Abstract
Cervical–petrous internal carotid artery (CP-ICA) pseudoaneurysms are rare and have different etiologies, presentations, and treatment options. A middle-aged patient with a history of chronic otitis media presented with acute otorrhagia and was found to have a left-sided CP-ICA pseudoaneurysm. The patient was a poor surgical candidate with difficult arterial access. The pseudoaneurysm was treated with stand-alone coiling via a left brachial approach with persistent contrast filling seen only in the aneurysm neck at the end of the procedure. The patient re-presented 12 days later with repeat hemorrhage and rapid enlargement of the neck remnant, and was treated with a covered stent via a transcervical common carotid artery cut-down. A covered stent may provide a more definitive treatment for CP-ICA pseudoaneurysms compared with standalone coiling.
Keywords: Aneurysm, Coil, Stent
Background
Cervical–petrous internal carotid artery (CP-ICA) aneurysms are rare, and only a small number of cases have been reported.1–25 CP-ICA aneurysms have a number of etiologies, a variety of presentations, and are amenable to a number of surgical and endovascular treatments. Herein we report a case of a CP-ICA aneurysm presenting with otorrhagia treated with two endovascular techniques.
Case presentation
A middle-aged patient with a history of end stage renal failure, atrial fibrillation, and chronic otitis media presented with bleeding from the left ear. The patient had experienced a similar episode 1 week prior that stopped spontaneously. The patient had a history of chronic otitis media without cholesteatoma, and had undergone tympanoplasty surgery 9 years earlier, but had persistent episodic drainage and severe sensorineural hearing loss despite the surgery. A CT scan of the head demonstrated evidence of chronic erosion of the left petrous temporal bone (figure 1). The current episode was controlled with external packing and reversal of warfarin and aspirin.
Figure 1.
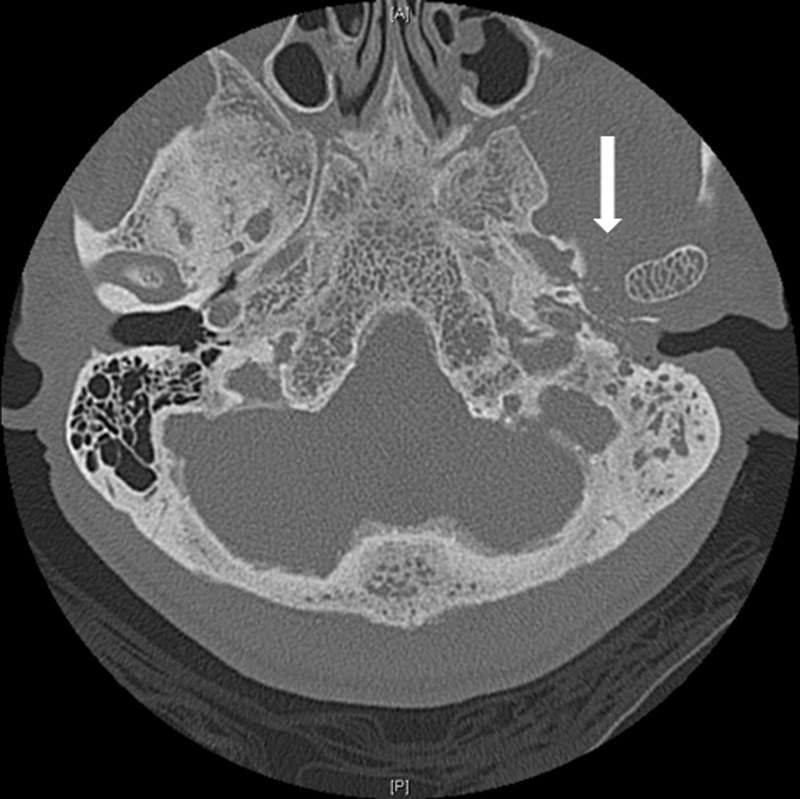
CT of the head, axial view. Chronic infection/inflammation of the left petrous temporal bone (arrow).
Treatment
Given the concern for a vascular lesion, the patient was taken directly for conventional angiography. Access was obtained via the left brachial artery as the patient could not straighten either leg and had an arteriovenous fistula in the right arm. A 5 F Simmons 2 Glidecath (Terumo, Somerset, New Jersey, USA) was used to obtain access to the left common carotid artery (CCA) (figure 2). Injection of the left CCA revealed a left CP-ICA pseudoaneurysm measuring approximately 17×10 mm (figure 3).
