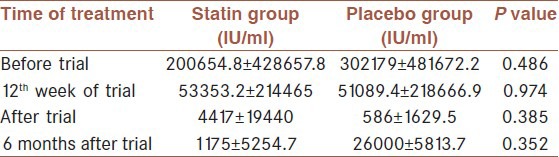
Table 3.
comparison between transaminases in statin and placebo group before and after treatment
DISCUSSION
In the current study, we didn't find any significant differences in the mean of viral load of hepatitis c in statin and placebo groups in 12th week of statin therapy, at the end of treatment and 6 months after treatment. Our findings also implied that early response to treatment (EVR) in statin and placebo were 70 and 75% and Sustain response to treatment (SVR) in statin and placebo was 95% in both groups.
A pilot study of 31 HCV-infected veterans who were given fluvastatin 20-320 mg/day for 2-12 weeks with weekly monitoring of HCV-RNA and liver tests reported modest reductions of viral load.[20] Furthermore, a pharmacoepidemiologic study found that the use of lovastatin was associated with a 40-50% lower incidence of moderate as well as severe liver injury among patients with preexisting liver disease.[21]
Even though, prior individual studies examined important aspects of the association between statin and lowering the severity if liver disease in HCV-infected patients, these studies either did not adjust for histological severity of liver disease, had generally short follow-up, or did not use placebo subjects. Clearly, more information is needed about the possible beneficial effect of statins in HCV-infected.[22]
The result of other in vivo studies has been same as our findings in this study. A study which has done by O’Leary et al. (2007) on 10 samples show that atorvastatin does not inhibit HCV-RNA replication in vivo.[17] Forde et al. in a cross-sectional study (2009) confirmed that statins don't have any apparent effect on HCV-RNA replication in vivo.[23]
Unlike in vivo studies the result of in vitro research has shown that statins can decrease HCV-RNA replication.[3] Ikeda et al. evaluated the effect of statins on HCV-RNA replication in OR6 cells.[24] Fluvastatin exhibited the strongest anti-HCV activity while atorvastatin and simvastatin showed moderate inhibitory effects. The combination of IFN and the statins exhibited strong inhibitory effects on HCV-RNA replication.[25,26]
There are some reasons for differences between in vitro and in vivo studies. As we told before various factors affect on antiviral treatment response in the human body such as obesity, insulin resistance and liver steatosis[15,16] so we need to do more studies to examine the effect of statins on HCV in relation to confounder factors. On the other hand, statins concentration in cell cultures is higher than their concentration in plasma. Studies have shown that serum levels of such agents after prolonged therapy are significantly lower than the statin concentrations those were used in replicon systems.[27]
In our study, response rate of patients were higher than western studies, there are some similar reports that show high response rate of combination therapy in Iranian patients, it may be due to lower viral load in comparison to western patients.[28,29]
In summary in our study statins did not have any significant effect on HCV virus number and virologic response. Our limitation in this study was few sample size, and further studies are necessary to examine the possible antiviral properties of statins and their potential role as adjuncts to standard HCV therapy.
AUTHORS’ CONTRIBUTION
All authors have contributed in designing and conducting the study. Ash, AB, SS, SSh, and Nkh collected the data and MM, MKh, MT, Ash, and BA did the analysis. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and are accountable for all aspects of the work.
