Abstract
A 76-year-old woman presented with symptoms suggestive of acute sinusitis. Previously, her breast carcinoma was treated with right lumpectomy, adjuvant chemotherapy and breast radiotherapy. She remained free from recurrence for the following 8 years. After initial treatment with antibiotics, the local symptom worsened with exophthalmos, eye blindness and development of an ulceration of the hard palate. MRI showed irregular enhancement of the nasal cavity extended to the maxillary sinus and ethmoidal lamina and concomitant infiltration of the orbit and skull base. A biopsy of the palatal ulcer showed a poorly differentiated adenocarcinoma and was compared with the histology of the primary breast tumour and it was concluded for the same morphology. After discussion at the multidisciplinary team, a specific chemotherapy has been activated with an initial local response. Further surgical resection was not thought appropriate and the patient has subsequently undergone palliative radiotherapy to the right paranasal lesions to improve local disease control.
Background
Metastases to the head and neck region are uncommon; in particular, involvement of nasal cavity and paranasal sinuses is exceedingly rare and, consequently, often associated with delayed diagnosis. The most frequent primary origin is renal cancer1 2; only few reports have described sinus metastases from breast, gastrointestinal, genitourinary and lower respiratory tract tumours.2 Symptoms from metastatic involvement of the paranasal sinuses are usually vague and unspecific.3 Generally, patients present with facial pain, epistaxis, nasal swelling/obstruction2–5 and/or orbital symptoms such as proptosis, diplopia, decreased vision and ptosis.4 5 The pathogenesis of metastasis to the paranasal sinuses is unclear; neoplastic cells likely spread to the central nervous system and paranasal region through blood and lymphatic vessels. Haematogenous spread is the most likely route; Debois also suggests the venous Batson route along the prevertebral venous plexus.6–8 Diagnosis is often difficult and patients are initially treated with antibiotics for clinically suspected infective sinusitis, usually with no symptoms’ improvement. Differential diagnoses should include inflammatory lesions, benign tumours (such as haemangiomas) and lymphoproliferative disorders. Sarcoidosis and Wegener granulomatosis can also involve paranasal sinuses.9
Breast cancer (BC) metastases occur in >50% of patients, but head and neck involvement is very rare.2 4 6 10 11 Nonetheless, BC metastases to larynx,12 nasopharynx,13 parotid gland,14 nose and paranasal sinuses2 have been occasionally reported. Metastases to this region may also remain undetected because of being ‘obscured’ by other, more clinically evident, concomitant disease sites.9 The most common metastatic sites are the maxillary, ethmoid, frontal and sphenoid sinuses and the nasal cavity.15 Metastases of the skull base with concomitant paranasal sinus involvement have also been reported. Noteworthy, a higher incidence is described in human epidermal growth factor receptor 2 (HER2)-positive as compared to HER2-negative patients.16
Treatment of these metastatic sites is inevitably palliative. Surgical resection is generally neither feasible nor recommended, unless with palliative intent (tumour debulking) in case of unmanageable local symptoms.15 A diagnostic biopsy is mandatory for confirming histology and biology, especially in patients with no history of cancer.13 14 Radiation and medical treatments are also palliative, though associated with high rates (60–80%) of clinical improvement of local symptoms and vision.17 18
The prognosis of these patients is rather poor, with a median survival ranging from 22 to 31 months in case of BC.17 19–21
We report the case of a patient with BC metastases to nasal cavity extended to the maxillary sinus and ethmoidal lamina and concomitant infiltration of the orbit and skull base.
Case presentation
A 76-year-old woman was diagnosed in 2004 with a pT2 (2.1 cm) pN0sn M0 ductal invasive carcinoma of the right breast, estrogen (ER) and progesterone (PgR) receptor negative, Ki-67 30%, HER2 positive (score 3+). The primary cancer treatment consisted of lumpectomy plus sentinel node biopsy, adjuvant anthracycline-based chemotherapy (four cycles of AC: doxorubicin and cyclophosphamide), followed by three cycles of CMF (cyclophosphamide, methotrexate and 5-fluorouracil); she received adjuvant breast radiotherapy (50 Gy to whole breast plus 16 Gy on tumour bed) at the end of chemotherapy. Adjuvant trastuzumab was not given as not yet available. She remained free from local and/or distant recurrence for the following 8 years.
In July 2012, she presented with symptoms suggestive of acute right sinusitis; a CT scan was performed which confirmed the clinical diagnosis. The patient received antibiotics with no benefit. The clinical picture worsened with headache, nasal obstruction, right exophthalmus, decreased right visual acuity and subsequent right eye blindness; the patient also developed ulceration of the right hard palate. In November, she underwent a new CT scan and MRI; the examinations showed irregular enhancement of the right maxillary and sphenoid sinuses, osteolysis of the right pterygoid process, medial and posterior wall of the right maxillary sinus and ethmoid cells, erosion of the hard palate and periorbital fat tissue infiltration.
