Abstract
A 77-year-old man presented with an acute worsening of chronic back pain. CT showed dense bilateral adrenal glands suggestive of adrenal haemorrhage which was confirmed by MRI. Despite appropriate glucocorticoid replacement for adrenal insufficiency, 7 days after admission this patient suffered an adrenal crisis. Owing to the timely diagnosis, appropriate treatment was given and the patient survived. Large bilateral adrenal haemorrhage however, can lead to cardiovascular collapse and death if not appropriately diagnosed and managed promptly. Despite its rarity, bilateral adrenal haemorrhage should always be considered as a differential for back pain in the setting of an acute illness due to its potentially fatal consequences.
Background
Diagnosis of adrenal haemorrhage is a challenge due to its low incidence (5 in 1 000 0001), the vagueness of its signs and symptoms, and its non-specific blood test abnormalities. For these reasons, it is often discovered incidentally on imaging for other suspected diagnoses. When back pain presents in the setting of an acute illness, a heightened level of suspicion is required to make a timely diagnosis.
Case presentation
A 77-year-old man presented to accident and emergency department with a 4-day history of back pain. The pain was sharp and of sudden onset which began in his lower back, moving upwards and radiating circumferentially forward to his epigastrium. The pain was accompanied by nausea, vomiting, confusion, fatigue and general malaise. He denied having fever.
This episode was an acute worsening of chronic back pain which had been going on for several years, presumed due to osteoarthritis. All of the symptoms described above were new, in addition to a 5 kg loss in weight over the past 3 weeks.
He had been seen in the community by his general practitioner (GP) at the time of onset who dipped his urine which was cloudy and contained protein +++. He did have urinary symptoms of frequency and frank haematuria but these were both in keeping with his type 2 diabetes and a prostate biopsy 2 weeks prior to this presentation. He had no dysuria. He was prescribed trimethoprim but the pain persisted and an acute deterioration of his mental state warranted a second home visit from his GP. Given his cardiovascular risk factors he was referred to hospital for a possible abdominal aortic aneurysm (AAA) or query pancreatitis.
On arrival at the hospital he was very confused. His observations were temperature 37.4, pulse 120 bpm, blood pressure 150/98 mm Hg, respiratory rate 28, oxygen saturations 94% on 15 L/min of O2, blood glucose (BM) 10.7 mmol/L. Examination was unremarkable other than an irregular pulse and a tender discolouration of his abdomen, possibly Cullen's sign suggestive of pancreatitis. He is known to have atrial fibrillation for which he takes warfarin.
Investigations
Blood tests showed raised inflammatory markers with a white cell count of 18×109/L and a C reactive protein of 166 mg/L. His electrolytes and creatinine were normal but he had a urea of 8.5 mmol/L and an estimated glomerular filtration rate of 47 mL/min. His liver function tests and amylase were normal. His International Normalised Ratio (INR) was 2.9. A random serum cortisol on admission was low at 376 nmol/L suggestive of adrenal insufficiency.
A focused assessment with sonography in trauma scan did not detect an obvious AAA or any free fluid in the abdomen though views were very poor due to abdominal girth and bowel gas. An urgent CT of the abdomen and pelvis (figure 1) was recommended and revealed dense bilateral adrenal glands.
Figure 1.
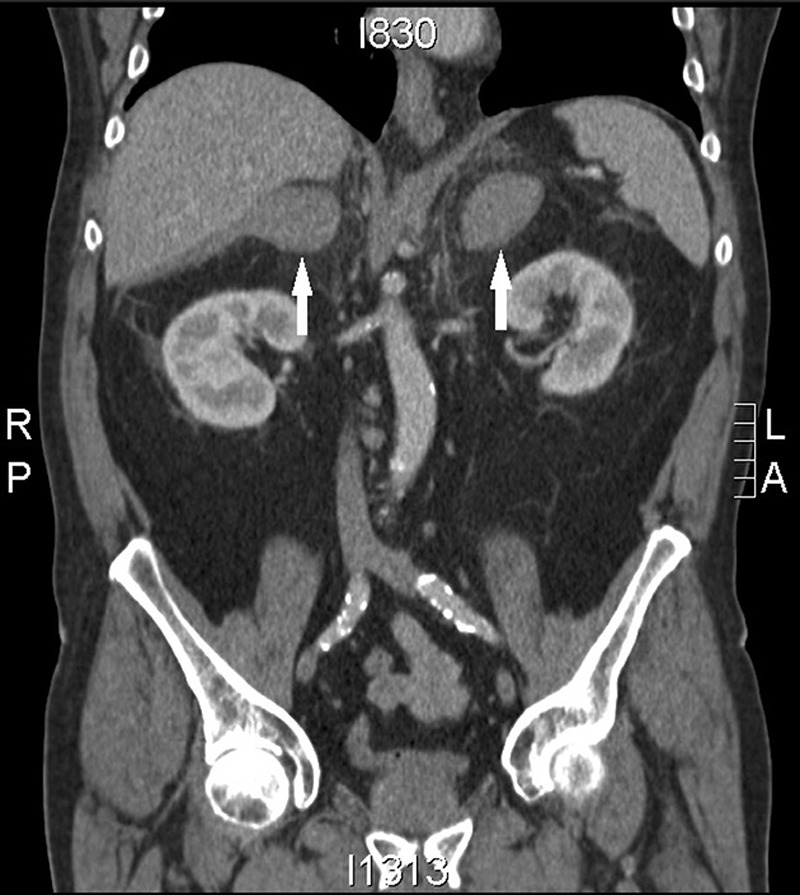
CT of the chest, abdomen and pelvis. Arrows highlighting the dense bilateral adrenal masses.
