Abstract
Chondrosarcoma is one of the most common malignant bone tumours in adults. However, it rarely occurs during pregnancy. Therefore, reports on surgical and medical management of this entity are hard to find. Different studies suggest a possible growth-enhancing effect of altered hormone levels on various bone tumours. The effect of pregnancy on growth characteristics of chondrosarcomas however remains unclear. We report a case of a 32-year-old pregnant woman with a newly occurred chondrosarcoma of the tibial head. Intense clinical monitoring and repeated MRI scans showed a tumour progression during pregnancy followed by the need of above-knee amputation after 30 weeks gestation. Spontaneous vaginal delivery after 38 weeks gestation was complicated by an amniotic infection syndrome and finally stopped, necessitating a caesarean section. Despite this there were no further complications to be mentioned. No local tumour recurrence or metastases could be detected in the staging CT scans following pregnancy.
Background
Chondrosarcoma is the third most common primary malignant bone tumour in adults.1 However, it is normally not seen in the long bones of pregnant women. Literature review only provides cases of pelvic or extraskeletal chondrosarcoma during pregnancy.2–4 As a result there are neither rich experiences nor common treatment rationale for women diagnosed with chondrosarcoma of the extremities during pregnancy. In addition it still remains unclear whether chondrosarcoma may show progression or dedifferentiation according to the patient's hormone status.2 5 6
Chondrosarcoma is divided into several subtypes, while 90% are considered as mainly slow growing tumours with low risk of metastases.7 Histologically it is graded G1–3 while G1 has recently been named atypical cartilaginous tumour (ACT/CS1). G1 chondrosarcomas or ACT/CS1 have a low metastatic potential (<1%) and a very good survival rate.8 G2 chondrosarcomas have an intermediate risk of metastases while those graded G3 tend to metastasise in up to 70% resulting in a poor prognosis.9 Except for some rare forms (eg, mesenchymal chondrosarcoma) these tumours are generally regarded as refractory to radiation or chemotherapy.10 Therefore, surgical treatment remains the most promising approach. While wide en bloc resection with or without reconstruction is recommended for G2 and G3 chondrosarcomas, ACT/CS1 confined to bone may be treated with intralesional curettage followed by bone grafting or polymethylmethacrylate (PMMA) injection to reduce morbidity and impairment.11 However, this can lead to a high rate of local recurrence, which makes it an option in thoroughly selected cases only.
The present case reports of a pregnant patient with G1 chondrosarcoma of the tibial head with fast progression during the course of pregnancy. This highlights potential complications of bone tumours that occur during pregnancy and may add to the understanding and treatment of such entities.
Case presentation
A 32-year-old woman who was 3 weeks’ pregnant presented at our outpatient clinic with right knee pain since an accidental fall 4 months ago. Pregnancy was first detected by a conventional home pregnancy test and verified during this first visit by a β-human chorionic gonadotropin test. MRI scans of the knee revealed a horizontal tear of the medial meniscus as well as a previously unknown mass of the tibial head (figure 1). There was no fever, weight loss or night sweat during the last few months. The patient had no relevant medical history except for a dust mite allergy. The only medication at that time was folic acid. Physical examination revealed a non-tender mass over the proximal tibia. No signs of local inflammation, erythema or lymphadenopathy could be detected. Blood supply, motor and sensory functions of the right leg were intact. Range of motion in the knee was Ext/Flex 0/0/130°.
Figure 1.
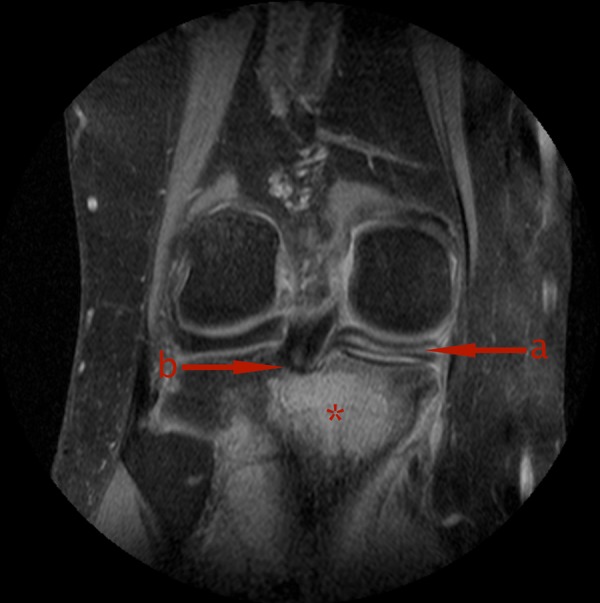
(A) Horizontal tear of the medial meniscus and (B) proximity of the unknown mass (*) of the tibial head to the root of the posterior cruciate ligament.