A biopsy of the palatal ulcer was performed and the histological examination showed a poorly differentiated adenocarcinoma.
The abdominal CT scan was negative for metastases while the chest CT scan showed multiple bilateral lesions, too small to be further characterised.
Based on the hypothesis of a tumour deriving from the respiratory cells of the sinus tract, the patient received two cycles of polychemotherapy (TCF: taxotere, cisplatin and 5-fluorouracyl), with rapid pain improvement. Based on the past diagnosis of BC, the pathologist was asked to compare the slides of the primary breast tumour with the palatal biopsy and concluded the two specimens had the same morphology; the subsequent immunohistochemical analyses confirmed a metastasis from the previous BC, with the same biological profile: ER and PgR negative and HER2 positive (score 3+; figure 1).
Figure 1.
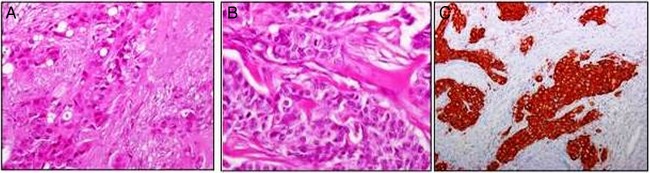
(A) Histological examination of the 2004 breast cancer revealed trabecular and solid aggregates of cells with abundant cytoplasm and prominent nuclei. Glandular differentiation was focally evident. (B) The 2012 nasal biopsy showed a proliferation of cells organised in a more solid pattern but with the same morphology as observed in the 2004 breast carcinoma. Mitoses were also evident. (C) The tumour was intensively positive for HER2 (score 3+).
After the revised pathological diagnosis, the medical treatment was changed to weekly paclitaxel plus q3 weeks trastuzumab. In February 2013, 3 months after the beginning of treatment, the patient underwent a re-evaluation CT scan which showed stable disease in the right maxillary sinus and an initial reduction of the ipsilateral orbital involvement. The patient completed five cycles of treatment and in April 2013 the facial MRI, chest and abdominal CT scans were repeated, showing minimal additional reduction of the orbital extension and a global reduction of the lung lesions. The palatal ulcer relieved and the right eye movements improved (figure 2); on the other hand, right side facial hypoesthesia and moderate pain along the trigeminal nerve persisted.
Figure 2.
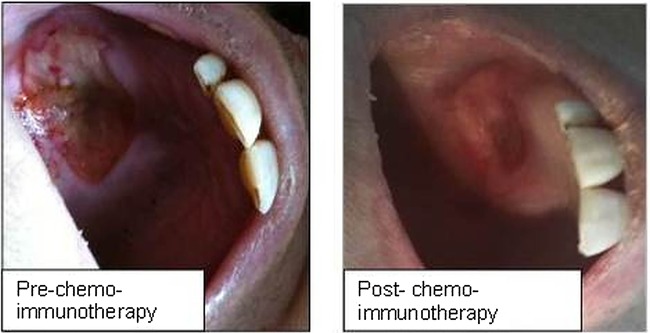
The palatal ulcer preimmunochemotherapy and postimmunochemotherapy.
Radiation therapy (RT) was added (30 Gy in 10 fractions) to the right paranasal lesions to improve local disease control. The head of the patient was fixed with a thermoplastic mask to the treatment bed, for improving the daily treatment reproducibility. RT was delivered with intensity modulated RT (Rapid Arc) technique using the Truebeam Varian Linear Accelerator. This sophisticated technique was chosen in order to spare the left eye/optic nerve and reduce as much as possible the dose to the ipsilateral eye/optic nerve (figure 3). Overall, the treatment was well tolerated and the patient experienced low acute skin and mucosal toxicities; 2 weeks after the end of RT, the patient reported further improvement in visual acuity and reduction of trigeminal neuralgic pain.
Figure 3.

Radiation therapy field.