MRI (figure 2) was advised to look at the density of the adrenal lesions. The T2 sequence confirmed dense adrenals with the most likely diagnosis being bilateral adrenal haemorrhage. This man had a previous MRI 2 months before admission to investigate the cause of his ongoing back pain. It revealed osteoarthritic and spondylotic changes of the spine but normal adrenals.
Figure 2.
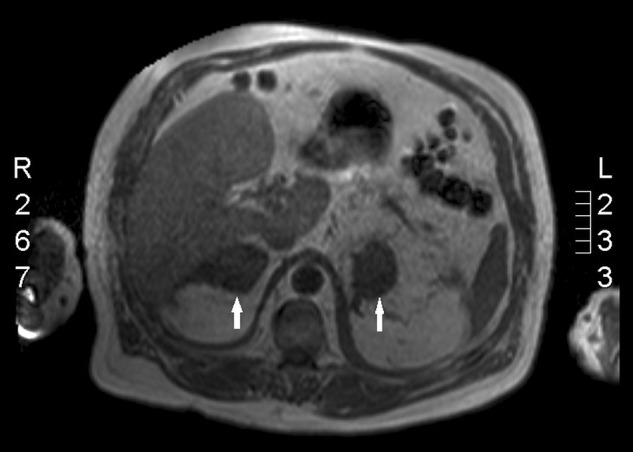
MRI showing the dense adrenal lesions (arrows). There are bilateral adrenal masses measuring 5.5 cm on the right and 4.7 cm on the left which return soft tissue signal on most sequences, but show low signal on the gradient echo T2 sequence. This is in keeping with the suspicion of adrenal haemorrhage.
Treatment
Hydrocortisone therapy was started to prevent adrenal insufficiency. Initial treatment was 200 mg/day by intravenous infusion with the intention of gradually reducing the dose. One week after admission, he was switched to oral hydrocortisone 20 mg once in the morning (OM) and 10 mg once in the night (ON). He was also started on oral fludrocortisone 100 μg due to deranged electrolytes. His warfarin was stopped on admission.
Outcome and follow-up
Initial response to treatment was good but shortly after switching to oral hydrocortisone there was an acute deterioration of his condition. He was found unresponsive, breathing and with a pulse, but with a Glasgow Coma Scale score of 4 and a BM of 3.3 mmol/L. It was managed as an Addisonian crisis with intravenous hydrocortisone 200 mg STAT followed by 100 mg four times a day. His repeat BM was 11.3 following an infusion of 50% dextrose and IM glucagon. Intravenous hydrocortisone was continued for 24 h and then the dose was gradually reduced to 40 mg OM and 20 mg ON orally for 1 week to settle on 20 mg OM and 10 mg ON from then on.
He was discharged on the hydrocortisone course as well as fludrocortisone 100 μg. He is to have outpatient follow-up in 1 month where a short synacthen and full pituitary function tests will be performed.
Discussion
Bilateral adrenal haemorrhage can be caused by blunt abdominal trauma, sepsis, antiphospholipid syndrome, thermal injuries, anticoagulation (usually associated with recent heparin use) or it can be idiopathic.2 The most likely cause in this case is thought to be the use of warfarin, despite the INR being in the therapeutic range. Adrenal haemorrhage resulting in acute adrenal insufficiency is a rare complication of anticoagulant therapy.2 Atrial fibrillation is not currently a recognised cause, though evidence is limited.
In healthy individuals cortisol secretion follows a circadian rhythm.3 Current glucocorticoid replacement therapy in patients with adrenal insufficiency does not precisely mimic this rhythm to provide appropriate physiological replacement.4 Over-replacement of glucocorticoids may lead to morbidity such as impaired glucose tolerance, osteoporosis, obesity and sleep disturbance, in addition to under-replacement leading to the possibility of a significant compromise in well-being as well as potentially fatal consequences if occurring in the presence of an intercurrent illness.5
To optimise glucocorticoid replacement therapy, it should be tailored to the needs of each patient. Improper therapy leaves patients susceptible to adrenal insufficiency and adrenal crisis. Patients with additional comorbidities are especially prone to crisis, and all are usually precipitated by major stress such as infection or surgery.6
When trying to prevent adrenal crisis, there are three main areas to be considered: (1) the daily maintenance dose of cortisol, (2) the daily dose required to mimic normal physiology and (3) the need for extra cortisol during intercurrent illness and mental or physical stress.5 In reality this proves challenging. Dose based adjustments should be centred around clinical signs and symptoms rather than objective biological serum markers assessing the tissue activity of cortisol, and these will often not be specific to adrenal insufficiency and can fluctuate day-to-day in response to the increased metabolic demand. Although difficult, treatment regimes should always be tailored to the patient as improper alignment can decrease long-term outcomes as well as significantly reduce quality of life.