Figure 2.
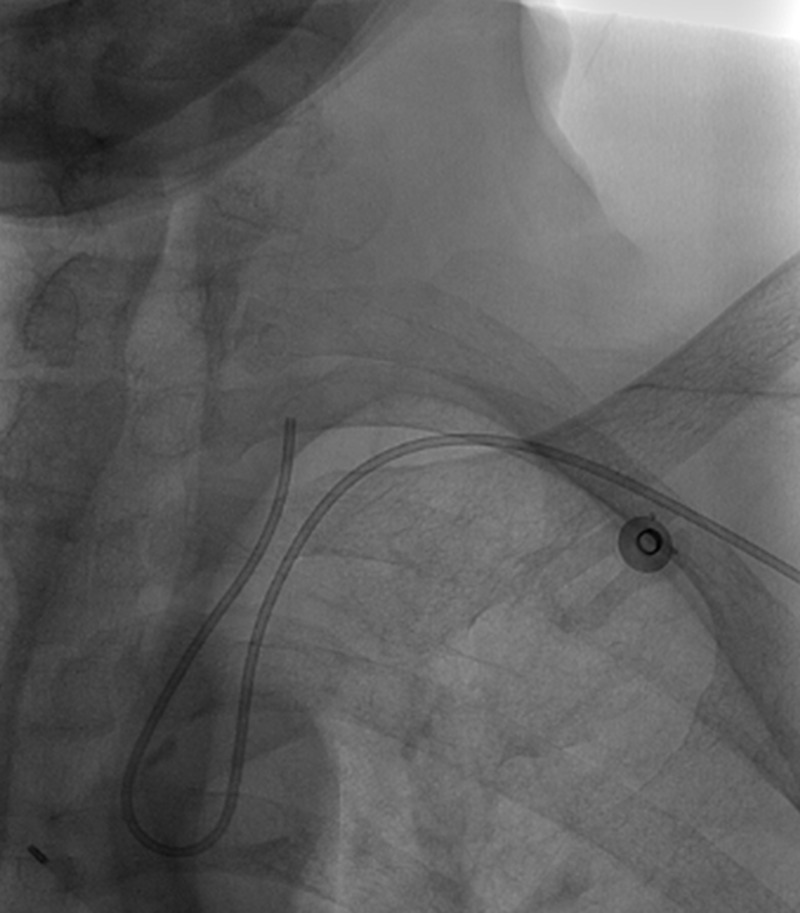
Angiogram, unsubtracted view. Left brachial approach to the left common carotid artery with Simmons II catheter.
Figure 3.
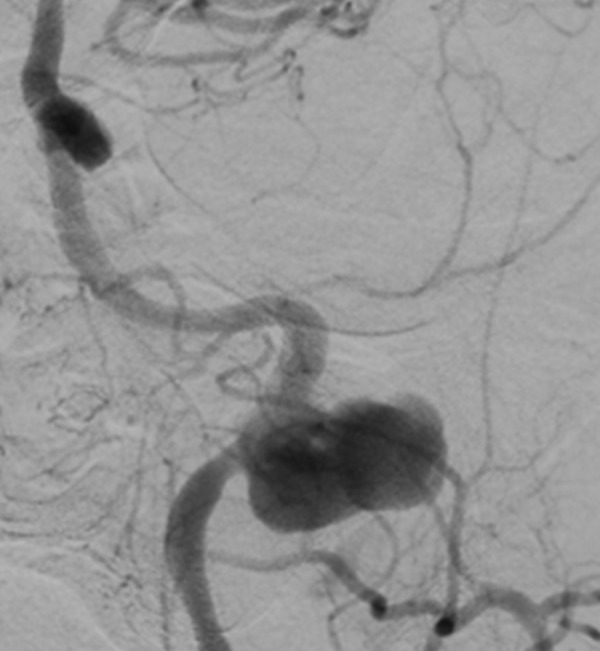
Angiogram, left common carotid artery injection, anteroposterior view. Left cervical–petrous internal carotid artery pseudoaneurysm projecting laterally.