Investigations
Owing to suspected malignancy of the mass we decided to perform an open bone biopsy. Histology of the biopsy revealed cancellous bone filled with hyaline cartilage tissue accompanied by focal tissue destruction. Lots of chondroid cell clusters showed multiple and some atypical nuclei. No mitoses could be detected. Altogether a well-differentiated chondrosarcoma (G1) could be diagnosed. Combining histological and radiological results it had to be regarded as stage IA according to Enneking at that time.12
Given the fact that the patient was 8 weeks pregnant by the time of the definite diagnosis we settled for an observant approach in agreement with the patient and her husband. After 12 more weeks a control MRI should be scheduled. Treatment options at that time were extensive intralesional curettage of the tibia and allogeneic bone grafting of the cavity or a more radical approach by wide local resection both preferably after delivery.
In the meantime physiotherapy was prescribed to preserve a largely painless movement of the knee. An additional knee brace should help stabilise the patient's knee to further reduce pain and a feeling of occasional instability.
Control MRI of the right leg after 23 weeks gestation showed a contrast-enhanced lesion of the tibial head with progredient extraosseous growth (figures 2 and 3). A newly occurred infiltration of the Hoffa fat pad (39×18 mm) and the medial collateral ligament with overlying tissue (41×31 mm) could be detected. Furthermore, a strong enhancement of the surrounding tissue of the tibial head as well as of the femoral condyles was noted. Inhomogeneous intramedullary distribution of contrast agent into the tibial diaphysis suggested a continuing expansion. No metastatic lymph nodes could be seen. The staging thus proceeded from stage IA to stage IB according to Enneking. At that time the range of motion was already reduced to Ext/Flex 0/0/100° with progredient swelling of the tibial head and pain. Owing to the beginning infiltration of the knee joint, previously offered treatment options were not adequate anymore. Therefore, an above-knee amputation was discussed with the patient and her husband.
Figure 2.
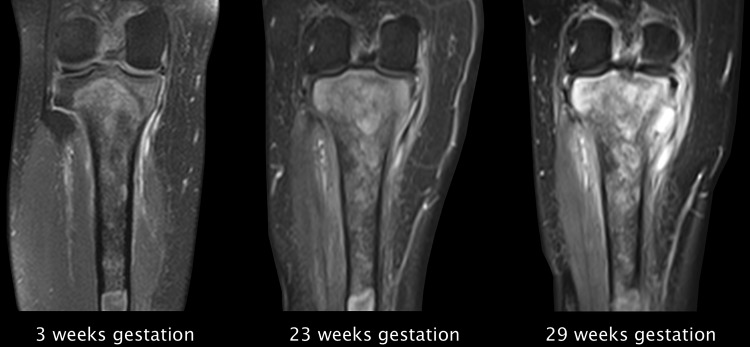
Coronal MRI sequences showing tumour progress during different stages of pregnancy. Beside the local tumour growth there is an increasing infiltration of the medial collateral ligament as well as signal alterations of circumferential tissues of the knee.
Figure 3.
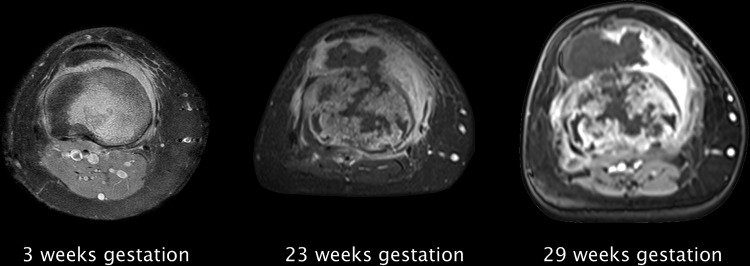
Axial MRI sequences showing tumour progress during different stages of pregnancy. There is a progressive infiltration of the Hoffa fat pad as well as an increasing joint effusion.