Hydrocortisone is used most commonly for glucocorticoid replacement in adrenal insufficiency. The current suggestion is 15–25 mg/day in divided doses with dose monitoring largely being based on clinical judgement.7 Even when given multiple times during the day, the serum cortisol profile is still far from paralleling the normal physiological cortisol circadian rhythm.5 Consequently, patients often report impaired health-related quality of life and often experience fatigue during the day before their next dose. These patients can experience socioeconomic health problems such as hospitalisation, time off from work and the need for disability allowance.5
The best patient outcomes have resulted from small, uncontrolled studies in patients with adrenal insufficiency who have undergone hydrocortisone replacement by an intravenous infusion from a programmable pump.3 5 8 Hydrocortisone infusions though are clearly not pragmatic in general clinical practice.
Whenever possible, hydrocortisone replacement therapy should be tailored to a patient's lifestyle and the signs and symptoms they exhibit. Patients in whom hydrocortisone is adequately replaced but who are still experiencing fatigue should be considered for multiple daily doses. Fludrocortisone can also be given as a mineralocorticoid substitute at a dose of 50–200 μg/day to achieve normotension, normokalaemia and plasma renin activity in the upper normal range.7
The main factor in preventing adrenal crisis is appropriate glucocorticoid replacement therapy. Another major factor in the precipitation of crisis is the lack of adequate education of the patient and caregiver as to what action to take in the event of an imminent crisis.
All patients and caregivers should be educated in how to recognise an incoming crisis and how to adjust their dose appropriately. For minor physical and psychological stressors, it is suggested to double the current dose for 24 h.5 When there is illness and in particular fever, the dose should be doubled or even tripled to atleast 30–60 mg/day, although there is currently a lack of evidence for this.5 As infectious disease is one of the major causes of adrenal crisis, treatment of possible or suspected infection should be aggressive and should include a sufficiently increased dose of hydrocortisone. In situations where there is a major stressor, like surgery or severe infection, hydrocortisone should be administered intravenously 100–400 mg/24 h.5
Reducing the dose of intravenous hydrocortisone to oral should be performed in a similar way as in this case. The exact regime should be based on clinical judgement and ideally it should have the input of a consultant endocrinologist. Medical professionals should be extra vigilant to the signs of developing illness and if detected the dose should be adjusted accordingly. During the switch from intravenous to oral, frequent BMs may prove a useful clinical indicator.
Patients with adrenal insufficiency following adrenal haemorrhage are at risk of adrenal crisis. Matching glucocorticoid therapy to an individual's cortisol circadian rhythm remains very challenging but efforts should be carried out to adjust hydrocortisone replacement to an individual's needs. Appropriate replacement and thorough and repeated education of patients and their partners of the causes and signs of a crisis is the best strategy to avoid this life-threatening emergency.7
Learning points.
Diagnosis of adrenal haemorrhage is often complicated by its non-specific presentation but it should always be considered as a possible cause of back pain in setting of an acute illness, especially in the absence of another obvious cause.
Appropriate hydrocortisone replacement therapy should be based on the symptoms a patient exhibits and their day to day needs.
Individuals with adrenal insufficiency should be made aware of how to detect an imminent adrenal crisis and how to adjust their dose of hydrocortisone accordingly.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003;361:1881–93 [DOI] [PubMed] [Google Scholar]
- 2.Picolos M, Nooka A, Davis A, et al. Bilateral adrenal haemorrhage: an overlooked cause of hypotension. J Emerg Med 2007;32:167–9 [DOI] [PubMed] [Google Scholar]
- 3.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 2009;94:1548–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debono M, Ross R, Newell-Price J. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol 2009;190:719–29 [DOI] [PubMed] [Google Scholar]
- 5.Grossman A, Johannsson G, Quinkler M, et al. Perspectives on the management of adrenal insufficiency: clinical insights from across Europe. Eur Society Endocrinol 2013;169:165–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White K, Arlt W. Adrenal crisis in treated Addison's disease: a predictable but under-managed event. Eur J Endocrinol 2010;162:597–602 [DOI] [PubMed] [Google Scholar]
- 7.Hahner S, Allolio B. Management of adrenal insufficiency in different clinical settings. Expert Opin Pharmacother 2005;6:2407–17 [DOI] [PubMed] [Google Scholar]
- 8.Lovas K, Gjesdal C, Christensen M, et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison's disease. Eur J Endocrinol 2009;160:993–1002 [DOI] [PubMed] [Google Scholar]