ACKNOWLEDGMENT
Authors are grateful to Professor Mohsen Janghorbani for his valuable comments on the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–8. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q, Li N, Han Q, Zhang P, Yang C, Zeng X, et al. Statin therapy improves response to interferon alfa and ribavirin in chronic hepatitis C: A systematic review and meta-analysis. Antiviral Res. 2013;98:373–9. doi: 10.1016/j.antiviral.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Vere CC, Streba CT, Streba L, Rogoveanu I. Statins in the treatment of hepatitis C. Hepat Mon. 2012;12:369–71. doi: 10.5812/hepatmon.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchet M, Seidah NG, Labonté P. SKI-1/S1P inhibition: A promising surrogate to statins to block hepatitis C virus replication. Antiviral Res. 2012;95:159–66. doi: 10.1016/j.antiviral.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Peng LF, Lin W, Choe WH, Sakamoto N, Kato N, et al. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology. 2007;132:311–20. doi: 10.1053/j.gastro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda M, Kato N. Life style-related diseases of the digestive system: Cell culture system for the screening of anti-hepatitis C virus (HCV) reagents: Suppression of HCV replication by statins and synergistic action with interferon. J Pharmacol Sci. 2007;105:145–50. doi: 10.1254/jphs.fm0070050. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE, van Heeswijk R, Alves K, Smith F, Garg V. Effect of the hepatitis C virus protease inhibitor telaprevir on the pharmacokinetics of amlodipine and atorvastatin. Antimicrob Agents Chemother. 2011;55:4569–74. doi: 10.1128/AAC.00653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrus MR, East J. Use of statins in patients with chronic hepatitis C. South Med J. 2010;103:1018–22. doi: 10.1097/SMJ.0b013e3181f0c6b4. [DOI] [PubMed] [Google Scholar]
- 10.Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: A distinct syndrome? Intern Emerg Med. 2008;3:99–108. doi: 10.1007/s11739-008-0127-1. [DOI] [PubMed] [Google Scholar]
- 11.Conjeevaram HS, Kleiner DE, Everhart JE, Hoofnagle JH, Zacks S, Afdhal NH, et al. Race, insulin resistance and hepatic steatosis in chronic hepatitis C. Hepatology. 2007;45:80–7. doi: 10.1002/hep.21455. [DOI] [PubMed] [Google Scholar]
- 12.Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106–15. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 13.Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–90. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–8. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G. Review article: Hepatitis C virus-associated steatosis – Pathogenic mechanisms and clinical implications. Aliment Pharmacol Ther. 2005;22(Suppl 2):52–5. doi: 10.1111/j.1365-2036.2005.02597.x. [DOI] [PubMed] [Google Scholar]
- 16.Lecube A, Hernández C, Simó R, Esteban JI, Genescà J. Glucose abnormalities are an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Am J Gastroenterol. 2007;102:2189–95. doi: 10.1111/j.1572-0241.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: A pilot clinical trial. Hepatology. 2007;45:895–8. doi: 10.1002/hep.21554. [DOI] [PubMed] [Google Scholar]
- 18.Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–9. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh S, Sanyal AJ. Hepatitis C and nonalcoholic fatty liver disease. Semin Liver Dis. 2004;24:399–413. doi: 10.1055/s-2004-860869. [DOI] [PubMed] [Google Scholar]
- 22.Maillard P, Krawczynski K, Nitkiewicz J, Bronnert C, Sidorkiewicz M, Gounon P, et al. Nonenveloped nucleocapsids of hepatitis C virus in the serum of infected patients. J Virol. 2001;75:8240–50. doi: 10.1128/JVI.75.17.8240-8250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forde KA, Law C, O’Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol. 2009;15:5020–7. doi: 10.3748/wjg.15.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–25. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- 25.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 26.Vere CC, Streba CT, Streba L, Rogoveanu I. Statins in the Treatment of Hepatitis C. Hepatitis Monthly. 2012;12:369–71. doi: 10.5812/hepatmon.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui JM, Kench J, Farrell GC, Lin R, Samarasinghe D, Liddle C, et al. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J Gastroenterol Hepatol. 2002;17:873–81. doi: 10.1046/j.1440-1746.2002.02813.x. [DOI] [PubMed] [Google Scholar]
- 28.Alavi Moghaddam M, Zali MR, Aalaei Andabili SH, Derakhshan F, Miri SM, Alavian SM. High rate of virological response to peginterferon α-2a-ribavirin among non-cirrhotic Iranian hemophilia patients with chronic hepatitis C. Iran Red Crescent Med J. 2012;14:466–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Alavian SM, Behnava B, Tabatabaei SV. The comparative efficacy and safety of peginterferon alpha-2a vs. 2b for the treatment of chronic HCV infection: A meta-analysis. Hepat Mon. 2010;10:121–31. [PMC free article] [PubMed] [Google Scholar]


