Abstract
Background:
Although the added value of increasing extent of glioblastoma resection is still debated, multiple technologies can assist neurosurgeons in attempting to achieve this goal. Intraoperative magnetic resonance imaging (iMRI) might be helpful in this context, but to date only one randomized trial exists.
Methods:
We included 14 adults with a supratentorial tumor suspect for glioblastoma and an indication for gross total resection in this randomized controlled trial of which the interim analysis is presented here. Participants were assigned to either ultra-low-field strength iMRI-guided surgery (0.15 Tesla) or to conventional neuronavigation-guided surgery (cNN). Primary endpoint was residual tumor volume (RTV) percentage. Secondary endpoints were clinical performance, health-related quality of life (HRQOL) and survival.
Results:
Median RTV in the cNN group is 6.5% with an interquartile range of 2.5-14.75%. Median RTV in the iMRI group is 13% with an interquartile range of 3.75-27.75%. A Mann-Whitney test showed no statistically significant difference between these groups (P =0.28). Median survival in the cNN group is 472 days, with an interquartile range of 244-619 days. Median survival in the iMRI group is 396 days, with an interquartile range of 191-599 days (P =0.81). Clinical performance did not differ either. For HRQOL only descriptive statistics were applied due to a limited sample size.
Conclusion:
This interim analysis of a randomized trial on iMRI-guided glioblastoma resection compared with cNN-guided glioblastoma resection does not show an advantage with respect to extent of resection, clinical performance, and survival for the iMRI group. Ultra-low-field strength iMRI does not seem to be cost-effective compared with cNN, although the lack of a valid endpoint for neurosurgical studies evaluating extent of glioblastoma resection is a limitation of our study and previous volumetry-based studies on this topic.
Keywords: Glioblastoma, intraoperative magnetic resonance imaging, neuronavigation, neuro-imaging, randomized controlled trial
INTRODUCTION
Glioblastoma is an infiltrating malignant brain tumor. Standard treatment consists of surgery, radiotherapy, and chemotherapy, leading to a median survival of 14.6 months.[21] The role of surgery is still under debate, though mounting evidence suggests that increased extent of tumor resection (EOTR) is associated with prolonged overall survival.[13]
Intraoperative magnetic resonance imaging (iMRI) is a tool to maximize EOTR, but many reports on its efficacy suffer from methodological flaws.[5] A recently published randomized controlled trial (RCT) demonstrated that iMRI leads to increased EOTR in comparison to conventional surgery, comparable to the use of 5-aminolaevulinic acid.[17]
In this paper, we present the results of an interim analysis of an international multicenter RCT that compares iMRI with conventional neuronavigation (cNN) for the neurosurgical treatment of glioblastoma. The objective is to assess whether iMRI-guided surgery leads to increased EOTR compared with cNN-guided surgery, and whether health-related quality of life (HRQOL) differs between these two approaches. We consulted the CONSORT (Consolidated Standards of Reporting Trials) 2010 guidelines for reporting of this trial.[16]
The study protocol is registered at ClinicalTrials.gov under number NCT00943007 and has been approved by the institutional ethics research boards of all participating centers.
MATERIALS AND METHODS
Participants
We included 14 patients between March 2010 and July 2012 from all participating centers for the interim analysis of this RCT. Inclusion criteria were: Supratentorial brain tumor-suspected to be glioblastoma on contrast-enhanced diagnostic MRI, indication for gross total resection (GTR) of the tumor, age 18 years or older, WHO Performance Scale (WPS) 2 or better, ASA class 3 or better, adequate knowledge of the Dutch or French language, and informed consent. Exclusion criteria were: Recurrent brain tumor, multiple brain tumor localizations, earlier skull radiotherapy, earlier chemotherapy for glioblastoma, chronic kidney disease or other renal function disorder, and a known MR-contrast allergy.
Sample size
To reduce the chance for type I errors (false positive) we used an alpha value of 0.05. To reduce the chance for type II errors (false negative) we used a beta value of 0.2 leading to a power of 0.8. We considered a 10% additional resection of the preoperative tumor volume as the minimal clinically relevant difference, with an estimated standard deviation of approximately 12%.[12,15] This led to 23 patients in each treatment group. To compensate for loss to follow-up we intended to include a total of 54 patients for the complete study.
