Abstract
Background:
Glioblastoma multiforme (GBM) is the most common and lethal primary malignancy of the central nervous system (CNS). Despite the proven benefit of surgical resection and aggressive treatment with chemo- and radiotherapy, the prognosis remains very poor. Recent advances of our understanding of the biology and pathophysiology of GBM have allowed the development of a wide array of novel therapeutic approaches, which have been developed. These novel approaches include molecularly targeted therapies, immunotherapies, and gene therapy.
Methods:
We offer a brief review of the current standard of care, and a survey of novel therapeutic approaches for treatment of GBM.
Results:
Despite promising results in preclinical trials, many of these therapies have demonstrated limited therapeutic efficacy in human clinical trials. Thus, although survival of patients with GBM continues to slowly improve, treatment of GBM remains extremely challenging.
Conclusion:
Continued research and development of targeted therapies, based on a detailed understanding of molecular pathogenesis can reasonably be expected to yield improved outcomes for patients with GBM.
Keywords: Glioblastoma multiforme, gene therapy, immunotherapy, molecularly targeted therapy
BIOLOGY OF GLIOBLASTOMA MULTIFORME
Glioblastoma multiforme (GBM), a World Health Organization (WHO) grade IV glioma, is the most common and lethal primary malignancy of the central nervous system (CNS). More than 10,000 new cases are reported annually in the United States.[39,270] Despite aggressive treatment including surgical resection, chemo- and radiotherapy, median survival time for patients with GBM is only 14.6 months.[237] GBM is an incurable disease that almost invariably leads to neurological demise and death. Due to its high degree of invasiveness, radical resection of the primary tumor mass is not curative. Infiltrating tumor cells invariably remain within the surrounding brain, leading to disease progression or recurrence, either locally or distant from the primary tumor.[264]
Experimental evidence supports the hypothesis that GBM contains a subpopulation of highly tumorigenic cells, glioblastoma stem cells (GSCs), from which recurrent GBM is thought to derive.[10,32,59,266] GSCs have the capacity to self-renew, differentiate into multiple lineages, and for tumorigenesis[32,33,127,170,175,200,225,226,230,231,267] Unlike the bulk of the rapidly dividing tumor cells, GSCs are thought to be relatively quiescent, rendering them resistant to conventional chemo- and radiotherapy.[32] Failure of conventional treatments combined with its poor prognosis highlights the need for novel approaches for GBM that are targeted at residual tumor cells in order to prevent recurrence.
Primary versus secondary GBM
GBM can be classified as primary or secondary. Primary GBM occurs de novo; without evidence of a less malignant precursor, whereas secondary GBM develops from an initially low-grade diffuse astrocytoma (WHO grade II) or anaplastic astrocytoma (WHO grade III). The majority of GBMs (~90%) are primary.[160] Patients with primary GBM tend to be older (mean age = 55 years) than patients with secondary GBM (mean age = 40 years). Secondary GBM is associated with better prognosis and increased overall survival time compared with primary GBM. Although largely indistinguishable based upon histopathology, primary and secondary GBMs evolve from different genetic precursors and harbor distinct genetic alterations.[268] Genetic alterations more typical for primary GBM are epidermal growth factor receptor (EGFR) overexpression, PTEN mutations, and loss of chromosome 10.[75,159,161,258] Whereas genetic alterations more commonly seen in secondary GBM include isocitrate dehydrogenase-1 (IDH1) mutations, TP53 mutations, and 19q loss.[7,77,118,155,159,160,161,268,280] Although different gene expression patterns in primary and secondary GBM were identified, characterization of the IDH1 mutation has allowed for reliable molecular differentiation of primary from secondary GBM.[9,158,160,269,280]
Prior to the discovery of IDH1 mutation in secondary GBM, primary and secondary GBM were distinguished based upon clinical findings. The diagnosis of primary GBM was made in patients without radiologic or histologic evidence of a less malignant precursor, whereas a diagnosis of secondary GBM was made in patients with preexisting low-grade astrocytoma or evidence of a less malignant precursor either radiographically or histologically.[160] In 2008, it was reported that IDH1 mutations occur in a disproportionately large portion of younger patients and in the majority of patients with secondary GBM.[164] Additionally, IDH1 mutations appear to be associated with better prognosis and increase in overall survival.[160] Interestingly, IDH1 mutations are also found in greater than 80% of diffuse astrocytoma and anaplastic astrocytomas, which are precursors of secondary GBM. In subsequent studies of primary and secondary GBM, IDH1 mutations were found in greater than 80% of secondary GBM and less than 5% of primary GBM.[9,158,269,280] Thus, IDH1 mutations are a reliable, objective molecular marker for secondary GBM over clinical and pathological criteria.[158,160]
GBM subtypes
Targeting the underlying genetic alterations leading to GBM is critical to develop effective treatment strategies. Although GBM is a highly heterogeneous tumor, high dimensional genomic profiling has allowed GBM to be categorized into four subtypes based upon characteristic genetic alterations and distinct molecular profiles. In order to better understand the pathogenesis of GBM, high dimensional genomic profiling has been used to identify genetic abnormalities driving GBM tumorigenesis. Using gene expression-based molecular classification of GBM that integrates multidimensional genomic data to establish patterns of aberrant gene expression and copy number alterations seen in GBM, four subtypes of GBM were identified. These GBM subtypes are classical, mesenchymal, proneural, and neural.[15,20,25,154,169,238,258]
Each subtype harbors distinct genetic alterations and expression profiles.[20,258] The classical subtype is strongly enriched in the gene expression pattern observed in astrocytes.[23,258] It is characterized by aberrant EGFR activity, leading to EGFR overexpression.[25,169,258] Additionally, loss of chromosome 10 is frequently observed in the classical subtype. Mutations in TP53, NF1, and IDH1 are uncommonly found in the classical subtype.[258] The mesenchymal subtype is also enriched in the gene expression pattern seen in astrocytes, but in addition, expresses mesenchymal markers as well as microglia markers.[23,258] It is characterized by alterations in the gene for NF1. PTEN deletions, which lead to alterations in the PI3K/AKT/mTOR intracellular signaling pathways, are also commonly seen in the mesenchymal subtype. EGFR overexpression is less commonly seen in the mesenchymal subtype compared with other GBM subtypes.[258] The proneural subtype is enriched in proneural genes as well as the gene expression patterns seen in oligodendrocytes.[23,258] It is characterized by alterations in TP53, platelet-derived growth factor receptor (PDGFR), and IDH1.[7,77,118,258,268,280] Although overexpression of PDGFR is observed in many GBMs, focal amplification of PDGFR with resultantly high levels of PDGFR expression is most frequently seen in the proneural subtype. The proneural subtype is also associated with a younger age at diagnosis.[258] Neural subtype gene expression pattern is the most similar to that of normal brain tissue. It is strongly enriched in the gene expression pattern seen in neurons, and expresses both astrocytic and oligodendrocytic markers.[23,258]
GBM without IDH1 mutation has been identified as classical, mesenchymal, proneural, and neural. The majority of GBM with IDH1 mutation have the proneural gene expression pattern; however, only 30% of GBM with proneural gene expression patterns have the IDH1 mutation.[160] These findings support the hypothesis that secondary GBM are a more homogeneous group characterized by an IDH1 mutation and a proneural pattern of gene expression, whereas primary GBM are a more heterogeneous group with a diverse array genetic mutations and aberrant gene expression profiles.[160]
Overall, the proneural subtype is diagnosed at a younger age than other subtypes.[258] This is as expected given that secondary GBM, almost always the proneural subtype, is generally diagnosed in younger patients.[160,169] The proneural subtype is associated with better prognosis and increased survival compared with the other subtypes.[169,258] Interestingly, however, aggressive treatment with chemo-and radiotherapy has been shown to significantly decrease mortality in patients with classical or mesenchymal subtypes, but has not been shown to significantly alter mortality in the proneural subtypes.[258] The discovery that GBM consists of various subtypes with different genetic alterations and gene expression patterns suggests that no single therapy will be efficacious across all subtypes. Due to the heterogeneity of GBM, it is likely that future therapies will be tailored to target the underlying molecular abnormalities seen in individual patients.
CURRENT STANDARD OF CARE AND TREATMENT
The current standard of care for patients with GBM includes maximal safe resection, followed by concurrent radiation therapy (RT) to the resection cavity and chemotherapy (with temozolomide (TMZ), followed by adjuvant TMZ).[39,236] [Table 1]. Surgical resection alone results in a median survival of approximately 6 months. Combined, surgical resection and RT extend median survival to 12.1 months. Addition of TMZ further extends the median survival to 14.6 months.[237]
Table 1.
Current standard of care

Surgical resection
Surgery remains an important component in the treatment of GBM. It allows for a histologic confirmation of the diagnosis as well as cytoreduction. Surgery may also serve a therapeutic role by reducing the intracranial pressure, and depending on the location of the tumor, occasionally leads to recovery of some neurological function. The principal contraindications to resective surgery are poor performance status (Karnofsky performance scale [KPS] <70), advanced age, eloquent location or extensive bihemispheric involvement.
The goal of surgery is to achieve gross total resection of the contrast enhancing component of the tumor, without compromising neurological function.[168] Gross total resection may not be possible based on anatomic structures invaded by the tumor. Advances in surgical imaging techniques, such as intraoperative magnetic resonance imaging (MRI), diffusion tensor imaging, awake craniotomy, cortical mapping, stereotactic guidance, and fluorescent-guided resection, have facilitated delineation of tumor borders and can help optimize maximal safe surgical resection[4,97,168] [Table 2]. Fluorescent-guided resection utilizes a pharmacologic agent that localizes to tumor but not the surrounding normal brain and fluoresces when exposed to light of a specific wavelength. This fluorescence can be used to guide tumor resection by identifying tumor tissue that may otherwise appear normal.[4,97,171,232] Studies comparing fluorescence-guided resection with standard white light resection have shown that patients undergoing fluorescence-guided resection were more likely to have a gross total resection and were more likely to be progression free at 6 months.[97,171,232] Although maximal surgical resection remains important, ultimately GBM does not have a surgical “answer”.
Table 2.
