Abstract
Background & objectives:
The prerequisite of radioimmunotherapy is stable binding of a radionuclide to monoclonal antibodies, which are specific to the tumour-associated antigen. Most B-cell lymphomas express CD20 antigen on the surface of the tumour cells, making it a suitable target for therapeutic radioactive monoclonal antibodies. In the present study, the immunoconjugate of biosimilar Rituximab (Reditux™) and macrocyclic chelator, p-SCN-Bz-DOTA, was prepared and radiolabelled with Lutetium-177 followed by quality control procedures.
Methods:
Rituximab(BioSim) was desalted with sodium bicarbonate (0.1M, pH 9.0) and incubated with DOTA-SCN (1:50). The effectiveness of the conjugation was evaluated by determining the number of chelators per antibody molecule. This conjugate was radiolabelled with Lutetium-177 and purified using PD10 column. The quality control parameters like pH, clarity, radiochemical purity, in vitro stability and sterility were studied. Immunoreactivity of 177Lu-DOTA-Rituximab (BioSim) was assessed using RAMOS cells. The radioimmunoconjugate (RIC) after stringent quality assurance was injected in three patients and the biodistribution profile was analysed.
Results:
An average of 4.25 ± 1.04 p-SCN-Bz-DOTA molecules could be randomly conjugated to a single molecule of Rituximab (BioSim). The radiochemical purity of the labelled antibody was > 95 per cent with preserved affinity for CD20 antigen. The final preparation was stable up to about 120 h when tested under different conditions. A favourable biodistribution profile was observed with liver showing the maximum uptake of the RIC.
Interpretation & conclusions:
A favourable radiochemical purity, stability and biodistribution of the radiolabelled immunoconjugate indicate that clinical trials for evaluation of toxicity and efficacy of 177Lu-DOTA-antiCD20 antibody-Rituximab (BioSim) in patients of relapsed and refractory non Hodgkin's lymphoma can be considered.
Keywords: CD20, Lutetium-177, mAb, NHL, radioimmunotherapy, Rituximab (BioSim)
In radioimmunotherapy (RIT), monoclonal antibodies (mAbs) are attached to a therapeutic radioisotope where these antibodies act as a carrier and target tumour cells1. RIT is also said to be more advantageous as compared to unlabelled therapeutic antibodies, given the additive effect of radiation-induced cytotoxicity and the ability of the associated radioactivity to kill the adjoining cancerous tumor cells that may not have bound the radiolabelled antibody2.
Non Hodgkin's lymphoma (NHL), being an inherently radiosensitive malignancy has provided the basis for RIT. In 2002, Yttrium-90 labelled ibritumomab tiuxetan (Zevalin; Biogen Idec, Inc., Cambridge, MA, USA) was approved by the United States Food and Drug Administration (FDA) for the treatment of patients with relapsed/refractory low-grade or follicular non-Hodgkin's lymphoma, or transformed B-cell NHL that did not respond to treatment with rituximab followed by Iodine-131 labelled tositumomab (Bexxar; Corixa Corp, Seattle, WA, USA) in 2003. However, the RIT with these murine antibodies was often limited by the development of human anti-mouse antibodies (HAMA), the relative inability of mouse antibodies to recruit human immune effector mechanism for tumour killing and subsequent downregulation of target the antigen. To overcome these limitations, antibodies were genetically engineered to produce “chimeric” and “human” antibodies are developed and used that mimic human antibodies more closely3,4,5. Rituximab is commercially available (as Rituxan in USA and as MabThera in Europe) chimeric mouse/human IgG1 monoclonal antibody directed against the B cell-specific transmembrane antigen CD20 expressed on pre-B and mature B lymphocytes and is approved for the treatment of B-cell NHL resistant to other chemotherapy treatments5.
Radionuclides such as 131I, 90Y, 188Re (Rhenium-188) and 117Lu (Lutetium-177) have been used to radiolabel mAbs that can be employed for the RIT of neoplastic lesions. The β-emission energy of 177Lu (βmean =166 keV) is lower than other radionuclides commonly used for this therapy (β mean 131I=191 keV; β mean 90Y= 699 keV, βmean 188Re = 770 KeV)6,7. The short-range, lower-energy beta emission and adequate half-life of 177Lu allows a concentrated dispensation of its dose in small lesions and is less damaging to the surrounding normal tissues. The other notable benefits of this radioisotope are that it produces low gamma energy radiation which allows gamma imaging and can be used for dosimetric estimations in humans8,9.