Interventions
Experimental group
Within 72 h before surgery, a standard neuronavigation MRI scan was made at 1.5 Tesla (T) or 3T according to the local neurooncology protocol (T1 1 mm isovoxel after administration of a gadolinium-based contrast agent). Patients were operated in the iMRI operating room setup, using the Stealth Station neuronavigation system, Medtronic Polestar N20 (0.15T) moveable magnet and the Starshield® tent as a mobile Faraday cage for shielding radiofrequency noise (Medtronic Navigation, Louisville, CO). The head was fixed in a MR compatible head holder, and specific MR compatible anesthesia equipment was used (monitor, ECG pads, thermometer). No other precautions needed to be taken because of iMRI, and regular instruments could be used. A first nonenhanced T1 iMRI scan was usually made before starting surgery, but this was not mandatory. When the neurosurgeon considered glioblastoma resection to be complete, at least one intraoperative T1 7 min 4 mm scan was made after administration of contrast agent. In all but one case a so-called “double-dose” of contrast was used (0.2 mmol/kg). The neurosurgeon judged whether the scan demonstrated residual tumor, and decided either to continue resection if feasible and perform a new scan afterwards, or to finish the procedure after the intraoperative scan. If residual tumor was suspected, the “resection-scan-cycle” was repeated until the neurosurgeon considered glioblastoma resection to be maximal. Within 48 h after surgery, a regular control MRI scan was made including a contrast-enhanced T1 multi-planar reconstruction MR scan (1 mm isovoxel).
Control group
The preoperative and postoperative imaging was the same as in the treatment group, only the surgical procedure differed. Patients were operated in a regular operating room setup using the Stealth Station neuronavigation system (Medtronic Navigation, Louisville, CO). The head was fixed in a standard Mayfield headclamp, and regular instruments were used. The surgery was finished at the point the neurosurgeon considered resection to be maximal.
Outcomes
Primary endpoint
Residual tumor volume (RTV) percentage is used as the primary endpoint to assess EOTR. Pre - and postoperative tumor volume was calculated by segmenting the hyperintense area on contrast-enhanced T1 MRI (including enclosed central necrosis) and subtracting the hyperintense area on native T1 MRI to compensate for blood in the resection cavity. Measurements were performed using OsiriX software (Pixmeo SARL, Bernex, Switzerland) on Mac OS X using a Wacom Bamboo pen mouse for contour drawing. Postoperative tumor volume was divided by preoperative tumor volume to calculate the fraction of RTV. Multiplying the fraction with 100% provided the RTV. In formula:
RTV = (postoperative contrast enhancement/preoperative contrast enhancement) ×100%
Secondary endpoints
We recorded baseline demographic characteristics (sex, age, length, weight) and corticosteroid use on study entry. Complications were monitored on the case record form. WPS was scored one day before surgery, one day after surgery and before discharge from the hospital. We also recorded HRQOL. The patient was asked to complete the EORTC QLQ-C30 questionnaire with the QLQ-BN20 brain cancer module, and the EuroQol EQ-5D questionnaires. The questionnaires were taken one day before surgery, before discharge and 3 months after surgery. Later we added “12 months after surgery” to allow for a more long-term follow up as well. Raw scores were calculated and converted to standardized scores using a linear transformation. Overall survival was recorded for all patients.
Randomization
Patients were randomized and allocated to either the cNN or iMRI group. Randomization was performed by the first author using TEN-ALEA software for randomization in clinical trials. This software is provided by the Trans European Network (http://www.tenalea.com/) and maintenance for The Netherlands is performed by the Netherlands Cancer Institute (http://www.nki.nl/). No randomization blocks were used. The neurosurgeon could not be blinded for the procedure. We did not intend to blind the physicians on the ward, nor the patients. Volumetric assessment of pre- and postoperative tumor volume was performed by a single blinded researcher.
Statistics
Descriptive statistics were used to express RTV, WPS and survival. Univariate analysis was used to express the difference between both treatment groups regarding RTV(Mann–Whitney test), WPS(Mann–Whitney test) and survival (Kaplan–Meier analysis using a log rank test). After checking whether the residuals of the regression analysis were normally distributed, multivariate analysis was used to express the independent contributions on the primary endpoint of age, sex, preoperative tumor volume, and histologically proven glioblastoma. For data entry and calculations, SPSS Statistics version 21 for Mac (IBM Corporation, Armonk, NY) was used.