Surgical aids

Chemo-and radiotherapy
The combination of RT plus TMZ is the most efficacious adjuvant therapy to prolong survival after primary resection. Treatment following surgery usually consists of 6 weeks of RT to the surgical cavity and TMZ, followed by 6 adjuvant cycles of TMZ.[234,237]
The current standard of care for RT in GBM is focal, fractionated external beam radiation therapy (EBRT) to the surgical resection cavity and to a 2 cm margin of surrounding brain tissue.[1] Usually, 60 Gy of RT is delivered in fractions of 2 Gy over 6 weeks.[128,222] Ionizing radiation induces single-strand and double-strand breaks in the DNA of proliferating cells. Other modes of delivering RT have being investigated, including brachytherapy, but none have been proven more effective than the current standard approach.[39]
TMZ, an oral alkylating chemotherapeutic agent, causes DNA damage and triggers a cascade of events leading to tumor cell apoptosis.[47] Recently, TMZ was added to the standard of care for GBM treatment. Previously, chemotherapy had no demonstrable clinical benefit, and RT alone remained the standard of care after surgical resection.[4] In 2005, a clinical trial demonstrated that concurrent RT and TMZ followed by adjuvant TMZ significantly prolonged the median survival more than that of radiation alone (14.6 months versus 12.1 months; P < 0.001). At the 5-year analysis of this study, more patients treated with TMZ were alive (9.8% versus 1.9%; P < 0.001).[237] These findings established the therapeutic benefit of TMZ in combination with RT, establishing the so-called “Stupp regimen” standard of care for GBM treatment.[236,237] Despite these advances, the median progression-free survival time is only 7 months.[39] When given in combination with RT, patients receive 75 mg/m2/day of TMZ for 6 weeks. For adjuvant therapy following completion of RT, patients receive 150 mg/m2/day of TMZ for 5 days every 28 days for at least 6 cycles.[237]
As mentioned earlier, TMZ derives its therapeutic benefit from adding a methyl group to purine bases of DNA, which leads to DNA damage and triggers a cascade of events that leads to tumor cell apoptosis.[47,283] The primary cytotoxic target of TMZ is O6-methylguanine. The methyl group added to O6-methylguanine can be removed by O6-methylguanine methyltransferase (MGMT), which is a DNA repair protein that functions to remove methyl groups from the O6 position of guanine. Removal of this methyl group confers resistance of tumor cells to TMZ and other alkylating chemotherapeutic agents by protecting cells from their DNA-damaging effects.[108,259,283] In some patients, MGMT expression has been decreased or silenced by methylation of the promoter regions of the MGMT gene, preventing it from removing methyl groups from the O6 position of guanine.[259] Thus, the methylation status of the promoter region of the MGMT gene is one of the main mechanisms contributing to TMZ sensitivity or resistance in patients with GBM.[34,94,140,253,259] Patients with an unmethylated MGMT are much less responsive to TMZ, whereas MGMT methylation confers sensitivity to TMZ in patients with GBM.[60,81,108,283]
Implantation of carmustine wafers into the resection cavity is another Food and Drug Administration (FDA) approved treatment of GBM.[272] Similar to TMZ, carmustine is a DNA alkylating agent.[47] Biodegradable wafers impregnated with carmustine line the tumor resection cavity, allowing for delivery of chemotherapy. Carmustine is released into the surrounding brain tissue immediately after tumor resection and its effects last for several weeks.[4] In clinical trials, carmustine wafers used in combination with radiation and TMZ have been shown to modestly prolong survival in subsets of patients. However, because there are complications associated with the use of wafers, including infection, swelling, need for removal, and impairment of wound healing,[88,141,272] they are not used routinely at most centers.
THERAPIES UNDER INVESTIGATION MOLECULARLY TARGETED THERAPIES
Genetically, GBM is a highly heterogeneous tumor harboring multiple recurrent and nonrecurrent genetic alterations.[8] Within the same tumor, cytogenetically related and unrelated clones coexist.[8] Due to recent progress in genomics, several aberrantly activated pathways and mutated genes have been implicated in the pathogenesis and malignant progression of GBM.[8,212,255] These findings have inspired the investigation of molecularly targeted therapies designed to target tumor-specific recurrent genetic alterations as a novel approach to treating GBM.[8,255] Many mutations occur in receptor tyrosine kinases (RTKs) or components of their downstream signaling pathways, making them potential targets for drug development and evaluation in clinical trials.[165,212,255] The majority of growth factor receptors are transmembrane glycoprotein RTKs with extracellular ligand-binding domains and intracellular kinase domains.[8,212] Activation of these RTKs triggers a cascade of downstream signaling events, and inappropriate activation of these signaling pathways is thought to drive tumor growth, survival, invasion into normal brain, and secretion of angiogenic factors[212] [Figure 1]. Thus, inhibition of these pathways and their downstream intracellular signaling components is the goal of molecularly targeted approaches to treatment of GBM. See Table 3 for a list of these various pathways.
Figure 1.
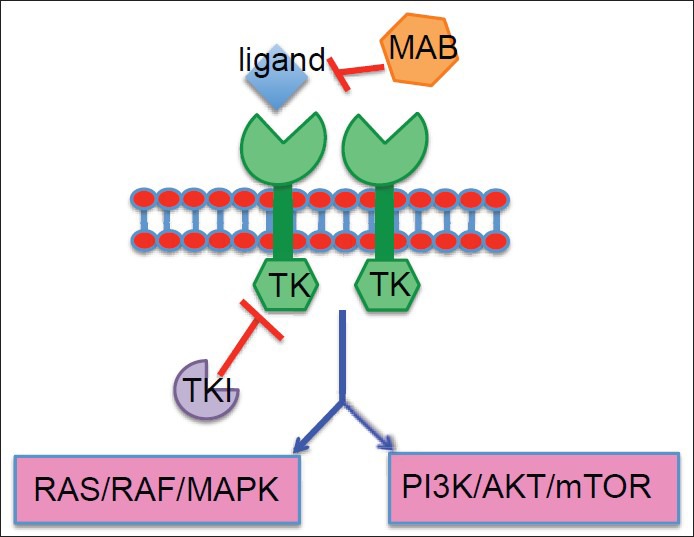
Activation of RTKs triggers a cascade of downstream signaling events, and inappropriate activation of these pathways drives tumor growth, survival, invasion into normal brain, and secretion of angiogenic factors. The hexagons labeled with TK represent the intracellular component of the RTK, and the TKI is shown inhibiting the TK activity. MABs are too large to cross cell membranes, so they are used to target cell surface proteins and other extracellular peptides.[59,61] The MAB is shown inhibiting the ligand from binding and activating the extracellular ligand-binding domain of the RTK
Table 3.
Molecularly targeted therapy
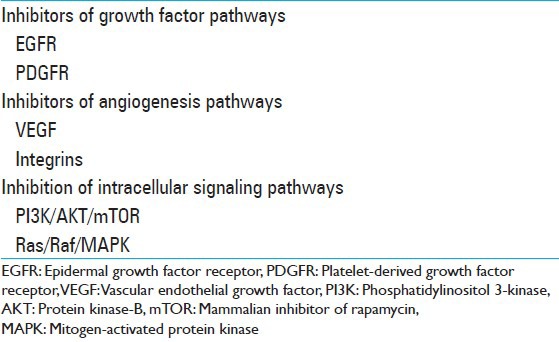
Molecularly targeted therapies can broadly be divided into small molecule inhibitors and monoclonal antibodies. Small molecule inhibitors are nonpolymeric, organic compounds able to cross cell membranes and target specific intracellular constituents.[47] Many small molecule inhibitors are tyrosine kinase inhibitors (TKIs), which function by selectively targeting the intracellular kinase domain of RTKs, blocking receptor activation of downstream signaling pathways. Single kinase inhibitors have activity against only one RTK, whereas as multikinase inhibitors have activity against several different RTKs. Conversely, monoclonal antibodies, too large to cross cell membranes, are used to target cell surface proteins and other extracellular peptides.[56,111,241] In clinical trials, targeted agents are generally studied as single agent therapies in recurrent GBM or used in combination with RT and TMZ for treatment of newly diagnosed GBM.[47] Overall, the results of multiple clinical trials with various molecularly targeted agents have demonstrated only modest therapeutic benefit, as discussed below.