The decision was made to proceed with standalone coiling. Through a 6 F Simmons 1 guide catheter (Cordis, Bridgewater, New Jersey, USA), a 2.6 F J-tipped PX Slim microcatheter (Penumbra, Alameda, California, USA) with a 0.014 inch Synchro2 Standard microwire (Boston Scientific, Natick, Massachusetts, USA) was used to obtain access to the aneurysm, which was coiled with seven 0.0200 inch PC 400 coils (Penumbra), achieving a packing density of 21.16% with only residual filling in the aneurysm neck (figure 4). The patient did well postoperatively and was discharged home 6 days after the procedure.
Figure 4.
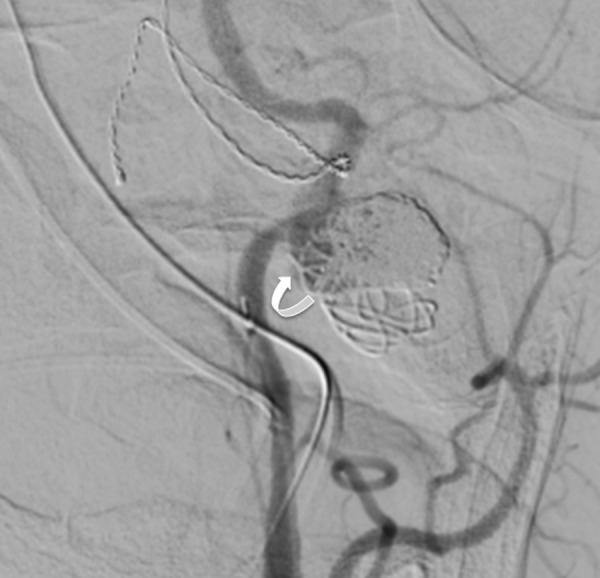
Angiogram, left common carotid artery injection, anteroposterior view. Status post coil embolization of the pseudoaneurysm with only contrast filling in the aneurysm neck at the end of the procedure (arrow).
The patient presented 12 days after discharge with another episode of bleeding from the left ear, which occurred following hemodialysis and ceased without intervention. The patient was taken to the angiography suite for further investigation. Following induction of anesthesia and intubation, the patient experienced a hypertensive episode associated with a significant hemorrhage from the left ear that was controlled with external packing. Anticipating that a stent would not be easily positioned using the previous approach, access to the left CCA was obtained by an emergent transcervical cut-down. A sheath was inserted, and the initial angiograms demonstrated compaction of the coil mass and increased filling of the proximal pseudoaneurysm sac without extravasation (figure 5).
Figure 5.
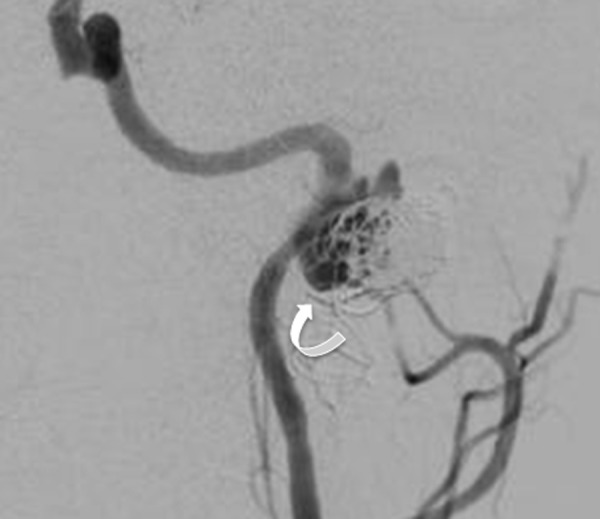
Angiogram, left common carotid artery injection, anteroposterior view. Coil compaction with increased contrast filling in the aneurysm neck and proximal dome (arrow).
A 6×50 mm Viabahn Endoprosthesis (Gore) covered stent was deployed and followed by angioplasty to 5 mm to ensure adequate apposition to the vessel wall and the pseudoaneurysm defect. Final angiograms demonstrated exclusion of the pseudoaneurysm from the circulation (figure 6) and no evidence of distal thromboembolic complications. Perioperative antibiotics were given. The patient was immediately started on aspirin and Plavix, had an uneventful postoperative course, and was discharged 4 days later.
Figure 6.