RESULTS
Interim analysis
The decision to perform an interim analysis was approved by the institutional ethics research boards of the coordinating center. It was not part of the original research protocol, which we modified for several reasons. The main reason was that we estimated that our minimally required difference of 10% would not be consistent with the actual results. Meanwhile iMRI did prolong surgery time by 1.5-2 h. Also surgery was hindered by device-related limitations in the iMRI group, like suboptimal ergonomics and intermittent malfunction of the ultrasonic aspirator (CUSA Excel Ultrasonic Surgical Aspirator; Integra Radionics, Burlington, MA, USA) due to magnetic interference. Further, we noticed that patient inclusion took significantly longer than expected based on a previous study.[6] This was mainly related to the indication for GTR as an inclusion criterion: Patients in which preoperatively was decided to leave a small area of contrast enhancement untouched because of the vicinity of eloquent areas or to avoid significant opening of the ventricular system, were excluded.
Baseline data
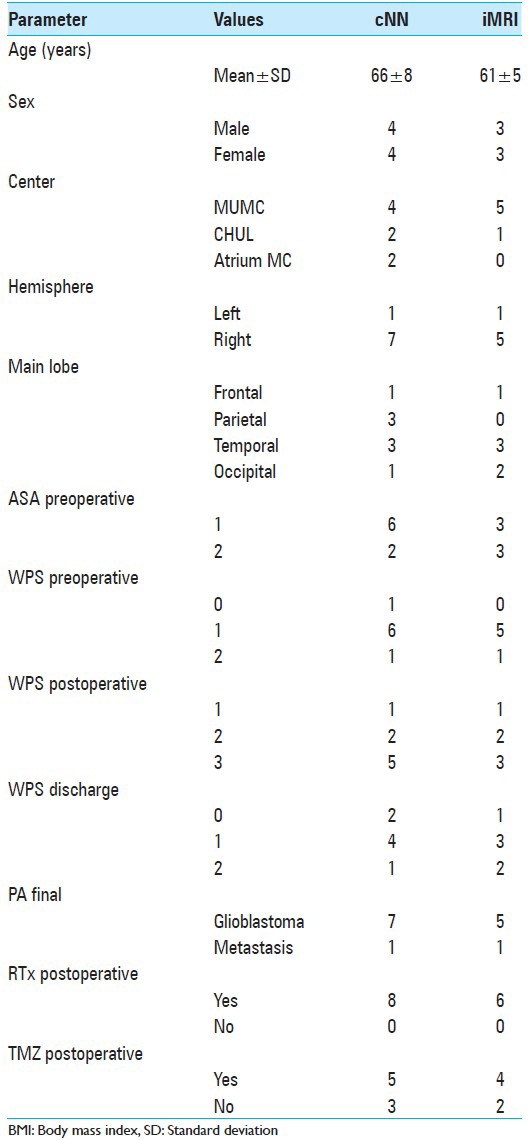
After randomization, eight patients were assigned to the cNN group and six patients to the iMRI group. Participant flow is visualized in Figure 1. Mean age for the cNN group was 66 ± 8 years compared with 61 ± 5 years for the iMRI group. Eleven patients were operated in the Netherlands and three in Belgium. In both treatment groups, one surgery was performed in the left hemisphere, all other surgeries were in the right hemisphere. Tumors were located in all lobes, except for in the iMRI group where no parietal tumors were included. Histopathology revealed glioblastoma in all patients except for two, having a metastasis: One in the cNN group and one in the iMRI group. All patients received radiotherapy postoperatively, and most received chemotherapy with temozolomide. Further details are provided in Table 1.
Figure 1.

Participant flow in the study
Table 1.
Study demographics
Outcomes
Outcomes were analyzed for all patients according to an intention to treat principle. Imaging data were complete for all patients, but questionnaires contained some missing data (in particular HRQOL questionnaires).