Inhibition of growth factor pathways
Amplification of EGFR signaling is one of the most common genetic alterations seen in GBM.[8,168] Over 60% of GBM overexpress EGFR, and in about half of these, overexpression is the result of a mutant form of the receptor, EGFRvIII, which has a constitutively active kinase domain.[8,167,168,212,255] EGFR overexpression promotes tumor growth, survival, angiogenesis, and invasion.[147,220] EGFR is also overexpressed in other cancers, and a variety of small molecule TKIs and some monoclonal antibodies have been investigated for their potential to block aberrant EGFR signaling.[8]
Gefitinib and erlotinib are oral small molecule EGFR TKIs that have been extensively studied in preclinical and clinical trials of GBM. Binding to intracellular kinase domain of EGFR, they inhibit the activation of its downstream signaling pathways. In preclinical models of GBM, gefitinib and erlotinib have demonstrated antiproliferative and antiinvasive effects, potent inhibition of EGFR, and prolonged survival. Unfortunately, in clinical trials, they have shown limited therapeutic efficacy.[8,166] Both gefitinib and erlotinib have been studied as monotherapy in recurrent GBM and in combination with RT and TMZ for newly diagnosed GBM, but neither agent provided any clinical benefit.[21,166,176,177,201,212,248] Several additional clinical trials using erlotinib in combination with radiation and TMZ have shown that erlotinib enhances tumor cell sensitivity to radiation.[8,47] Other studies, however, have not shown the same results.[47] Overall, small molecule EGFR TKIs have provided minimal to no benefit in clinical outcomes.[85,176,177,188,189,201,252]
Cetuximab is a chimeric murine-human monoclonal antibody that blocks activation of EGFR by its ligands.[165] Cetuximab binding also leads to internalization and downregulation of EGFR, and it has been suggested that cetuximab may have antibody dependent cytotoxic effects on the GBM cells it binds.[76] In preclinical models of GBM, cetuximab decreased growth and prolonged survival. Unfortunately, in clinical trials, cetuximab has shown limited therapeutic benefit and has not improved patient outcomes.[42,65,76,89,90,157]
Overexpression of PDGFR signaling is another frequent alteration found in GBM. PDGF stimulates tumor growth through autocrine signaling, promoting angiogenesis through paracrine effects on adjacent endothelial cells.[162] Imatinib is a small molecule multikinase inhibitor that binds PDGFR as well as several other RTKs. Similar to EGDF TKIs, imatinib blocks the PDGFR kinase domain to prevent activation of its downstream signaling pathways. Unfortunately, similar to EGFR inhibitors, clinical trials with imatinib demonstrated a lack of therapeutic efficacy and minimal clinical benefit.[192,271] Initially, these results were attributed to the presence of drug efflux pumps at the blood-brain barrier actively removing imatinib from the brain.[165] Thus, subsequent clinical trials administering hydroxyurea with imatinib were conducted. Results of these trials, however, showed that addition of hydroxyurea did not improve outcomes.[61,193,195]
Inhibition of angiogenic pathways
GBM is a highly vascularized tumor characterized by extensive angiogenesis.[168,199] Vascular endothelial growth factor (VEGF), a critical mediator of angiogenesis, is highly overexpressed in GBM.[31,62,68,69,165,184] VEGF has been correlated with clinical outcomes, including time to recurrence and survival.[214,264] Several small molecule inhibitors and monoclonal antibodies have been developed to block this signaling.[255] Antiangiogenic therapies are currently the most advanced group of targeted therapies for GBM, and there have been multiple clinical trials demonstrating their therapeutic efficacy.[168,184]
Bevacizumab (trade name Avastin) is a humanized monoclonal antibody that binds and neutralizes the VEGF ligand, preventing activation of its receptors.[56,121,168,180,241,260] In clinical trials of recurrent GBM, bevacizumab demonstrated clinical benefit and improved progression-free survival.[72,168,260,261,262] In 2009, based on the results of two clinical trials, bevacizumab was approved by the FDA for use as monotherapy in recurrent GBM. Bevacizumab is thought to improve outcomes by reducing angiogenesis, decreasing growth of tumor cells expressing VEGF, disrupting the microvasculature of the tumor leading to increased tumor hypoxia, and increasing tumor cell sensitivity to RT.[24,67,105,184] It is currently undergoing clinical trials for use in combination with radiation and TMZ for treatment of newly diagnosed GBM. To date, the results of these clinical trials have shown some promising results, but additional trials are also currently underway to confirm clinical benefit.[35,112,168,262,263] Results of a phase III trial recently presented at American Society of Clinical Oncology (ASCO) demonstrated that the addition of bevacizumab to RT and TMZ does not improve overall survival in patients with newly diagnosed GBM over that of RT and TMZ alone. The addition of bevacizumab was shown to slightly improve progression-free survival in patients with newly diagnosed GBM; however, this did not reach statistical significance. Compared with RT and TMZ alone, RT and TMZ in combination with bevacizumab was associated with higher rates of toxicities. Although full interpretation of the long-term results of this study are ongoing, current results suggest that addition of bevacizumab to RT and TMZ for treatment of patient with newly diagnosed GBM does not improve prognosis.[82,184]
Cedarinib, sunitinib, and vatalanib are multikinase TKIs with predominant VEGF inhibition that have been used to block angiogenesis in GBM. Cedarinib and sunitinib are inhibitors of VEGF, PDGFR, and c-kit. Despite encouraging results in preclinical trials, clinical trials with these agents have demonstrated limited therapeutic benefit, and in some trials, were associated with high toxicity rates.[12,110,156,194,198] In clinical trials, similar to cedarinib and sunitinib, vatalanib, an oral inhibitor of VEGF and PDGFR, provided no clinical benefit.[194]
Cilengitide is another novel antiangiogenic therapy being studied in GBM. It is a cyclized pentapeptide small molecule inhibitor that selectively blocks activation of the ανβ3 and ανβ5 integrins. The integrins are a family of cell adhesion molecule transmembrane glycoprotein receptors that mediate cell-matrix and cell-cell interactions. In GBM, the ανβ3 and ανβ5 integrins are widely overexpressed in GBM cells and tumor vasculature, and in addition to VEGF, they are key mediators of angiogenesis, tumor growth, and metastasis.[13,213,224] Several clinical trials have shown that cilengitide is well tolerated with minimal toxicity, and ongoing clinical trials are investigating its therapeutic efficacy and clinical benefit.[196,197,213,235] The results of a phase III trial recently presented at ASCO showed that the addition of cilengitide to RT and TMZ for treatment of newly diagnosed GBM does not improve progression-free survival or overall survival compared with RT and TMZ alone. In this trial, cilengitide was well tolerated and its safety profile was confirmed. To date, results of this trial suggest that the addition of cilengitide to RT and TMZ for treatment of newly diagnosed GBM does not improve prognosis.[233]
Inhibition of intracellular signaling pathways
Downstream signaling of many RTKs, including EGFR and PDGFR, lead to activation of the PI3K/AKT/mTOR and RAS/RAF/MAPK secondary messenger systems. Dysregulation of these intracellular signaling pathways often occurs in GBM.[8,39] PTEN and NFI are endogenous inhibitors of PI3K and RAS, and these inhibitors are often lost or mutated in GBM.[47,142] Other signaling molecules in the PI3K and RAS pathways are also commonly mutated in GBM. These alterations further contribute to inappropriate P13K and RAS activity in GBM. The identification of these alterations, however, allows for the investigation of other approaches besides inhibition of RTK to interfere with aberrant signaling pathway activity.
PI3K signals through activation of AKT and subsequently mTOR. Perifosine is a small molecule inhibitor of AKT that has been shown in preclinical trials to reduce signaling through the PI3K pathway. Currently, clinical trials with perifosine are being conducted to evaluate its clinical efficacy.[39,172] Rapamycin, temsirolimus, sirolimus, and everolimus are all small molecule inhibitors of mTOR. In clinical trials, these agents have been well tolerated, but they have demonstrated limited therapeutic efficacy.[41,47,78,179,218,265] RAS signals through activation of RAF and MAPK proteins. The rate-limiting step in RAS signaling is farnesylation. Accordingly, inhibitors of farnesyl transferase, including tipifarnib and lonafarnib, have been investigated in GBM.[40,218,223] Preclinical studies have shown reduced signaling through the RAS pathway, but in clinical trials, they have not provided benefit.[40,47,218] Downstream of RAS, RAF signaling occurs through activation of its kinase domains. Sorafenib is a multikinase TKI of RAF, VEGF, PDGFR, and several other kinases. In clinical trials, sorafenib was been well tolerated, but only had limited therapeutic benefit.[165,198]
Multi-targeted kinase inhibitors and combination therapy
Clinical trials of small molecule TKIs have demonstrated limited efficacy in patients with GBM. This is likely the result, at least in part, of multiple mutations and pathways driving tumor growth. Due to the intratumor heterogeneity, lack of a dominant single oncogenic “driver” mutation and redundancy of signaling pathways in cancer cells has become increasingly clear that targeting a single receptor or signaling pathway is unlikely to succeed in GBM.[212] Therefore, several strategies have been developed to improve the therapeutic efficacy of targeted molecular therapies. These strategies include using combinations of multiple single-targeted agents and designing small molecule compounds that target multiple kinases simultaneously. These strategies have the potential for greater efficacy by inhibiting multiple pathways, but they also have an increased risk of toxicity from systemic inhibition.[39] Several multi-target agents have been described in the above sections based on the primary pathway they target. Several clinical trials have evaluated the use of multi-kinase inhibitors and combinations of molecularly target agents. Unfortunately, while toxicities often increased substantially, no “combination therapy” has demonstrated superior clinical benefit over single agents.[12,47,61,110,156,192,193,194,195,198,212,271]
IMMUNOTHERAPY
Immunotherapy attempts to harness the immune system to selectively destroy tumor cells. It includes both passive and active strategies.[256] Passive immunotherapy uses antibodies, immune cells, or other components of the immune system to target the tumor cells.[39,148,149] This approach does not require activation of the patient's native immune response; rather, immune cells are activated ex vivo and then reinjected.[241] Passive immunotherapy involves use of monoclonal antibodies, cytokine-mediated therapies, and adoptive cell transfer approaches.[254] Monoclonal antibodies were discussed earlier and will be further discussed later in the “Gene Therapy” section that follows. Active immunotherapy attempts to stimulate the patient's native immune response against the tumor. Active immunotherapy includes peptide-based and cell-based approaches. These therapies are often referred to as cancer vaccines. Currently, most immunologic therapies available are antibody-based therapies, some of which were discussed earlier. There are, however, several cancer vaccines being evaluated as treatment for GBM in clinical trials.[241] See Table 4 for a list of the various active and passive immunotherapeutic approaches.
Table 4.
Immunotherapy

The immune system can be divided into the innate and the adaptive immune systems.[111] The innate immune system consists of macrophages, monocytes, neutrophils, basophils, eosinophils, natural killer (NK) cells, and complement. The cells of the innate immune response are able to recognize pathogen-associated molecular patterns and defend against the foreign invaders.[241] The adaptive immune system consists of T and B lymphocytes and antigen presenting cells (APCs), and in contrast to the innate immune system, the cells of the adaptive immune system must be activated by antigens. T lymphocytes can be divided into cytotoxic and helper. Activation of cytotoxic T lymphocytes leads to cell-mediated killing, whereas activation of T helper lymphocytes leads to release of cytokines and recruitment of more immune cells. Activated B lymphocytes are responsible for production of antibodies and antibody-mediated cell cytotoxicity. APCs internalize antigen by phagocytosis or endocytosis, then present a fragment to bind and activate T cells. Dendritic cells (DCs) are the most active APCs, and they are often referred to as professional APCs.[254]
Passive immunotherapy: Adoptive cell transfer
Adoptive cell transfer uses stimulated immune effector cells to generate cell-based cytotoxic responses to attack GBM cells.[256,275] In this approach, autologous immune cells are activated ex vivo and readministered.[63,168,241,256] The cells can be given systemically or intratumorally. The two main immune cells used in adoptive cell transfer are lymphocyte-activated killer (LAK) cells and cytotoxic T lymphocytes (CTLs).[63,168,241] LAK cells are usually obtained by culture of autologous peripheral lymphocytes in the presence of interleukin-2 (IL-2) to promote maturation and activation, yielding a polyclonal population of both T and NK cells.[241] Although these cells yield a cytotoxic immune reaction, it is nonspecific, and not exclusively directed at tumor cells.[63,275] CTLs are obtained from peripheral blood mononuclear cells or tumor-infiltrating lymphocytes that are stimulated ex vivo with autologous tumor cells, generating activated CTLs with specificity to the tumor.[63,168,241]
Some of the earliest attempts at immunotherapy for treatment of GBM involved LAK cells and/or CTLs.[241] Although results were mixed, CTLs appear to be better tolerated than LAK cells, likely because of their high specificity of targeting tumor cells. Overall, results of clinical trials with LAK cells and CTLs have demonstrated minimal therapeutic efficacy and have not had an impact on survival.[11,17,18,57,58,92,93,96,104,117,123,133,143,168,173,174,183,228,246,247,256,274,275]
Active immunotherapy: Cancer vaccines
Active immunotherapy is similar in concept to vaccination. It boosts the patient's native immune response against the tumor cells by priming it with antigen exposure.[1,278] Several sources of GBM-related antigen may be used in active immunotherapy, which include intact tumor cells, tumor cell lysate, tumor-derived peptides and mRNA, and synthetic peptides.[256] Active immunotherapy approaches include peptide-based therapies and cell-based therapies[1,255,278] [Figure 2]. In peptide-based therapies, GBM-related antigens are administered to the patient as a vaccine to stimulate an immune response.[39,278] The antigens used for cancer vaccines are usually small peptides, around nine amino acids in length, which are capable of activating cytotoxic T lymphocytes. Rindopepimut is an injectable peptide vaccine designed to stimulate an immune response against a specific EGFRvIII antigen.[54,264] EGFRvIII variant is a constitutively active, mutant form of EGFR expressed in GBM.[37] A segment of this mutant peptide has been used as a vaccine to generate EGFRvIII-specific antibodies.[37,95,208] In clinical trials of patients with GBM expressing EGFRvIII, rindopepimut has shown some promising results, but clinical trials have not been completed.[208,209,210]
Figure 2.