Another MRI of the right leg after 29 weeks gestation revealed an increase of the extraosseous parts with a progredient infiltration of the Hoffa fat pad (57×29 mm) and the medial collateral ligament (49×32 mm) (figures 2 and 3). A thickening of the medial vastus as well as an increasing joint effusion of the knee could also be noted already. Owing to the fast progression of the tumour we decided not to wait until delivery and performed a planned above-knee amputation during pregnancy.
Treatment
Above-knee amputation was performed under spinal anaesthesia after 30 weeks gestation with obstetric stand-by. Previously a gynaecological consultation was obtained to ensure the child's well-being. Histology confirmed the diagnosis of a well-differentiated chondrosarcoma (G1) pT2 pNX L0 V0 Pn0 R0 with infiltration of the Hoffa fat pad and the footprint of the anterior cruciate ligament and the root of the posterior cruciate ligament, reaching about 100 mm distal intramedullary.
For postoperative monitoring the patient was transferred to the obstetric intermediate care unit. Postoperative pain control was achieved with an epidural catheter and a femoral paravascular 3-in-1 nerve block.13 Cardiotocography (CTG) showed regular uterine contractions and an elevation of the baseline fetal heart rate (FHR) to 165 bpm. For this reason it was decided to end epidural analgesia and only continue the 3-in-1 nerve block. Normalised actions and FHR could be recorded by CTG about an hour after this measure.
During the following days stump conditioning was performed using elastic bandages and supportive physiotherapy. Wound healing showed satisfactory results after 14 days and the patient was discharged from the hospital. An interim prosthesis was fitted as soon as possible to ensure mobility of the patient. No anomalies were recorded during the last few weeks of pregnancy.
Outcome and follow-up
The patient was admitted to our University Hospital due to stalled labour in the expulsive phase after 38 weeks gestation. She was not given any steroids prior to delivery because this attempt was set in a birthing centre. An amniotic infection syndrome was diagnosed with a fever (38.9°C), leukocytosis and steadily increasing FHR as measured by CTG. Instrumental delivery was deemed inappropriate and it was decided to carry out an emergency caesarean section. A healthy full-term infant was developed. The APGAR score was 08/09/10 with an umbilical cord pH of 7.25. Birth weight was 3105 g. Histology of placenta and amnion revealed a moderate chorioamnionitis with an incipient omphalovasculitis and segmental funiculitis. In addition, hints for circulatory disorders of the placenta and intrauterine hypoxia could be found, connected with a premature placental separation.
Control sonography 3 days postpartum to check uterine regression did not yield any complications. CT scans of thorax and abdomen with contrast did not show any suspect masses, lesions suggestive of metastases or lymphadenopathy. Especially lungs, liver and regional lymph nodes were without any suspicious findings.
A long-term follow-up CT scan of the lower extremities, thorax and abdomen is planned after 1 year.
Discussion
We demonstrated the difficulties of decision-making in patients diagnosed with chondrosarcoma of the extremities during pregnancy. Owing to the rare occurrence of this disease combination there are no common guidelines in the literature.
Main concerns at first presentation are about survival rates and possible complications as well as fetal risks. In addition, extended staging diagnostics including CT scans are not an option because of too high radiation exposures. Survival rates of pregnant women diagnosed with chondrosarcoma of the extremities are not likely to differ from those of non-pregnant women. However, no epidemiologic data exist to prove this. Complications during pregnancy and delivery have only been reported for pelvic chondrosarcomas until today. They include metastasising to the pancreas,4 recurrent pain and fetal growth restriction.14 In case of considerable local tumour growth mechanical complications of vaginal delivery also seem possible. Placental and fetal metastasising of chondrosarcomas of the extremities has not been reported to date. An adverse effect of altered maternal hormone levels on tumour growth however cannot be ruled out until today.5 6 Therefore, a treatment with the least achievable delay appears to be necessary.