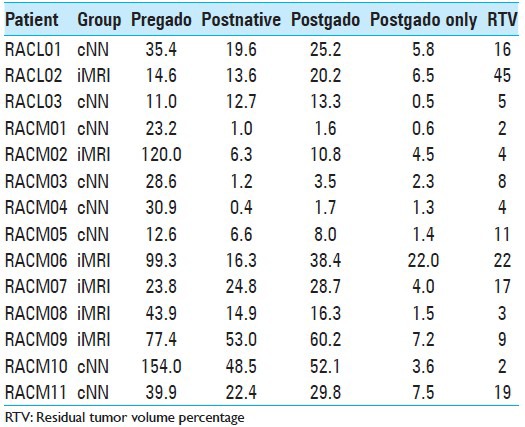
Tumor volumetry results are displayed in Table 2. The first three columns represent volumetry results for preoperative contrast-enhanced MRI, postoperative native MRI and postoperative contrast-enhanced MRI. The “postgado only” column represents postoperative contrast enhancement (supposed to be residual tumor) and the last column displays rounded RTV values. Median RTV in the cNN group is 6.5% with an interquartile range of 2.5-14.75%. Median RTV in the iMRI group is 13% with an interquartile range of 3.75-27.75%. A Mann-Whitney test showed no statistically significant difference between these groups (P =0.28). When both patients with a metastasis are excluded for further analysis, median RTV in the cNN group is 5% with an interquartile range of 2–16% and median RTV in the iMRI group is 9% with an interquartile range of 3.5-33.5%. Also, for the glioblastoma population, a Mann-Whitney test did not show a statistically significant difference between these groups (P =0.43). Multivariate analysis does not reveal any significant influence of sex, age, preoperative tumor volume, or histological diagnosis on RTV.
Table 2.
Tumor volumetry data (in cm3)
Median preoperative WPS is 1 for both treatment groups, ranging from 0 to 2. Median WPS one day postoperatively is 3 in the cNN group and 2.5 in the iMRI group, ranging from 1 to 3 in both groups. Median WPS before discharge (approximately one week after surgery in both groups) is 1 in both groups, ranging from 0 to 2. For the latter, data for one patient in the cNN group are missing. Using a Mann-Whitney test, mean rank for the WPS before discharge is 6.3 in the cNN group and 7.8 in the iMRI group. The difference is not statistically significant (P =0.53).
Median survival in the cNN group is 472 days, with an interquartile range of 244-619 days. Median survival in the iMRI group is 396 days, with an interquartile range of 191-599 days. The corresponding log rank P 0.81. When both patients with a metastasis are excluded for further analysis, median survival is 539 days in the cNN group and 396 days in the iMRI group, with a log rank P 0.68. One glioblastoma patient in the cNN group opted for euthanasia 8 months after surgery due to late disease-related complications. If this patient is excluded from the latter analysis, median survival remains unchanged, with a log rank P 0.55.
Figure 2a shows a Kaplan–Meier curve that displays cumulative survival in time for both treatment groups, and Figure 2b shows the same curve excluding patients with a metastasis.
Figure 2.
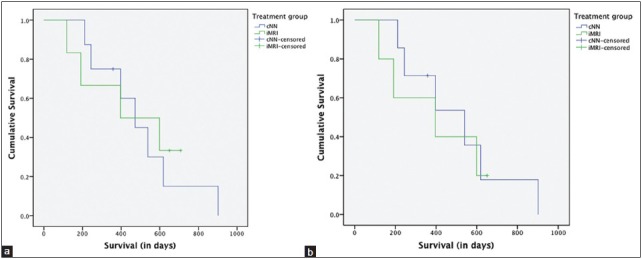
Kaplan–Meier curve for (a) all patients, and (b) patients with a histologically proven glioblastoma
HRQOL scores are left out of this paper. Explorative data analysis does not show a clear advantage in HRQOL for either treatment group. After consultation of a health-technology assessment expert we decided to refrain from any further statistical analyses due to the small sample size.
Adverse events
One patient from the iMRI group suffered from postoperative hemorrhage, which led to prolonged hospital stay and cognitive impairment. No other adverse events or serious adverse events were reported.
DISCUSSION
Our study is the second RCT on glioblastoma surgery using iMRI. This paper reports our results from an interim analysis of 14 patients. Volumetric assessment of the primary endpoint (RTV) does not show any advantage of iMRI-guided resection over cNN-guided resection: There is no statistical difference between both groups and mean RTV is higher in the iMRI group than in the cNN group. Clinical performance does not differ between both groups, and neither does survival in our population. Regarding HRQOL no firm conclusions could be drawn, although there is no clear tendency favoring one treatment group. Study inclusion has been halted based on the results of this interim analysis. The combination of the minimal difference between iMRI-based treatment and cNN-based treatment on the one hand and the slow inclusion rate on the other hand would lead to a much longer timeframe to achieve the statistically significant results we initially aimed for. Based on the present interim analysis, however, we do not expect a different conclusion, and by the time that statistical significance would be reached, the technology used in this study is likely to be replaced by new devices. Therefore, the necessary additional investments associated with the investigated iMRI technology (mainly increased time consumption in the operating theatre) does not seem justified in our opinion, and could even be considered as ethically inadequate.