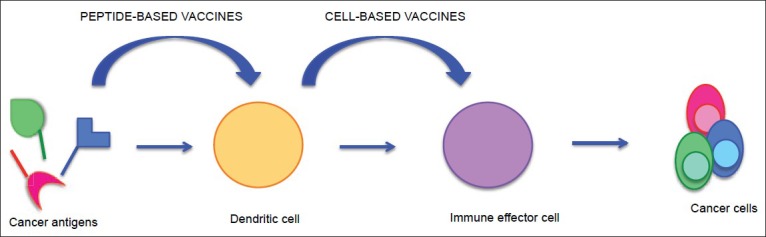
In peptide-based therapies, GBM-related antigens are administered to the patient as a vaccine to stimulate an immune response. Once administered, DCs, which are professional APCs, internalize these GBM-related antigens and present them to the immune effector cells to stimulate an immune response. In cell-based therapies, APCs are activated with GBM-related antigens, rather than the antigen itself, to prime the immune system and then injected into the patients to generate an immune response
In cell-based therapies, APCs are activated with GBM-related antigens, rather than the antigen itself, to prime the immune system and then injected into the patients to generate an immune response.[38,168,275,278] As DCs are professional APCs, they are typically used.[254,256,278] The DCs are typically collected from the patient's autologous peripheral blood mononuclear cells, cultured in the presence of growth factors and matured, activated ex vivo with tumor-related peptides, and then readministered to generate an antitumor response.[38,39,63,136,275,276,278] These activated cells can be delivered intradermally, to the lymph nodes or intratumorally.[241] Several clinical trials of cell-based therapies are underway. Overall, DC vaccines appear to be well tolerated, but have not yet resulted in significant therapeutic benefit.[5,6,27,30,48,115,132,136,181,278,279,281,282]
GENE THERAPY
Gene therapy for the treatment of cancer involves the delivery of genetic material, which includes transgenes, toxins, and viruses, into tumor cells for therapeutic purposes. The genetic material is typically packaged within a vector for delivery into cells. See Table 5 for a list of various gene therapy approaches. There are a variety of different vectors, which include both viral and nonviral vectors. Some gene therapies have shown promising results in preclinical trials, but clinical trials have been unable to demonstrate any significant therapeutic efficacy.[151,242] Lack of clinical benefit is, at least in part, likely due to the low transfection efficiency of most viral vectors.
Table 5.
Gene therapy

Vectors
Vectors are classified as viral or nonviral. Viral vectors were the first and most commonly used vectors in gene therapy for GBM. Viral vector-based delivery systems take advantage of the ability of viruses to deliver their genetic information into the host cell.[151] As part of their normal life cycle, viruses bind host cells and, through transduction, introduce their genetic material. For use as vectors in gene therapy, viruses undergo modification; components of their viral genomes are removed and replaced with the therapeutic genetic material to be delivered to the target cell. Depending on which parts of the viral genome are removed, viruses can be engineered into replication-competent or replication-deficient vectors. To generate replication-deficient vectors, the coding regions of the viral genome required for viral replication are removed.[45,151] Thus, replication-deficient vectors can transduce their host cell, but are unable to replicate within their host. Conversely, replication-competent vectors contain coding regions needed for viral replication, enabling them to continue replication and propagate viral infection to surrounding cells.[151]
Vectors ideally transduce tumor cells while sparing normal cells in order to avoid toxicity to the surrounding brain.[45,122,168] Different viruses have been used as vectors, each with their own advantages and disadvantages. The viral vectors available for gene therapy can be divided into integrating and nonintegrating vectors. Vectors based on retroviruses and lentiviruses integrate their viral genetic material into the chromosomal DNA of the host cell. Vectors based on adenoviruses and herpes simplex viruses type 1 (HSV-1) are able to deliver their genetic material into the nucleus of their host cell, but they are unable to integrate it into the host cell DNA. Retroviruses and adenoviruses are among the earliest and most widely studied viral vectors for use in gene therapy.[29,249,273]
Retroviruses are a large family of enveloped RNA viruses.[119] They contain a reverse transcriptase that, following transduction, allows them to integrate their genetic material in the host DNA.[119] Retroviruses selectively infect actively dividing cells, which allows them to target rapidly dividing GBM cells while sparing normal brain cells.[129] A major drawback is that they may be unable to target the relatively quiescent GSCs that may be responsible for tumor resistance to treatment and recurrence.[45] Another drawback of retroviruses is that they are unstable and difficult to produce at high titers. In order to increase viral titers within the brain, virus-producing cells (VPCs), which continuously produce replication-deficient retrovirus, are often injected into the brain instead of direct viral injection.[187,190] VPCs, however, have a short lifespan and a limited ability to migrate within the brain.[187] Lentiviruses, a subpopulation of retroviruses, are able to infect dividing as well as nondividing cells, allowing them to overcome the drawbacks of traditional retroviruses.[22,119,129]
Adenoviruses, large double-stranded DNA viruses, are unlike retroviruses and lentiviruses. They are unable to integrate their DNA into the host genome following transduction.[45,119,151] They are, however, able to infect dividing and quiescent cells.[64] They express their transgenes at high levels and can be produced at high titers. Herpes simplex virus-1 (HSV1), a member of the herpesvirus family, is also a large, nonintegrating, double-stranded DNA virus. It has neurotropic properties that enable it to efficiently infect both quiescent and proliferative cells of the CNS.[43,64,163] HSV1 has one of the highest vector packing capacities for delivery of genetic material into cells, and this capacity can be utilized for simultaneous delivery of multiple genes with one vector.[64,102,103,126]
In addition to viruses, stem cells have been explored as vectors for delivery of gene therapies in GBM. The three types of stem cells studied as vectors in GBM are neural, embryonic, and mesenchymal.[242] Stem cell vectors appear promising because of inherent tumor trophic properties, which may enhance delivery of genetic material to tumor cells and increase therapeutic efficacy.[2,3,114,122,242,284] Nanoparticles have also been explored as vectors for delivery of gene therapies in GBM. Liposomes are the most widely used nanoparticle vectors. These vectors act by delivering genes to a targeted site based upon their size, charge, and high surface to volume ratio, which provides a powerful source for diffusion.[242]
Conditionally cytotoxic gene therapy
Conditionally cytotoxic gene therapy, also referred to as enzyme-prodrug activating therapy or suicide gene therapy, is the most commonly used gene therapy approach in GBM.[242] In conditionally cytotoxic approaches, the transgene for a noncytotoxic enzyme is delivered into tumor cells, and this enzyme remains noncytotoxic until the administration of a noncytotoxic prodrug. Upon prodrug administration, the enzyme converts the noncytotoxic prodrug into a toxic metabolite that induces tumor cell death[242,255] [Figure 3]. For enzyme-prodrug activating therapy to be successful, the enzyme transgene must be delivered exclusively into tumor cells, and its enzymatic activity must be high enough to kill tumor cells without causing toxicity to surrounding normal cells or inducing a systemic immune response.[122] In most enzyme-prodrug therapies, there is a significant bystander effect. This occurs when the cytotoxic metabolite is transmitted, via gap junctions or by diffusing through extracellular space, to cells that were not originally transduced with the enzyme.[28] Since expression of the enzyme gene will not occur in all tumor cells, this bystander effect is important in amplifying the cytotoxicity of the enzyme-prodrug therapy.[144]
Figure 3.