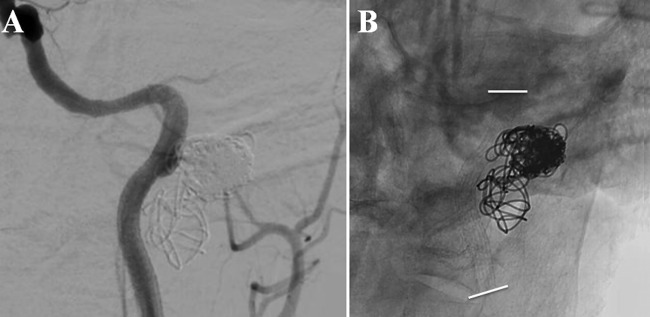
(A) Angiogram, left common carotid artery injection, anteroposterior view. Status post placement of a covered stent with reconstruction of the vessel wall and no aneurysm filling. (B) Unsubtracted view demonstrating stent (delineated by white lines).
Discussion
CP-ICA aneurysms have a number of different etiologies. Many of these aneurysms are pseudoaneurysms that arise as a result of trauma,2 9 21 iatrogenic injury,10 15 16 22 23 infection,7 19 radiation,8 11 22 25 or genetic abnormalities, such as fibromuscular dysplasia14 and Ehlers–Danlos syndrome.24 In our case, chronic otitis media is a possible etiology with progressive erosion into the left ICA, although we cannot rule out iatrogenic injury given a history of tympanoplasty.
CP-ICA aneurysms can present in a variety of different ways, including headache, cranial neuropathies,1 20 23 Horner syndrome,20 transient ischemic attack,4 pulsatile tinnitus,13 hearing loss,6 13 epistaxis,3 4 8 11 13 15 22 and otorrhagia.4 6–8 10 15 19 25 They may also be asymptomatic. A single case of both subarachnoid hemorrhage and epistaxis secondary to a ruptured petrous ICA aneurysm was reported.22 Occasionally the bleeding (whether it be epistaxis, otorrhagia, or subarachnoid hemorrhage) associated with a ruptured CP-ICA pseudoaneurysm can be life threatening, as occurred during our patient's second admission.
A number of different surgical and endovascular techniques have been used to treat CP-ICA aneurysms. Traditional surgical clipping of petrous aneurysms has not been greatly utilized due to the difficult location within the petrous bone. Surgical or endovascular ICA occlusion is an option if the patient passes a balloon test occlusion (BTO) or in the setting of life threatening hemorrhage when all other methods have failed.2 25 Surgical bypass has been used in conjunction with surgical or endovascular entrapment.1 6 17 19 22 Surgical excision of a cervical ICA aneurysm with end to end anastomosis has also been performed.5 Endovascular techniques have included balloon embolization,3 6 coil embolization,7 8 20 stand-alone stenting (typically with a covered stent),8–11 14 21 23 stent-assisted coiling,26 and flow diversion.27–29 The bleeding27 and thromboembolic complication29 rate may be higher with flow diversion.
In our case, an endovascular approach was favored given that the patient was an extremely poor surgical candidate. The first procedure was technically challenging through a left brachial approach. The Simmons catheters offered a method for accessing the left CCA from the left subclavian artery (although this was still difficult). This approach facilitated standalone coiling but did not provide the support for a covered stent or a flow diverting stent. A BTO was contemplated during the first procedure but was not attempted given the anticipated difficulty of maneuvering a diagnostic catheter to the appropriate vessels in addition to the fact that the patient was under general anesthesia and could not be assessed neurologically. Given the great difficulty with arterial access and navigating through the great vessels during the first procedure, a transcervical approach was utilized in the second procedure. Had a BTO plus carotid sacrifice been an option, it likely would have been the safer approach given the risks of a carotid cut-down and covered stent placement in this particular patient.
Although the previous cases of stand-alone coiling did not report treatment failure, we experienced rapid coil compaction, aneurysm enlargement, and re-rupture. We initially chose not to aggressively pack the neck of the pseudoaneurysm with coils in an attempt to avoid ICA obstruction and thromboembolic complications. It is possible that the weakened pseudoaneurysm wall allowed for rapid enlargement and re-rupture of this neck remnant. This case would suggest that a covered stent may provide a more definitive treatment for CP-ICA pseudoaneurysms. It should be noted that treatment of infected pseudoaneurysms with covered stents carries a risk of infectious complications,30 but has been safe in our experience.31 This risk was deemed acceptable given the emergent circumstances and lack of other, better treatment options.