Despite the small sample size, there is still a clear conclusion from this interim analysis, which differs from the conclusion of the only other iMRI RCT by Senft et al.[17] These authors concluded that iMRI-guided glioblastoma resection leads to GTR in 96% of cases compared with 68% in the control group (P =0.023). Their control group consisted of a mixed population of cNN-guided resection and resection without neuronavigation. A post hoc exploratory analysis did not demonstrate a significant difference between both arms in the control group. Their definition of GTR was a tumor volume of less than 0.175 cm3 detected by a contrast-enhanced T1-weighted MRI, the same as used by Stummer et al.[21,20] The median postoperative volume of contrast-enhancing tissue was 0 cm3 in the iMRI group and 0.03 cm3 in the control group. No further details on their volumetry methodology are provided in the article. To our opinion, the authors’ conclusion favoring iMRI-guided glioblastoma resection should be seen in the context of a minimal difference in postoperative tumor volume between both treatment groups. No significant difference exists in progression-free survival, and most importantly: There is a lack of a valid methodology for volumetric assessment of glioblastoma resection.[6,13,23] In our opinion, tumor volumes reported in the study of Senft are within the error limits of tumor volumetry. Further, a clear definition of “tumor” is one of the challenges to be solved besides improving accuracy of glioblastoma volumetry itself. Both these factors are key in defining a valid endpoint to measure glioblastoma resection.
The widely cited study from Lacroix et al. states that a minimum of 98% glioblastoma resection is needed for survival benefit.[5,11] In that study, the authors used a method by which they assessed intraobserver agreement, but not interobserver agreement.[18] The same comments applies to the study by Kuhnt et al.[9,16] Sanai et al. reported a minimum of 78% glioblastoma resection needed for survival benefit.[12,14,15] These authors also used a volumetric approach based on manual segmentation of contrast-enhancing tissue on T1-weighted imaging, but did not describe any correction for hyperintense signal on native T1-weighted imaging, and did not report on intraobserver and interobserver agreement of their methodology. There is an ongoing discussion regarding an optimal approach to define and measure glioblastoma resection, and no consensus has been reached yet.[6,9,7,14,19,23] Therefore, in contrast to studies on radiotherapy and chemotherapy,[22,24] neurosurgical studies cannot yet benefit from a valid endpoint to measure glioblastoma resection, which limits external validity of individual study results. To minimize the error in respect to our own published data, we decided to have tumor volumes measured by a single (blinded) observer. We previously described this approach to have high intraobserver agreement, but low interobserver agreement.[6] Nevertheless, this type of approach is still the most commonly used approach to tumor volumetry in neurosurgical studies.[9,10,12]
A more fundamental discussion is to what extent further investments should be made to increase EOTR for glioblastoma surgery. Glioblastoma is a nonfocal disease in which tumor cells can be found far beyond the contrast-enhancing area.[1,2,3,8] As long as there is no valid endpoint to quantify glioblastoma resection, no firm conclusions can be drawn regarding the added value of GTR (or complete resection of enhancing tumor [CRET] as described by Vogelbaum et al.).[23] The minimal increase in patient survival after four decades of glioblastoma surgery (despite all sorts of technical equipment), in combination with no consensus on how to measure our results, may indicate that expanding technical innovation for glioblastoma resection should not have our highest priority at this moment.[4]
Limitations
The main limitation of this study is the lack of a valid endpoint for glioblastoma volumetry, as discussed before. We used the best available tumor volume definition but, still, our volumetry results should not be considered as absolute values, but more as a tendency in which the exact quantification can differ from the data provided in this article. Nevertheless, based on our previous study that analyzed intraobserver and interobserver agreement of glioblastoma volumetry, we are confident that the tendency reported in this article is a correct reflection of the results.[6]
Our interim analysis has a small sample size, which limits statistical significance in our endpoints. In particular, differences between HRQOL in both treatments groups cannot be tested due to the small sample size aggravated by missing data.