Outcome and follow-up
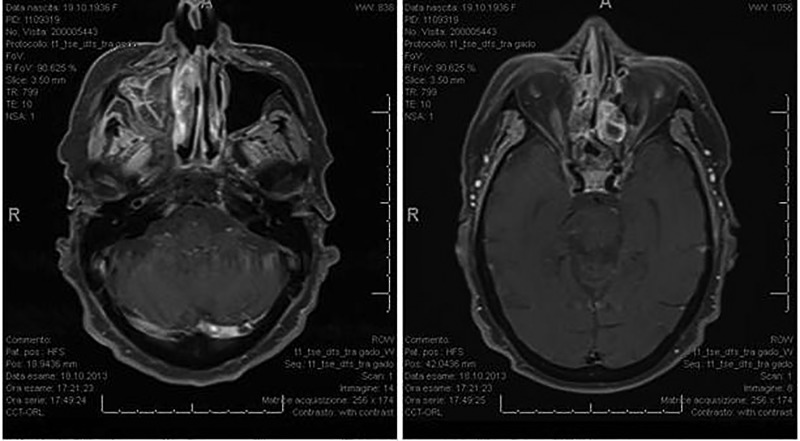
The patient is currently receiving maintenance single agent trastuzumab. The last radiological evaluation in October 2013 showed stable disease of the sinus metastases and further decrease in the number of lung lesions (figures 4–7). The clinical signs of disease disappeared and the patient reports only mild right side residual facial paraesthesia.
Figure 4.
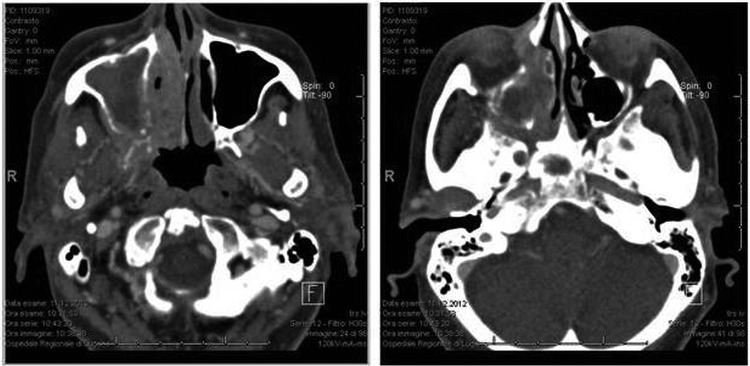
CT scan before chemoimmunotherapy with paclitaxel and trastuzumab (December 2012).
Figure 5.

MRI before chemoimmunotherapy with paclitaxel and trastuzumab (January 2013).
Figure 6.

MRI after chemoimmunotherapy with paclitaxel and trastuzumab (July 2013).
Figure 7.
MRI after maintenance therapy with trastuzumab (October 2013).
Discussion
This case highlights the key complementary role of imaging and pathology to discriminate new primaries from metastases in patients with a history of BC. Some metastatic sites from BC are rare and hardly diagnosed, but effort should be made to rule out the nature of suspicious lesion before starting any treatment. The available literature reports that metastatic BC to the sinusal region is often associated with extremely poor prognosis; if diagnosed early, treatment might indeed prolong survival and especially improve quality of life.
BC metastases to the paranasal sinuses can mimic a primary cancer of the nasal cavity, both tumours showing epithelial differentiation. However, primary tumours commonly show neoplastic changes in the overlying respiratory epithelium and do not express ER and PgR receptors or HER2. Expanding the panel of immunohistochemistry markers may be crucial in this specific setting to achieve a correct diagnosis. HER2-positive patients, on the other hand, have a higher incidence of brain metastases compared to patients with HER2-negative BC; as a consequence, a BC metastasis should always be suspected and ruled out in patients with a history of HER2-positive disease.16
When evaluating lesions of the paranasal sinus, CT scan may provide some hints about their benign or malignant nature, that is, bone erosion and remodelling, angiogenesis or invasion of the sphenopalatine foramen being signs of malignancy. MRI better shows the true extent of the disease and provides more accurate information, such as infiltration of the skull base and potential leptomeningeal involvement. Combined positron emission tomography/CT can additionally be used to detect a possibly different primary tumour.22 23
A meticulous analysis of the patient's medical history and a close collaboration between oncologists, radiation oncologists and pathologists, as made possible by the routine multidisciplinary team meetings, are crucial in discriminating a primary from a secondary tumour, allowing to plan an adequate and timely treatment strategy.
Learning points.
Breast cancer metastases occur in >50% of patients including the bone, lungs, liver and the brain, but head and neck involvement is very rare.2 4 6 10 11 A high index of suspicion should be employed, especially where there is a medical history of malignancy.
The common presenting symptoms of paranasal sinus metastasis mimic those of rhinosinusitis and, therefore, can lead to a delay in diagnosis. The choice of appropriate treatment, in such cases, is extremely difficult since the surgical approach cannot be radical, surgery usually being limited to obtaining a tumour biopsy for differential diagnosis. The vast majority of these patients undergo palliative radiotherapy in combination with chemotherapy.
In the literature, the interval between the diagnosis of breast cancer and paranasal metastasis has been reported to range from 3 months to 12 years and the mechanism of metastasis to the paranasal sinuses is unclear.