As chemotherapy and radiation are not practicable options in chondrosarcomas, an induced preterm delivery (before 37 weeks gestation) only offers the advantage of an earlier surgical treatment. This will always have to be balanced against fetal health of course. Data on hormonal treatment or biologics during pregnancy do not exist up to now. Despite its ethical issues, termination of pregnancy may also be an option especially during the first trimester, but has not been recommended routinely. Surgical treatment of non-obstetric diseases during pregnancy is regarded as relatively safe today, especially when performed in regional anaesthesia.15 Given the lack of other valid therapy options this provides the strongest arguments for a surgical approach with maintenance of pregnancy in case of chondrosarcomas of the extremities.
Patient's perspective.
Regular follow-ups have already been scheduled. CT screening will be repeated after 1 year to rule out tumour recurrence or development of metastases.
Learning points.
Chondrosarcoma of the extremities rarely occurs during pregnancy and thus has to be treated interdisciplinarily.
Diagnosis can reasonably be made and monitored by MRI only and has to be supported by an early histopathological confirmation.
Hormone-dependent growth behaviour of certain types of chondrosarcomas seems very likely as seen in the present case. Further experimental studies are needed to investigate this.
Treatment options are limited to surgical intervention, which has to be performed as early as possible to minimise the risk of further local growth or metastasising.
Acknowledgments
The authors thank Dr Christian Foelsch for performing the surgery and Dr Siegmund Koehler and his team for the obstetrical stand-by.
Footnotes
Contributors: PPR collected the clinical data for the case report. PPR and TE drafted the manuscript. JS and SF-W revised the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dorfman HD, Czerniak B. Bone cancers. Cancer 1995;75:203–10 [DOI] [PubMed] [Google Scholar]
- 2.Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg 1999;119:22–9 [DOI] [PubMed] [Google Scholar]
- 3.O'Brien J, Thornton J, Cawley D, et al. Extraskeletal myxoid chondrosarcoma of the cerebellopontine angle presenting during pregnancy. Br J Neurosurg 2008;22:429–32 [DOI] [PubMed] [Google Scholar]
- 4.Mikhail MG, Lim KB. Dedifferentiated chondrosarcoma metastasizing to the pancreas in pregnancy. Acta Obstet Gynecol Scand 1989;68:467–8 [DOI] [PubMed] [Google Scholar]
- 5.Grifone TJ, Haupt HM, Podolski V, et al. Immunohistochemical expression of estrogen receptors in chondrosarcomas and enchondromas. Int J Surg Pathol 2008;16:31–7 [DOI] [PubMed] [Google Scholar]
- 6.Meijer D, Gelderblom H, Karperien M, et al. Expression of aromatase and estrogen receptor alpha in chondrosarcoma, but no beneficial effect of inhibiting estrogen signaling both in vitro and in vivo. Clin Sarcoma Res 2011;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini A, Guerra G, Mavrogenis AF, et al. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol 2012;106:929–37 [DOI] [PubMed] [Google Scholar]
- 8.Hogendoorn PCW, Bovee JM, Nielsen GP. Chondrosarcoma (grades I-III), including primary and secondary variants and periosteal chondrosarcoma. In: Fletcher CD, Bridge JA, Hogendoorn PCW, et al., eds World Health Organization classification of tumours of soft tissue and bone. Lyon: IARC, 2013:264 [Google Scholar]
- 9.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 1977;40:818–31 [DOI] [PubMed] [Google Scholar]
- 10.Mavrogenis AF, Gambarotti M, Angelini A, et al. Chondrosarcomas revisited. Orthopedics 2012;35:e379–90 [DOI] [PubMed] [Google Scholar]
- 11.Streitbürger A, Ahrens H, Balke M, et al. Grade I chondrosarcoma of bone: the Münster experience. J Cancer Res Clin Oncol 2009;135:543–50 [DOI] [PubMed] [Google Scholar]
- 12.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986;204:9–24 [PubMed] [Google Scholar]
- 13.Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the ‘3-in-1 block’. Anesth Analg 1973;52:989–96 [PubMed] [Google Scholar]
- 14.Somoye G, Havenga S. Chondrosarcoma in the left hemipelvis imitating a pelvic ovarian mass in pregnancy: a case report. Clin Exp Obstet Gynecol 2010;37:65–6 [PubMed] [Google Scholar]
- 15.Gerstenfeld TS, Chang DT, Pliego AR, et al. Nonobstetrical abdominal surgery during pregnancy in Women's Hospital. J Matern Fetal Med 2000;9:170–2 [DOI] [PubMed] [Google Scholar]