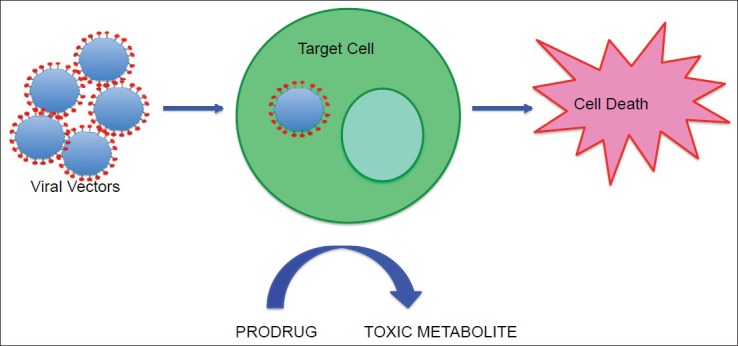
This figure shows conditionally cytotoxic gene therapy, also referred to as enzyme-prodrug activating therapy or suicide gene therapy, which is the most commonly used gene therapy. In conditionally cytotoxic approaches, as described in the text, the transgene for a noncytotoxic enzyme is delivered into tumor cells using a vector, and this enzyme remains noncytotoxic until the administration of a noncytotoxic prodrug. Upon prodrug administration, the enzyme converts the noncytotoxic prodrug into a toxic metabolite that induces tumor cell death
In GBM, the most commonly studied enzyme/prodrug combination is the herpes simplex virus-1 thymidine kinase (HSV1-TK)/ganciclovir (GCV) system.[242] HSV1-TK is a thymidine kinase that converts the prodrug GCV into a toxic DNA analog that integrates into replicating DNA triggering tumor cell death.[14,83,255] In clinical trials of HSV1-TK/GCV for GBM, replication-deficient retroviruses and replication-deficient adenoviruses have been used to successfully deliver the HSV1-TK transgene into the tumor cavity.[99,122,151,242] As retroviruses can be unstable and difficult to produce at high titers, trials with retroviral vectors typically involve implantation of VPCs producing retroviruses expressing HSV1-TK into the tumor resection cavity to increase titers. Once implanted, these cells produce retroviruses expressing the HSV1-TK transgene, which diffuse into the surrounding brain tissue to infect tumor cells. Adenoviruses, in contrast to retroviruses, can be produced at high titers. Thus, trials with adenoviral vectors usually involve direct injection of virus expressing the HSV1-TK transgene into the surgical resection cavity. Compared with retroviruses, adenoviruses have demonstrated higher gene transfer efficiency into GBM cells.[211] Overall, numerous clinical trials using either retroviral or adenoviral vectors have shown that HSV1-TK/GCV therapy is well tolerated, but its therapeutic efficacy has been limited.[70,80,87,98,100,101,134,135,178,185,186,210,211,221,229,242,245]
Directly cytotoxic gene therapy
Directly cytotoxic gene therapy, otherwise known as targeted toxin therapy, utilizes surface molecules overexpressed in GBM to target toxins directly into tumor cells to cause tumor cell death. This can be accomplished through viral vector-mediated delivery of transgenes for highly toxic proteins or with recombinant molecules: Immunotoxins. Immunotoxins consist of a tumor-specific monoclonal antibody or ligand conjugated to a toxin.[63,148,255,264] The antibody or ligand component binds selectively to surface molecules overexpressed in GBM and induces internalization of the immunotoxin. Upon internalization, the toxin triggers a cascade of intracellular events that lead to tumor cell apoptosis. Commonly used toxins are Pseudomonas exotoxin (PE) and Diptheria exotoxin (DT).[264] Immunotoxins target surface molecules overexpressed in GBM: EGFR, interleukin-13 (IL-13), urokinase-type plasminogen activator (uPA), and transferrin.[16,19,46,50,52,63,106,109,137,150,202,277] Despite promising results in preclinical studies, clinical trials of immunotoxins have not shown significant clinical benefit.[124,125,131,153,182,191,203,204,205,206,207,243,251]
One of the most studied immunotoxons in GBM is IL-13-PE, a recombinant protein of the cytokine IL-13 fused with the PE toxin. In normal cells, IL-13 binds to a heterodimeric receptor complex composed of IL-13 and IL-4 receptors. The majority of GBM cells, however, overexpress a mutant form of the IL-13 receptor, IL-13Rα2.[28] Unlike the normal receptor for IL-13, the mutant IL-13Rα2 form binds IL-13 in the absence of IL-4, and it binds IL-13 with higher affinity.[49,50,51,52,53,145,146] As with other immunotoxins, results of clinical trials with IL-13-PE have shown no improvement in patient outcomes.[124,153,264]
Monoclonal antibodies can be conjugated to radionuclides, small molecule inhibitors, enzymes that require prodrug administration for activation, and chemotherapeutic agents for antibody-mediated targeted delivery into tumor cells.[239] Radioimmunoconjugates, which consist of tumor-specific monoclonal antibodies conjugated to radioactive iodine, are in essence radiolabeled antibodies that allow targeted destruction of GBM cells through radiation-mediated DNA damage. Radioimmunoconjugates also target surface molecules overexpressed in GBM, including EGFR and integrin ανβ3. Similar to immunotoxins, clinical trials of radioimmunoconjugates have not demonstrated clinical benefits.[130,257]
Immunostimulatory gene therapy
Immunomodulatory gene therapy uses the genetic material of cytokines, lymphocytes, or other immune modulators to enhance the host immune response to the tumor. Immunomodulatory approaches are based on the expectation that antitumor immune responses, activated by tumor-specific antigen expression, would effectively eliminate tumor cells. Unfortunately, often tumors develop mechanisms to evade these immune responses, creating an immune suppressive tumor microenvironment.[28,122] Thus, the goal of immunostimulatory gene therapy is to improve the immune response against the tumor. Several immunostimulatory approaches have been tested in GBM, including cytokine-mediated gene therapy and immune cell recruitment strategies.
Cytokines are a group of immune effector molecules that play a critical role in initiating, supporting, and inhibiting specific immune pathways. Cytokine-mediated gene therapy aims to use these cytokines to facilitate immune surveillance and stimulate cell-mediated immune responses against tumor cells. It involves specific delivery of transgenes for various immunostimulatory agents into tumor cells.[151] Tumor cell expression of the gene is used to induce more potent immune responses to the tumor cell.[242]
Immune cell recruitment strategies attempt to recruit DCs or other APCs into the tumor microenvironment in order to prime an immune response against GBM. One strategy to do this combines cytokine-mediated gene therapy with cytotoxic gene therapy. This approach involves delivery of fms-like tyrosine kinase-3 ligand (Flt3L), a cytokine that stimulates recruitment of DCs, as well as HSV1-TK into the surgical resection cavity.[122] Following administration of the GCV, the dying tumor cells release the peptides that will activate the DCs that were recruited to the tumor microenvironment by Flt3L.[26,44,242] This approach has mainly been studied in preclinical trials and has demonstrated an immune response with tumor regression and enhanced survival.[26,44] Clinical trials evaluating this approach in patients with GBM have recently begun.[44,122,242]
Oncolytic virotherapy
In oncolytic virotherapy, unlike in other approaches, viral toxicity is directly responsible for tumor cell death. Oncolytic viral vectors are engineered as conditionally replicating viruses that specifically infect and replicate within tumor cells, while sparing the surrounding normal brain.[242] Oncolytic virotherapy takes advantage of the innate ability of lytic viruses to lyse and kill their host cells following infection.[122,151,255] Upon lysis, virions spread to infect the surrounding tumor cells, propagating their toxicity[45,242] [Figure 4]. In order to be safe and effective, oncolytic viral vector replication and lytic destruction must be limited to tumor cells only, while being relatively avirulent in normal brain cells.[45,151] This can be accomplished by attenuating the virus to allow restriction of its replication to rapidly dividing cells, permitting them to infect and lyse tumor cells but not normal, nonreplicating brain cells. Additional molecular strategies used to accomplish this include deletion of components of the viral genome necessary for viral replication that are not expressed in normal cells but aberrantly expressed in cancer cells, insertion of promoter sequences into the viral genome that drive the expression of replication genes under the control of unique transcription factors overexpressed in cancer cells, and engineering viral capsid proteins that use unique molecular targeting to selectively transduce cancer cells.[151] Conditionally replicating HSV1 and adenoviruses vectors are the most commonly studied oncolytic viruses in clinical trials of GBM; however, other oncolytic viruses, such as reovirus, measles virus, and Newcastle disease virus, are also being investigated as therapeutic options for GBM.[28,71,151,152]
Figure 4.
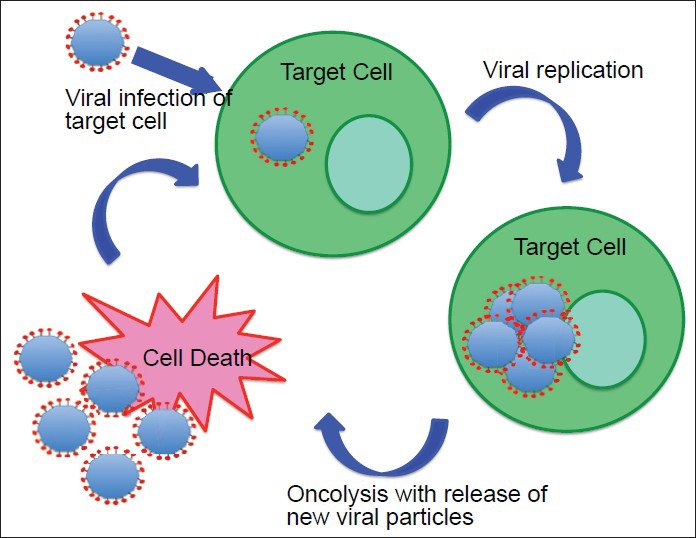
This figure shows conditionally cytotoxic gene therapy, also referred to as enzyme-prodrug activating therapy or suicide gene therapy, which is the most commonly used gene therapy. In conditionally cytotoxic approaches, as described in the text, the transgene for a noncytotoxic enzyme is delivered into tumor cells using a vector, and this enzyme remains noncytotoxic until the administration of a noncytotoxic prodrug. Upon prodrug administration, the enzyme converts the noncytotoxic prodrug into a toxic metabolite that induces tumor cell death
The first oncolytic virus tested in GBM was conditionally replicating HSV1.[84,242] HSV is a neurotropic virus; hence, it is critical that it is attenuated such that it only infects tumor cells in order to prevent toxicity to surrounding normal brain cells. Since it was first studied, HSV has been modified in different ways to achieve an adequate safety profile.[242] G207 is one of the most widely studied oncolytic HSV vector. It has been genetically engineered to replicate only within rapidly dividing cells.[28] Its HSV1-TK gene has been left intact, which allows oncolytic virotherapy with G207 to be combined with GCV to further increase its cytotoxic effects.[122] In clinical trials, G207 was able to infect and replicate within tumor cells, but it was unable to kill tumor cells efficiently. Oncolytic HSV vectors genetically engineered to encode transgenes for cytotoxic or immunostumulatory proteins are also being explored.[86,240] Overall, clinical trials with G207 and other oncolytic HSV vectors have shown high safety profiles, but they have demonstrated limited therapeutic efficacy.[138,139,244]
Conditionally replicating adenoviruses have also been studied.[242] Adenoviruses have been engineered to specifically replicate and lyse tumor cells in several different ways.[122] An advantage of adenoviruses to HSV oncolytic viruses is that they are naturally nonneurotropic, which enhances their safety profile. ONYX-015 is an adenovirus that has been modified to only replicate in cells with a p53 mutation.[36,79,219,255] Ad5-Delta is another adenovirus that only replicates in cells with defective retinoblastoma function.[2,3,73,74,79,250] Clinical trials using conditionally replicating adenoviruses have just begun, but early results indicate a favorable safety profile.[36]
A major drawback of oncolytic viruses is the host immune response, which limits their ability to spread to surrounding cells and reduces their transduction efficiency. Currently, direct delivery of oncolytic virus to the tumor is the only method that has been used in clinical trials. Preclinical trials are investigating novel delivery of these viruses into the bloodstream, which include the use of neural and mesenchymal stem cells as vectors.[55,66,120]
OTHER NOVEL THERAPIES
A variety of other novel approaches for the treatment of GBM are currently being investigated. The NovoTTF-100A is a device that uses alternating electric fields to disrupt cell division, and it is currently being studied as a treatment option for GBM.[116] The use of thermal lasers to denature tumor tissues is another area being investigated.[39] Laser interstitial thermal therapy (LITT) is a minimally invasive thermoablative procedure that uses a laser to create low-powered thermal energy that heats and destroys tumor cells.[91,227] LITT has been used for several decades for the treatment of GBM and other tumors; however, its use has been limited due to several technical limitations, mainly difficulty in regulating the dose of LITT delivered to individual patients.[107,113,215,216,217,227] In order to overcome these technical limitations, the NeuroBlate System (Monteris Medical, Inc.) was developed with technical advances, such as real-time MRI-guided thermography to facilitate detection of thermal damage to tissue, that allow for safer and more precise delivery of LITT for treatment of GBM. Recently, there have been several Phase I clinical trials done to evaluate the safety of the NeuroBlate System.[91,227] Results of these trials have demonstrated that the NeuroBlate System LITT has a favorable safety profile in patients with GBM; however, additional studies assessing clinical efficacy and outcomes still need to be conducted.[91,227]
CONCLUSION
GBM is the most common and lethal primary malignancy of the CNS. Even with surgical resection and aggressive treatment with chemo-and radiotherapy, the prognosis remains very poor. Due to continued advancements, the understanding of the complex biology of GBM and its pathogenesis, a wide variety of novel therapeutic approaches have been developed and are currently under study as potential treatments for GBM. Despite promising results in preclinical trials, many of these therapies have provided limited or no therapeutic efficacy in human clinical trials. Thus, although survival of patients with GBM continues to slowly improve, treatment of GBM remains extremely challenging. Continued research and development of new molecular targeted and immunotherapeutic approaches, based on a detailed understanding of molecular pathogenesis, can reasonably be expected to lead to increased survival and more favorable prognosis of patients with GBM.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/64/132138
Contributor Information
Taylor A. Wilson, Email: Taylor.Wilson5@gmail.com.