Tissue biopsies are necessary to reach the correct diagnosis. Also important is a close collaboration between oncologists, radiologists and pathologists.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer 1981;48:1487–91 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein JM, Montgomery MD, Balogh K., Jr Metastatic tumours to maxilla, nose, and paranasal sinuses. Laryngoscope 1966;76:621–50 [DOI] [PubMed] [Google Scholar]
- 3.Weber AL, Strnton AC. Malignant tumors of the paranasal sinuses: radiologic, clinical, and histopathologic evaluation of 200 cases. Head Neck Surg 1984;6:761–76 [DOI] [PubMed] [Google Scholar]
- 4.Nelson EG, Goldman ME, Hemmati M. Metastatic carcinoma of the ethmoid sinus. Otolaryngol Head Neck Surg 1990;103:120–3 [DOI] [PubMed] [Google Scholar]
- 5.Lopez JI, Nevado M, Eizaguirre B, et al. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. A clinicopathologic study of 6 cases. Tumori 1990;76:250–4 [DOI] [PubMed] [Google Scholar]
- 6.Batson OV. The function of the vertebral veins and their role in the spread of metastasis. Ann Surg 1988;112:138–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fyrmpas G, Televantou D, Papageorgiou V, et al. Unsuspected breast carcinoma presenting as orbital complication of rhinosinusitis. Eur Arch Otorhinolaryngol 2008;265:979–82 [DOI] [PubMed] [Google Scholar]
- 8.Marchioni D, Monzani D, Rossi G, et al. Breast carcinoma metastases in paranasal sinuses, a rare occurrence mimicking a primary nasal malignancy case report. Acta Otorhinolaryngol Ital 2004;24:87–91 [PubMed] [Google Scholar]
- 9.González F, López-Couto C. Orbital metastases. A report of four cases and a review of the literature. Arch Soc Esp Oftalmol 2006;81:451–62 [DOI] [PubMed] [Google Scholar]
- 10.Monserez D, Vlaminck S, Kuhweide R, et al. Symmetrical ethmoidal metastases from ductal carcinoma of the breast, suggesting transcribrosal spread. Acta Otorhinolaryngol Belg 2001;55:251–7 [PubMed] [Google Scholar]
- 11.Debois JM. TxNxM1: the anatomy and clinics of metastatic cancer. Boston: Kluwer Academic Publishers, 2002 [Google Scholar]
- 12.Wanamaker JR, Kraus DH, Eliachar I, et al. Manifestations of metastatic breast carcinoma to the head and neck. Head Neck 1993;15:257–62 [DOI] [PubMed] [Google Scholar]
- 13.Saab GA, Abdul-Karim FW, Samara M. Breast carcinoma metastatic to the nasopharynx. J Laryngol Otol 1987;101:723–5 [DOI] [PubMed] [Google Scholar]
- 14.Raut V, Sinnathuray AR, McClean G, et al. Metastatic breast carcinoma in the parapharyngeal space. J Laryngol Otol 2001;115:750–2 [DOI] [PubMed] [Google Scholar]
- 15.Bernstein JM, Montgomery WW, Balogh K. Metastatic tumors to the maxilla, nose and paranasal sinuses. Laryngoscope 1966;76:621–50 [DOI] [PubMed] [Google Scholar]
- 16.Sanna G, Franceschelli L, Rotmensz N, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res 2007;27:2865–9 [PubMed] [Google Scholar]
- 17.Ahmad SM, Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol 2007;18:405–13 [DOI] [PubMed] [Google Scholar]
- 18.Bellmann C, Fuss M, Holz FG, et al. Stereotactic radiation therapy for malignant choroidal tumors. Ophthalmology 2000;107:358–65 [DOI] [PubMed] [Google Scholar]
- 19.Shields JA, Shields CL, Brotman HK, et al. Cancer metastatic to the orbit: the 2000 Robert M. Curtis Lecture. Ophthal Plast Reconstr Surg 2001;17: 346–54 [DOI] [PubMed] [Google Scholar]
- 20.Johnston J, George M, Karkos PD, et al. Late metastasis to macroscopically normal paranasal sinuses from breast cancer. Ecancermedicalscience 2013;7:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey S, Baer S. A rare case of breast cancer metastasising to the nasopharynx and paranasal sinuses. Int J Surg Case Rep 2012;3:460–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwee TC, Basu S, Alavi A. PET and PET/CT for unknown primary tumors. Methods Mol Biol 2011;727:317–33 [DOI] [PubMed] [Google Scholar]
- 23.Keller F, Psychogios G, Linke R, et al. Carcinoma of unknown primary in the head and neck: comparison between positron emission tomography (PET) and PET/CT. Head Neck 2011;33:1569–75 [DOI] [PubMed] [Google Scholar]