Learning points.
Cervical–petrous internal carotid artery (ICA) pseudoaneurysms are rare lesions that have a variety of etiologies, presentations, and treatments.
A high degree of suspicion is necessary for patients presenting with otorrhagia.
Coil embolization is not an unreasonable option if the ICA is not narrowed, but this case would suggest that the aneurysm neck should be completely occluded in addition to the dome.
Complete exclusion of the pseudoaneurysm with a covered stent, however, may be a more definitive treatment.
Footnotes
Competing interests: AKP is a consult to Penumbra and this had no influence on the case report.
Patient consent: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.McGrail KM, Heros RC, Debrun G, et al. Aneurysm of the ICA petrous segment treated by balloon entrapment after EC-IC bypass. Case report. J Neurosurg 1986;65:249–52 [DOI] [PubMed] [Google Scholar]
- 2.Fleischer AS, Guthkelch AN. Management of high cervical-intracranial internal carotid artery traumatic aneurysms. J Trauma 1987;27:330–2 [DOI] [PubMed] [Google Scholar]
- 3.Willinsky R, Lasjaunias P, Pruvost P, et al. Petrous internal carotid aneurysm causing epistaxis: balloon embolization with preservation of the parent vessel. Neuroradiology 1987;29:570–2 [DOI] [PubMed] [Google Scholar]
- 4.Costantino PD, Russell E, Reisch D, et al. Ruptured petrous carotid aneurysm presenting with otorrhagia and epistaxis. Am J Otol 1991;12:378–83 [PubMed] [Google Scholar]
- 5.Tokimura H, Todoroki K, Asakura T, et al. Coexistence of extracranial internal carotid artery aneurysm and multiple intracranial aneurysms—case report. Neurol Med Chir (Tokyo) 1992;32:292–5 [DOI] [PubMed] [Google Scholar]
- 6.Umezu H, Seki Y, Aiba T, et al. Aneurysm arising from the petrous portion of the internal carotid artery: case report. Radiat Med 1993;11:251–5 [PubMed] [Google Scholar]
- 7.Kawakami K, Kayama T, Kondo R, et al. A case of mycotic ICA petrous portion aneurysm treated with endovascular surgery. No Shinkei Geka 1996;24:253–7 [PubMed] [Google Scholar]
- 8.Cheng KM, Chan CM, Cheung YL, et al. Endovascular treatment of radiation-induced petrous internal carotid artery aneurysm presenting with acute haemorrhage. A report of two cases. Acta Neurochir (Wien) 2001;143:351–5 [DOI] [PubMed] [Google Scholar]
- 9.Scavée V, De Wispelaere JF, Mormont E, et al. Pseudoaneurysm of the internal carotid artery: treatment with a covered stent. Cardiovasc Intervent Radiol 2001;24:283–5 [DOI] [PubMed] [Google Scholar]
- 10.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a Symbiot covered stent: technical case report. Neurosurgery 2002;50:658–62 [DOI] [PubMed] [Google Scholar]
- 11.Auyeung KM, Lui WM, Chow LC, et al. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol 2003;24:1449–52 [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JK, Gottfried ON, Amini A, et al. Aneurysms of the petrous internal carotid artery: anatomy, origins, and treatment. Neurosurg Focus 2004;17:E13. [DOI] [PubMed] [Google Scholar]
- 13.Moonis G, Hwang CJ, Ahmed T, et al. Otologic manifestations of petrous carotid aneurysms. AJNR Am J Neuroradiol 2005;26:1324–7 [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen JE, Grigoriadis S, Gomori JM. Petrous carotid artery pseudoaneurysm in bilateral carotid fibromuscular dysplasia: treatment by means of self-expanding covered stent. Surg Neurol 2007;68:216–20 [DOI] [PubMed] [Google Scholar]
- 15.Schmerber S, Vasdev A, Chahine K, et al. Internal carotid false aneurysm after thermocoagulation of the gasserian ganglion. Otol Neurotol 2008;29:673–5 [DOI] [PubMed] [Google Scholar]