Matthias A. Karajannis, Email: Matthias.Karajannis@nyumc.org.
David H. Harter, Email: David.Harter@nyumc.org.
REFERENCES
- 1.Aguilar LK, Arvizu M, Aguilar-Cordova E, Chiocca EA. The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr Treat Options Oncol. 2012;13:437–50. doi: 10.1007/s11864-012-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed AU, Thaci B, Alexiades NG, Han Y, Qian S, Liu F, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19:1714–26. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed AU, Tyler MA, Thaci B, Alexiades NG, Han Y, Ulasov IV, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm. 2011;8:1559–72. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: Overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26:825–53. doi: 10.1016/j.hoc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54:519–25. doi: 10.1002/pbc.22319. [DOI] [PubMed] [Google Scholar]
- 6.Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: A pilot study. J Neurooncol. 2010;99:261–72. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 7.Arjona D, Rey JA, Taylor SM. Early genetic changes involved in low-grade astrocytic tumor development. Curr Mol Med. 2006;6:645–50. doi: 10.2174/156652406778195017. [DOI] [PubMed] [Google Scholar]
- 8.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 10.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 11.Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989;70:175–82. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–23. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–9. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, Debatin KM. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci U S A. 1999;96:8699–704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–12. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigner DD, Brown M, Coleman RE, Friedman AH, Friedman HS, McLendon RE, et al. Phase I studies of treatment of malignant gliomas and neoplastic meningitis with 131I-radiolabeled monoclonal antibodies anti-tenascin 81C6 and anti-chondroitin proteoglycan sulfate Me1-14 F (ab’) 2--a preliminary report. J Neurooncol. 1995;24:109–22. doi: 10.1007/BF01052668. [DOI] [PubMed] [Google Scholar]
- 17.Blancher A, Roubinet F, Grancher AS, Tremoulet M, Bonate A, Delisle MB, et al. Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin-2 and LAK cells. Eur Cytokine Netw. 1993;4:331–41. [PubMed] [Google Scholar]
- 18.Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994;39:193–7. doi: 10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady LW, Miyamoto C, Woo DV, Rackover M, Emrich J, Bender H, et al. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: A phase II trial. Int J Radiat Oncol Biol Phys. 1992;22:225–30. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- 20.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PloS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown PD, Krishnan S, Sarkaria JN, Wu W, Jaeckle KA, Uhm JH, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–9. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchschacher GL, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95:2499–504. [PubMed] [Google Scholar]
- 23.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: Efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–14. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6:236–46. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro MG, Candolfi M, Kroeger K, King GD, Curtin JF, Yagiz K, et al. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2011;11:155–80. doi: 10.2174/156652311795684722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers R, Gillespie GY, Soroceanu L, Andreansky S, Chatterjee S, Chou J, et al. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci U S A. 1995;92:1411–5. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18:1048–54. doi: 10.1016/j.jocn.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry IH, O’Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409–15. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, McKay RM, Parada LF. Malignant glioma: Lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinot OL, Barrie M, Fuentes S, Eudes N, Lancelot S, Metellus P, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25:1470–5. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]
- 35.Chinot OL, de La Motte Rouge T, Moore N, Zeaiter A, Das A, Phillips H, et al. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334–40. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 36.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19:713–23. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow KH, Gottschalk S. Cellular immunotherapy for high-grade glioma. Immunotherapy. 2011;3:423–34. doi: 10.2217/imt.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279–83. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 40.Cloughesy TF, Wen PY, Robins HI, Chang SM, Groves MD, Fink KL, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: A North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–6. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 41.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Combs SE, Heeger S, Haselmann R, Edler L, Debus J, Schulz-Ertner D. Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)--phase I/II trial: Study protocol. BMC Cancer. 2006;6:133. doi: 10.1186/1471-2407-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corey L. Laboratory diagnosis of herpes simplex virus infections. Principles guiding the development of rapid diagnostic tests. Diagn Microbiol Infect Dis. 1986;4(3 Suppl):111–9S. doi: 10.1016/s0732-8893(86)80049-9. [DOI] [PubMed] [Google Scholar]
- 44.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutter JL, Kurozumi K, Chiocca EA, Kaur B. Gene therapeutics: The future of brain tumor therapy? Expert Rev Anticancer Ther. 2006;6:1053–64. doi: 10.1586/14737140.6.7.1053. [DOI] [PubMed] [Google Scholar]
- 46.Day ED, Lassiter S, Woodhall B, Mahaley JL, Mahaley MS., Jr The localization of radioantibodies in human brain tumors. I. Preliminary exploration. Cancer Res. 1965;25:773–8. [PubMed] [Google Scholar]
- 47.Day SE, Waziri A. Clinical trials of small molecule inhibitors in high-grade glioma. Neurosurg Clin N Am. 2012;23:407–16. doi: 10.1016/j.nec.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 48.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 49.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Debinski W, Gibo DM, Slagle B, Powers SK, Gillespie GY. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15:481–6. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 51.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271:22428–33. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 52.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–8. [PubMed] [Google Scholar]
- 53.Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48:103–11. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 54.Del Vecchio CA, Wong AJ. Rindopepimut, a 14-mer injectable peptide vaccine against EGFRvIII for the potential treatment of glioblastoma multiforme. Curr Opin Mol Ther. 2010;12:741–54. [PubMed] [Google Scholar]
- 55.Dembinski JL, Spaeth EL, Fueyo J, Gomez-Manzano C, Studeny M, Andreeff M, et al. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Ther. 2010;17:289–97. doi: 10.1038/cgt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. doi: 10.1089/cbr.2010.0902. [DOI] [PubMed] [Google Scholar]
- 57.Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother. 2009;32:914–9. doi: 10.1097/CJI.0b013e3181b2910f. [DOI] [PubMed] [Google Scholar]
- 58.Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27:398–404. doi: 10.1097/00002371-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Dirks PB. Brain tumor stem cells: Bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916–24. doi: 10.1200/JCO.2008.17.6792. [DOI] [PubMed] [Google Scholar]
- 60.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, et al. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389–407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: A patient series. Ann Oncol. 2005;16:1702–8. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 62.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 63.Ehtesham M, Black KL, Yu JS. Recent progress in immunotherapy for malignant glioma: Treatment strategies and results from clinical trials. Cancer Control. 2004;11:192–207. doi: 10.1177/107327480401100307. [DOI] [PubMed] [Google Scholar]
- 64.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56:155–62. doi: 10.1227/01.neu.0000145865.25689.55. [DOI] [PubMed] [Google Scholar]
- 66.Ferguson SD, Ahmed AU, Thaci B, Mercer RW, Lesniak MS. Crossing the boundaries: Stem cells and gene therapy. Discov Med. 2010;9:192–6. [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara N. VEGF-A: A critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–63. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- 69.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 70.Floeth FW, Shand N, Bojar H, Prisack HB, Felsberg J, Neuen-Jacob E, et al. Local inflammation and devascularization--in vivo mechanisms of the “bystander effect” in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther. 2001;8:843–51. doi: 10.1038/sj.cgt.7700382. [DOI] [PubMed] [Google Scholar]
- 71.Forsyth P, Roldan G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 72.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 73.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–60. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 74.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 75.Fujisawa H, Reis RM, Nakamura M, Colella S, Yonekawa Y, Kleihues P, et al. Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest. 2000;80:65–72. doi: 10.1038/labinvest.3780009. [DOI] [PubMed] [Google Scholar]
- 76.Fukai J, Nishio K, Itakura T, Koizumi F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008;99:2062–9. doi: 10.1111/j.1349-7006.2008.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 78.Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 79.Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Terrier-Lacombe MJ, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–72. [PubMed] [Google Scholar]
- 80.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: Preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–89. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 81.Gerson SL. MGMT: Its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 82.Gilbert M, editor. Chicago: 2013 ASCO Annual Meeting; 2013. RTOG 0825: Phase III double. blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM) [Google Scholar]
- 83.Gomez-Manzano C, Jiang H, Alonso M, Yung WK, Fueyo J. Gene therapy. Handb Clin Neurol. 2012;104:331–8. doi: 10.1016/B978-0-444-52138-5.00021-9. [DOI] [PubMed] [Google Scholar]
- 84.Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9:505–17. doi: 10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–7. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 86.Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S, et al. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J Gene Med. 2007;9:99–106. doi: 10.1002/jgm.999. [DOI] [PubMed] [Google Scholar]
- 87.Harsh GR, Deisboeck TS, Louis DN, Hilton J, Colvin M, Silver JS, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: A gene-marking and neuropathological study. J Neurosurg. 2000;92:804–11. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- 88.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Chemotherapeutic wafers for High Grade Glioma. Cochrane Database Syst Rev. 2008;3:CD007294. doi: 10.1002/14651858.CD007294. [DOI] [PubMed] [Google Scholar]
- 89.Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro Oncol. 2010;12:508–16. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasselbalch B, Lassen U, Poulsen HS, Stockhausen MT. Cetuximab insufficiently inhibits glioma cell growth due to persistent EGFR downstream signaling. Cancer Invest. 2010;28:775–87. doi: 10.3109/07357907.2010.483506. [DOI] [PubMed] [Google Scholar]
- 91.Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: Single-institution series. Neurosurgery. 2013;73:1007–17. doi: 10.1227/NEU.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayes RL, Arbit E, Odaimi M, Pannullo S, Scheff R, Kravchinskiy D, et al. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol. 2001;39:31–42. doi: 10.1016/s1040-8428(01)00122-6. [DOI] [PubMed] [Google Scholar]
- 93.Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–52. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 94.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 95.Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087–98. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holladay FP, Heitz-Turner T, Bayer WL, Wood GW. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J Neurooncol. 1996;27:179–89. doi: 10.1007/BF00177482. [DOI] [PubMed] [Google Scholar]
- 97.Hoover JM, Chang SM, Parney IF. Clinical trials in brain tumor surgery. Neuroimaging Clin N Am. 2010;20:409–24. doi: 10.1016/j.nic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 98.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: A randomised, controlled study. Mol Ther. 2004;10:967–72. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Iwami K, Natsume A, Wakabayashi T. Gene therapy for high-grade glioma. Neurol Med Chir. 2010;50:727–36. doi: 10.2176/nmc.50.727. [DOI] [PubMed] [Google Scholar]