Finally it should be noted that our data result from an 0.15T iMRI system with its specific advantages and disadvantages. We do not expect different results on high-field strength systems, however, only because of a higher spatial resolution, but the use of other imaging modalities might lead to different conclusions. We also cannot make a valid comparison with 5-aminolevulinic acid (5-ALA) guided surgery,[20] which might be an interesting control group for a future comparative study with iMRI.
CONCLUSIONS
This interim analysis of a RCT on iMRI-guided glioblastoma resection compared with cNN-guided glioblastoma resection does not show an advantage with respect to EOTR, clinical performance, and overall survival for the iMRI group. Although the lack of a valid endpoint to measure glioblastoma resection prevents firm conclusions to be drawn, the added value of (ultra-low-field strength) iMRI for this nonfocal disease is to be debated seriously and does not seem to be cost-effective. Before evaluating new technological developments, research of the near future should primarily focus on developing a valid endpoint to compare surgical results, between different centers and with different technologies, as well as the assessment of survival benefits with increased EOTR.
ACKNOWLEDGMENTS
The authors are grateful to Arno Skrabanja, PhD, for his methodological support in writing the protocol and performing the randomization, and to Prof. Carmen Dirksen, PhD, for her methodological support in health-technology assessment. Hilly Tjon A Fat and Natasja Janssen-Weijenberg have been most helpful in collecting survival data.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/70/132572
DISCLOSURE
This study is part of the PhD thesis of the first author, and has been financially supported by Medtronic Navigation. Medtronic Navigation was not involved in writing the protocol, had no access to the data, was not involved in writing the manuscript, and had no veto right for submission.
REFERENCES
- 1.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007;114:443–58. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan G, Sun B, Wu Z, Guo Q, Guo Y. In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin Radiol. 2004;59:77–85. doi: 10.1016/j.crad.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–74. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 4.Kelly PJ. Gliomas: Survival, origin and early detection. Surg Neurol Int. 2010;1:96. doi: 10.4103/2152-7806.74243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubben PL, Meulen Ter KJ, Schijns OE, Laak-Poort Ter MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: A systematic review. Lancet Oncol. 2011;12:1062–70. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- 6.Kubben PL, Postma AA, Kessels AGH, van Overbeeke JJ, van Santbrink H. Intraobserver and interobserver agreement in volumetric assessment of glioblastoma multiforme resection. Neurosurgery. 2010;67:1329–34. doi: 10.1227/NEU.0b013e3181efbb08. [DOI] [PubMed] [Google Scholar]
- 7.Kubben P, van Santbrink H. Glioblastoma resection. J Neurosurg. 2012;116:1163–4. doi: 10.3171/2011.8.JNS11637a. [DOI] [PubMed] [Google Scholar]
- 8.Kubben PL, Wesseling P, Lammens M, Schijns OE, Ter Laak-Poort MP, van Overbeeke JJ, et al. Correlation between contrast enhancement on intraoperative magnetic resonance imaging and histopathology in glioblastoma. Surg Neurol Int. 2012;3:158. doi: 10.4103/2152-7806.105097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neurooncology. 2011;13:1339–48. doi: 10.1093/neuonc/nor133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhnt D, Ganslandt O, Schlaffer SM, Buchfelder M, Nimsky C. Quantification of glioma removal by intraoperative high-field magnetic resonance imaging: An update. Neurosurgery. 2011;69:852–63. doi: 10.1227/NEU.0b013e318225ea6b. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 12.Nimsky C, Fujita A, Ganslandt O, Keller Von B, Fahlbusch R. Volumetric Assessment of Glioma Removal by Intraoperative High-field Magnetic Resonance Imaging. Neurosurgery. 2004;55:358–71. doi: 10.1227/01.neu.0000129694.64671.91. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 14.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JP, Trantakis C, Rubach M, Schulz T, Dietrich J, Winkler D, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme-a quantitative radiological analysis. Neuroradiology. 2005;47:489–500. doi: 10.1007/s00234-005-1397-1. [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 18.Shi WM, Wildrick DM, Sawaya R. Volumetric measurement of brain tumors from MR imaging. J Neurooncol. 1998;37:87–93. doi: 10.1023/a:1005944724470. [DOI] [PubMed] [Google Scholar]
- 19.Solheim O, Jakola AS, Gulati S, Salvesen O. Glioblastoma resection. J Neurosurg. 2012;116:1164–6. doi: 10.3171/2011.8.JNS11637b. [DOI] [PubMed] [Google Scholar]
- 20.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, den Bent van MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–43. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 24.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]