- 16.Saylam G, Tulgar M, Saatci I, et al. Iatrogenic carotid artery pseudoaneurysm presenting with conductive hearing loss. Am J Otolaryngol 2009;30:141–4 [DOI] [PubMed] [Google Scholar]
- 17.Ferroli P, Bisleri G, Nakaji P, et al. Endoscopic radial artery harvesting for U-clip EC-IC bypass in the treatment of a giant petrous internal carotid artery aneurysm: technical case report. Minim Invasive Neurosurg 2009;52:186–9 [DOI] [PubMed] [Google Scholar]
- 18.Palacios E, Gómez J, Alvernia JE, et al. Aneurysm of the petrous portion of the internal carotid artery at the foramen lacerum: anatomic, imaging, and otologic findings. Ear Nose Throat J 2010;89:303–5 [PubMed] [Google Scholar]
- 19.Oyama H, Hattori K, Tanahashi S, et al. Ruptured pseudoaneurysm of the petrous internal carotid artery caused by chronic otitis media. Neurol Med Chir (Tokyo) 2010;50:578–80 [DOI] [PubMed] [Google Scholar]
- 20.Mangat SS, Nayak H, Chandna A. Horner's syndrome and sixth nerve paresis secondary to a petrous internal carotid artery aneurysm. Semin Ophthalmol 2011;26:23–4 [DOI] [PubMed] [Google Scholar]
- 21.Yeh CH, Lin MS, Chiu MC, et al. Endovascular treatment of a huge cervical carotid artery pseudoaneurysm with Wallgraft prosthesis. Ann Vasc Surg 2011;25:265.e1–3 [DOI] [PubMed] [Google Scholar]
- 22.Endo H, Fujimura M, Inoue T, et al. Simultaneous occurrence of subarachnoid hemorrhage and epistaxis due to ruptured petrous internal carotid artery aneurysm: association with transsphenoidal surgery and radiation therapy: case report. Neurol Med Chir (Tokyo) 2011;51:226–9 [DOI] [PubMed] [Google Scholar]
- 23.Hacein-Bey L, Blazun JM, Jackson RF. Carotid artery pseudoaneurysm after orthognathic surgery causing lower cranial nerve palsies: endovascular repair. J Oral Maxillofac Surg 2013;71:1948–55 [DOI] [PubMed] [Google Scholar]
- 24.Chen JB, Sun H, Zhou LX, et al. Successful endovascular treatment of carotid aneurysms in a patient with vascular Ehlers–Danlos syndrome. J Neurol Surg A Cent Eur Neurosurg 2013;74(Suppl 1):e85–8 [DOI] [PubMed] [Google Scholar]
- 25.Bien AG, Cress MC, Nguyen SB, et al. Endovascular treatment of a temporal bone pseudoaneurysm presenting as bloody otorrhea. J Neurol Surg Rep 2013;74:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinar C, Bozkaya H, Parildar M, et al. Endovascular management of vascular injury during transsphenoidal surgery. Interv Neuroradiol 2013;19:102–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amenta PS, Starke RM, Jabbour PM, et al. Successful treatment of a traumatic carotid pseudoaneurysm with the pipeline stent: Case report and review of the literature. Surg Neurol Int 2012;3:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadkhodayan Y, Shetty VS, Blackburn SL, et al. Pipeline embolization device and subsequent vessel sacrifice for treatment of a bleeding carotid pseudoaneurysm at the skull base: a case report. J Neurointerv Surg 2013;5:e31. [DOI] [PubMed] [Google Scholar]
- 29.Tsang AC, Leung KM, Lee R, et al. Primary endovascular treatment of post-irradiated carotid pseudoaneurysm at the skull base with the pipeline embolization device. J Neurointerv Surg 2014. pii: neurintsurg-2014-011154 [DOI] [PubMed] [Google Scholar]
- 30.Shah H, Gemmete JJ, Chaudhary N, et al. Acute life-threatening hemorrhage in patients with head and neck cancer presenting with carotid blowout syndrome: follow-up results after initial hemostasis with covered-stent placement. AJNR Am J Neuroradiol 2011;32:743–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baril DT, Ellozy SH, Carroccio A, et al. Endovascular repair of an infected carotid artery pseudoaneurysm. J Vasc Surg 2004;40:1024–7 [DOI] [PubMed] [Google Scholar]