- 100.Izquierdo M, Cortes ML, Martin V, de Felipe P, Izquierdo JM, Perez-Higueras A, et al. Gene therapy in brain tumours: Implications of the size of glioblastoma on its curability. Acta Neurochir Suppl. 1997;68:111–7. doi: 10.1007/978-3-7091-6513-3_21. [DOI] [PubMed] [Google Scholar]
- 101.Izquierdo M, Martin V, de Felipe P, Izquierdo JM, Perez-Higueras A, Cortes ML, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther. 1996;3:491–5. [PubMed] [Google Scholar]
- 102.Jacobs A, Breakefield XO, Fraefel C. HSV-1-based vectors for gene therapy of neurological diseases and brain tumors: Part I. HSV-1 structure, replication and pathogenesis. Neoplasia. 1999;1:387–401. doi: 10.1038/sj.neo.7900055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacobs A, Breakefield XO, Fraefel C. HSV-1-based vectors for gene therapy of neurological diseases and brain tumors: Part II. Vector systems and applications. Neoplasia. 1999;1:402–16. doi: 10.1038/sj.neo.7900056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: Phase I trial. Cancer Res. 1986;46:2101–4. [PubMed] [Google Scholar]
- 105.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 106.Joshi BH, Leland P, Asher A, Prayson RA, Varricchio F, Puri RK. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001;61:8058–61. [PubMed] [Google Scholar]
- 107.Kahn T, Harth T, Kiwit JC, Schwarzmaier HJ, Wald C, Modder U. In vivo MRI thermometry using a phase-sensitive sequence: Preliminary experience during MRI-guided laser-induced interstitial thermotherapy of brain tumors. J Mag Reson Imaging. 1998;8:160–4. doi: 10.1002/jmri.1880080128. [DOI] [PubMed] [Google Scholar]
- 108.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–99. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 109.Kalofonos HP, Pawlikowska TR, Hemingway A, Courtenay-Luck N, Dhokia B, Snook D, et al. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J Nucl Med. 1989;30:1636–45. [PubMed] [Google Scholar]
- 110.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–52. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kanaly CW, Ding D, Heimberger AB, Sampson JH. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg Clin N Am. 2010;21:95–109. doi: 10.1016/j.nec.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008;89:113–8. doi: 10.1007/s11060-008-9599-0. [DOI] [PubMed] [Google Scholar]
- 113.Kangasniemi M, McNichols RJ, Bankson JA, Gowda A, Price RE, Hazle JD. Thermal therapy of canine cerebral tumors using a 980 nm diode laser with MR temperature-sensitive imaging feedback. Lasers Surg Med. 2004;35:41–50. doi: 10.1002/lsm.20069. [DOI] [PubMed] [Google Scholar]
- 114.Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–86. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 115.Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–9. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 116.Kirson ED, Dbaly V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–7. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4:329–36. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 118.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kootstra NA, Verma IM. Gene therapy with viral vectors. Annu Rev Pharmacol Toxicol. 2003;43:413–39. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- 120.Kranzler J, Tyler MA, Sonabend AM, Ulasov IV, Lesniak MS. Stem cells as delivery vehicles for oncolytic adenoviral virotherapy. Curr Gene Ther. 2009;9:389–95. doi: 10.2174/156652309789753347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kroeger KM, Muhammad AK, Baker GJ, Assi H, Wibowo MK, Xiong W, et al. Gene therapy and virotherapy: Novel therapeutic approaches for brain tumors. Discov Med. 2010;10:293–304. [PMC free article] [PubMed] [Google Scholar]
- 123.Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: A report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 126.Latchman DS. Gene delivery and gene therapy with herpes simplex virus-based vectors. Gene. 2001;264:1–9. doi: 10.1016/s0378-1119(01)00322-5. [DOI] [PubMed] [Google Scholar]
- 127.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 128.Leibel SA, Sheline GE. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66:1–22. doi: 10.3171/jns.1987.66.1.0001. [DOI] [PubMed] [Google Scholar]
- 129.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–6. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L, Quang TS, Gracely EJ, Kim JH, Emrich JG, Yaeger TE, et al. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. 2010;113:192–8. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 131.Li YM, Hall WA. Targeted toxins in brain tumor therapy. Toxins. 2010;2:2645–62. doi: 10.3390/toxins2112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 133.Lillehei KO, Mitchell DH, Johnson SD, McCleary EL, Kruse CA. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991;28:16–23. doi: 10.1097/00006123-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 134.Long Z, Li LP, Grooms T, Lockey C, Nader K, Mychkovsky I, et al. Biosafety monitoring of patients receiving intracerebral injections of murine retroviral vector producer cells. Hum Gene Ther. 1998;9:1165–72. doi: 10.1089/hum.1998.9.8-1165. [DOI] [PubMed] [Google Scholar]
- 135.Long Z, Lu P, Grooms T, Mychkovsky I, Westley T, Fitzgerald T, et al. Molecular evaluation of biopsy and autopsy specimens from patients receiving in vivo retroviral gene therapy. Hum Gene Ther. 1999;10:733–40. doi: 10.1089/10430349950018490. [DOI] [PubMed] [Google Scholar]
- 136.Luptrawan A, Liu G, Yu JS. Dendritic cell immunotherapy for malignant gliomas. Rev Recent Clin Trials. 2008;3:10–21. doi: 10.2174/157488708783330530. [DOI] [PubMed] [Google Scholar]
- 137.Mahaley MS, Jr, Mahaley JL, Day ED. The localization of radioantibodies in human brain tumors. II. Radioautography. Cancer Res. 1965;25:779–93. [PubMed] [Google Scholar]
- 138.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 140.Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–64. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 141.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–8. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–81. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 143.Merchant RE, Grant AJ, Merchant LH, Young HF. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988;62:665–71. doi: 10.1002/1097-0142(19880815)62:4<665::aid-cncr2820620403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 144.Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: Role of gap-junctional intercellular communication. Cancer Res. 2000;60:3989–99. [PubMed] [Google Scholar]
- 145.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neurooncol. 2003;64:117–23. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 146.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–99. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mischel PS, Shai R, Shi T, Horvath S, Lu KV, Choe G, et al. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene. 2003;22:2361–73. doi: 10.1038/sj.onc.1206344. [DOI] [PubMed] [Google Scholar]
- 148.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009;6:527–38. doi: 10.1016/j.nurt.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mohanam S, Sawaya RE, Yamamoto M, Bruner JM, Nicholson GL, Rao JS. Proteolysis and invasiveness of brain tumors: Role of urokinase-type plasminogen activator receptor. J Neurooncol. 1994;22:153–60. doi: 10.1007/BF01052890. [DOI] [PubMed] [Google Scholar]
- 151.Mohyeldin A, Chiocca EA. Gene and viral therapy for glioblastoma: A review of clinical trials and future directions. Cancer J. 2012;18:82–8. doi: 10.1097/PPO.0b013e3182458b13. [DOI] [PubMed] [Google Scholar]
- 152.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: An overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 153.Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher-Schroder E, et al. Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol. 2011;101:267–77. doi: 10.1007/s11060-010-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 155.Nakamura M, Yang F, Fujisawa H, Yonekawa Y, Kleihues P, Ohgaki H. Loss of heterozygosity on chromosome 19 in secondary glioblastomas. J Neuropathol Exp Neurol. 2000;59:539–43. doi: 10.1093/jnen/59.6.539. [DOI] [PubMed] [Google Scholar]
- 156.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103:491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 157.Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20:1596–603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 158.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–7. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 159.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004;64:6892–9. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 160.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–72. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 161.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–53. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15:275–86. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 163.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97:2208–13. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patel M, Vogelbaum MA, Barnett GH, Jalali R, Ahluwalia MS. Molecular targeted therapy in recurrent glioblastoma: Current challenges and future directions. Expert Opin Investig Drugs. 2012;21:1247–66. doi: 10.1517/13543784.2012.703177. [DOI] [PubMed] [Google Scholar]
- 166.Peereboom DM, Shepard DR, Ahluwalia MS, Brewer CJ, Agarwal N, Stevens GH, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98:93–9. doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 167.Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12:3935–41. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 168.Perry J, Okamoto M, Guiou M, Shirai K, Errett A, Chakravarti A. Novel therapies in glioblastoma. Neurol Res Int. 2012;2012 doi: 10.1155/2012/428565. 428565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 170.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 171.Pichlmeier U, Bink A, Schackert G, Stummer W Group ALAGS. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10:1025–34. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pitter KL, Galban CJ, Galban S, Tehrani OS, Li F, Charles N, et al. Perifosine and CCI 779 co-operate to induce cell death and decrease proliferation in PTEN-intact and PTEN-deficient PDGF-driven murine glioblastoma. PloS One. 2011;6:e14545. doi: 10.1371/journal.pone.0014545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Plautz GE, Barnett GH, Miller DW, Cohen BH, Prayson RA, Krauss JC, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89:42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 174.Plautz GE, Miller DW, Barnett GH, Stevens GH, Maffett S, Kim J, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–18. [PubMed] [Google Scholar]
- 175.Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 176.Prados MD, Chang SM, Butowski N, DeBoer R, Parvataneni R, Carliner H, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–84. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Prados MD, McDermott M, Chang SM, Wilson CB, Fick J, Culver KW, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: A phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–78. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 179.Prasad G, Sottero T, Yang X, Mueller S, James CD, Weiss WA, et al. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro Oncol. 2011;13:384–92. doi: 10.1093/neuonc/noq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 181.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Puri RK. Development of a recombinant interleukin-4-Pseudomonas exotoxin for therapy of glioblastoma. Toxicol Pathol. 1999;27:53–7. doi: 10.1177/019262339902700111. [DOI] [PubMed] [Google Scholar]
- 183.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–57. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 184.Rahmathulla G, Hovey EJ, Hashemi-Sadraei N, Ahluwalia MS. Bevacizumab in high-grade gliomas: A review of its uses, toxicity assessment, and future treatment challenges. OncoTargets Ther. 2013;6:371–89. doi: 10.2147/OTT.S38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 186.Rainov NG, Kramm CM, Banning U, Riemann D, Holzhausen HJ, Heidecke V, et al. Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Ther. 2000;7:1853–8. doi: 10.1038/sj.gt.3301311. [DOI] [PubMed] [Google Scholar]
- 187.Rainov NG, Ren H. Clinical trials with retrovirus mediated gene therapy--what have we learned? J Neurooncol. 2003;65:227–36. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 188.Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12:95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, et al. A phase I trial of erlotinib in patients with nonprogressive glioblastoma multiforme postradiation therapy, and recurrent malignant gliomas and meningiomas. Neuro Oncol. 2010;12:87–94. doi: 10.1093/neuonc/nop017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–61. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 191.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–65. [PubMed] [Google Scholar]
- 192.Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–65. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reardon DA, Dresemann G, Taillibert S, Campone M, van den Bent M, Clement P, et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101:1995–2004. doi: 10.1038/sj.bjc.6605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, et al. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115:2188–98. doi: 10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Reardon DA, Egorin MJ, Quinn JA, Rich JN, Gururangan S, Vredenburgh JJ, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–68. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 196.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–7. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 197.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: An integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–35. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reardon DA, Vredenburgh JJ, Coan A, Desjardins A, Peters KB, Gururangan S, et al. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105:621–7. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reijneveld JC, Voest EE, Taphoorn MJ. Angiogenesis in malignant primary and metastatic brain tumors. J Neurol. 2000;247:597–608. doi: 10.1007/s004150070128. [DOI] [PubMed] [Google Scholar]
- 200.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 201.Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–42. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 202.Riva P, Arista A, Franceschi G, Frattarelli M, Sturiale C, Riva N, et al. Local treatment of malignant gliomas by direct infusion of specific monoclonal antibodies labeled with 131I: Comparison of the results obtained in recurrent and newly diagnosed tumors. Cancer Res. 1995;55(23 Suppl):5952–6s. [PubMed] [Google Scholar]
- 203.Rustamzadeh E, Hall WA, Todhunter DA, Low WC, Liu H, Panoskaltsis-Mortari A, et al. Intracranial therapy of glioblastoma with the fusion protein DTIL13 in immunodeficient mice. Int J Cancer. 2006;118:2594–601. doi: 10.1002/ijc.21647. [DOI] [PubMed] [Google Scholar]
- 204.Rustamzadeh E, Hall WA, Todhunter DA, Vallera VD, Low WC, Liu H, et al. Intracranial therapy of glioblastoma with the fusion protein DTAT in immunodeficient mice. Int J Cancer. 2007;120:411–9. doi: 10.1002/ijc.22278. [DOI] [PubMed] [Google Scholar]
- 205.Rustamzadeh E, Li C, Doumbia S, Hall WA, Vallera DA. Targeting the over-expressed urokinase-type plasminogen activator receptor on glioblastoma multiforme. J Neurooncol. 2003;65:63–75. doi: 10.1023/a:1026238331739. [DOI] [PubMed] [Google Scholar]
- 206.Rustamzadeh E, Vallera DA, Todhunter DA, Low WC, Panoskaltsis-Mortari A, Hall WA. Immunotoxin pharmacokinetics: A comparison of the anti-glioblastoma bi-specific fusion protein (DTAT13) to DTAT and DTIL13. J Neurooncol. 2006;77:257–66. doi: 10.1007/s11060-005-9051-7. [DOI] [PubMed] [Google Scholar]
- 207.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 208.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–75. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–9. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 212.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110:13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 213.Scaringi C, Minniti G, Caporello P, Enrici RM. Integrin inhibitor cilengitide for the treatment of glioblastoma: A brief overview of current clinical results. Anticancer Res. 2012;32:4213–23. [PubMed] [Google Scholar]
- 214.Schmidt NO, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen EM, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–8. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 215.Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, Fiedler VU, Niehoff H, Ulrich SD, et al. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging. 2005;22:799–803. doi: 10.1002/jmri.20446. [DOI] [PubMed] [Google Scholar]
- 216.Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, Fiedler VU, Niehoff H, Ulrich SD, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: Preliminary results in 16 patients. Eur J Radiol. 2006;59:208–15. doi: 10.1016/j.ejrad.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 217.Schwarzmaier HJ, Yaroslavsky IV, Yaroslavsky AN, Fiedler V, Ulrich F, Kahn T. Treatment planning for MRI-guided laser-induced interstitial thermotherapy of brain tumors--the role of blood perfusion. J Magn Reson Imaging. 1998;8:121–7. doi: 10.1002/jmri.1880080124. [DOI] [PubMed] [Google Scholar]
- 218.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–47. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 219.Shah AC, Benos D, Gillespie GY, Markert JM. Oncolytic viruses: Clinical applications as vectors for the treatment of malignant gliomas. J Neurooncol. 2003;65:203–26. doi: 10.1023/b:neon.0000003651.97832.6c. [DOI] [PubMed] [Google Scholar]
- 220.Shai R, Shi T, Kremen TJ, Horvath S, Liau LM, Cloughesy TF, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–23. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 221.Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, et al. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10:2325–35. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 222.Shapiro WR, Green SB, Burger PC, Mahaley MS, Jr, Selker RG, VanGilder JC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 223.Shaw RJ, Cantley LC. Ras, PI(3) K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 224.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: The keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–13. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 225.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 226.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 227.Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J Neurosurg. 2013;118:1202–19. doi: 10.3171/2013.1.JNS1291. [DOI] [PubMed] [Google Scholar]
- 228.Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: Preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9:e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 229.Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–8. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 230.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stiles CD, Rowitch DH. Glioma stem cells: A midterm exam. Neuron. 2008;58:832–46. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 232.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 233.Stupp R, editor. Chicago: 2013 ASCO; 2013. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma and methylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter: Key results of the multicenter, randomized, open-label, controlled, phase III CENTRIC study. [Google Scholar]
- 234.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 235.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–8. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 236.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, et al. Changing paradigms--an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–80. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 237.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 238.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 239.Teicher BA, Chari RV. Antibody conjugate therapeutics: Challenges and potential. Clin Cancer Res. 2011;17:6389–97. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 240.Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13:705–14. doi: 10.1038/sj.gt.3302717. [DOI] [PubMed] [Google Scholar]
- 241.Thomas AA, Ernstoff MS, Fadul CE. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012;18:59–68. doi: 10.1097/PPO.0b013e3182431a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tobias A, Ahmed A, Moon KS, Lesniak MS. The art of gene therapy for glioma: A review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry. 2013;84:213–22. doi: 10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17:157–64. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 244.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 246.Tsuboi K, Saijo K, Ishikawa E, Tsurushima H, Takano S, Morishita Y, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res. 2003;9:3294–302. [PubMed] [Google Scholar]
- 247.Tsurushima H, Liu SQ, Tuboi K, Matsumura A, Yoshii Y, Nose T, et al. Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res. 1999;90:536–45. doi: 10.1111/j.1349-7006.1999.tb00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Uhm JH, Ballman KV, Wu W, Giannini C, Krauss JC, Buckner JC, et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int J Radiat Oncol Biol Phys. 2011;80:347–53. doi: 10.1016/j.ijrobp.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ulasov IV, Tyler MA, Rivera AA, Nettlebeck DM, Douglas JT, Lesniak MS. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–603. doi: 10.1002/jmv.00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 251.Vallera DA, Li C, Jin N, Panoskaltsis-Mortari A, Hall WA. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J Natl Cancer Inst. 2002;94:597–606. doi: 10.1093/jnci/94.8.597. [DOI] [PubMed] [Google Scholar]
- 252.van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–74. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.van den Bent MJ, Dubbink HJ, Sanson M, van der Lee-Haarloo CR, Hegi M, Jeuken JW, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: A report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27:5881–6. doi: 10.1200/JCO.2009.24.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Van Gool S, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S. Dendritic cell therapy of high-grade gliomas. Brain Pathol. 2009;19:694–712. doi: 10.1111/j.1750-3639.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Van Meir EG, Bellail A, Phuphanich S. Emerging molecular therapies for brain tumors. Semin Oncol. 2004;31:38–46. doi: 10.1053/j.seminoncol.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 256.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol 2010. 2010 doi: 10.1155/2010/689171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Veeravagu A, Liu Z, Niu G, Chen K, Jia B, Cai W, et al. Integrin alphavbeta3-targeted radioimmunotherapy of glioblastoma multiforme. Clin Cancer Res. 2008;14:7330–9. doi: 10.1158/1078-0432.CCR-08-0797. [DOI] [PubMed] [Google Scholar]
- 258.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Villalva C, Cortes U, Wager M, Tourani JM, Rivet P, Marquant C, et al. O6-Methylguanine-Methyltransferase (MGMT) promoter methylation status in glioma stem-like cells is correlated to temozolomide sensitivity under differentiation-promoting conditions. Int J Mol Sci. 2012;13:6983–94. doi: 10.3390/ijms13066983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 261.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 262.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, Reardon DA, Peters KB, Herndon JE, 2nd, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82:58–66. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 263.Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE, 2nd, Marcello J, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–24. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17:181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 266.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 268.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–23. doi: 10.1111/j.1750-3639.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 269.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–53. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 271.Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 272.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–12. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 274.Wood GW, Holladay FP, Turner T, Wang YY, Chiga M. A pilot study of autologous cancer cell vaccination and cellular immunotherapy using anti-CD3 stimulated lymphocytes in patients with recurrent grade III/IV astrocytoma. J Neurooncol. 2000;48:113–20. doi: 10.1023/a:1006456421177. [DOI] [PubMed] [Google Scholar]
- 275.Xu X, Stockhammer F, Schmitt M. Cellular-based immunotherapies for patients with glioblastoma multiforme. Clin Dev Immunol 2012. 2012 doi: 10.1155/2012/764213. 764213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yajima N, Yamanaka R, Mine T, Tsuchiya N, Homma J, Sano M, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005;11:5900–11. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- 277.Yamamoto M, Sawaya R, Mohanam S, Bindal AK, Bruner JM, Oka K, et al. Expression and localization of urokinase-type plasminogen activator in human astrocytomas in vivo. Cancer Res. 1994;54:3656–61. [PubMed] [Google Scholar]
- 278.Yamanaka R. Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med. 2008;14:228–35. doi: 10.1016/j.molmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 279.Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: Results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–9. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–9. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 282.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–7. [PubMed] [Google Scholar]
- 283.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: Mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–14. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 284.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, et al. Neural stem cell tropism to glioma: Critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–29. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]