Forrer et al10 have developed a freeze-dried kit formulation for the instant preparation of 177Lu-labelled Rituximab. In another study11, DOTA-NHS was used to radiolabel the anti-CD20 antibody with 177Lu. Stopar et al12 have reported on the radiolabelling of Rituximab with 99mTc. Preliminary results have been published on the biokinetics of 188Re-labelled anti-CD20 mAb in patients13. A biosimilar Rituximab (Reditux™) is now commercially available in several countries. Therefore, it is important to demonstrate that Reditux exhibits similar imaging and RIT characteristics as the original Rituximab preparation. In the present study, a simplified protocol was used to label Rituximab (BioSim) with 177Lu. The objective was to show whether the 177Lu-labelled biosimilar mAb can be used for the imaging and radioimmunotherapy of relapsed and refractory B-cell NHL in humans.
Material & Methods
Patients: Three patients attending the Medical Oncology outpatient department of Institute Rotary Cancer Hospital, All India Institute of Medical Sciences (AIIMS), New Delhi, India, with documented B cell non Hodgkin's lymphoma were selected for the study. The duration of the study was from June 2011 to August 2012. Patients with relapsed or refractory NHL to the conventional lines of chemotherapeutic regimen, < 25 per cent lymphoma involvement of the bone marrow based on histopathology, Karnofsky performance status (KPS) of greater than 60, platelet count > 1 × 109 cells/l, WBC count > 2500/µl and not having received any treatment for at least four weeks were included in the study. Refractory disease was defined as failure to achieve partial response (PR) or complete response (CR) after at least first line of conventional chemotherapeutic regimen. Recurrence of lesions within 6 months after achieving complete response to conventional chemotherapeutic regimen was categorized as relapse. Pregnancy, lactation and terminal illness (anticipated life expectancy of less than 3 months) were the exclusion criteria. The patients were administered with single dose of 177Lutetium-Rituximab (BioSim) in the department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi. Written informed consent was obtained from all the patients participating in the study. The study protocol was approved by institutional ethics committee.
Radioisotope and chemicals: High purity Lutetium chloride (177LuCl3) was obtained from Bhabha Atomic Research Center, Atomic Energy Regulatory Board, Mumbai, India. The specific activity was > 540mCi/mg (carrier free) and radionuclidic purity > 99 per cent. Anti-CD20 antibody Rituximab (BioSim; Reditux™) was obtained as solution (10 mg/ml) from Dr Reddy's Laboratories, Hyderabad, India and was used without purification. The product is a “biosimilar” to Rituximab and not same as RITUXAN® or MABTHERA®. The bifunctional chelating agent 4-isothiocyanate-benzyl 1,4,7,10-tetraazacyclododecane-N’, N”, N”’,N”” tetraacetic acid (p-SCN-Bz-DOTA) was obtained from Macrocyclics Inc., Richardson, TX, USA (stock # B205). Other chemicals such as ammonium acetate, sodium bicarbonate, phosphate buffer components, methanol, sodium carbonate, ascorbic acid, sodium carbonate, Folin-Colciteau reagent, EDTA, acetonitrile (ACN), Di-ethyltriaminepenta-acetic acid (DTPA), citric acid, metal free water were all of AR grade and were used without further purification.
Radio-chromatography was performed using thin layer chromatography (TLC) scanner (Bioscan AR2000, Paris, France). Analytical high performance liquid chromatography (HPLC) was performed on GE Akta Purifier (GE Healthcare Life Sciences, USA) consisting of injector, Iso pump and VWD detector connected to Bioscan radioactive detector. Gamma counter (Biodex, New York, USA) was used for counting the TLC strip segments.
Conjugation of DOTA-SCN to Rituximab (BioSim): The labelling procedure was performed in Radiochemistry unit of the Nuclear Medicine department, AIIMS, New Delhi, under strict aseptic and sterile conditions. One ml Reditux (10 mg/ml) was loaded on the PD10 Desalting column (GE Healthcare, Buckinghamshire, UK) and collected using 0.1M NaHCO3 (pH 9) as eluent to exchange the buffer. Antibody (6 mg) was incubated with p-SCN-Bz-DOTA in different molar ratios (1:10; 1:50) for 30 min at 37°C in the thermomixer. Following incubation, the conjugation mixture was purified using another PD10 column (with 0.25M ammonium acetate, pH 5-5.5) to remove unconjugated DOTA-SCN. The resulting solution was filtered using 0.22μm millipore filter and stored at 4° C for labelling with radioisotope later. No centrifugation was required for the synthesis of immunoconjugate.
Determination of number of chelators per antibody: The number of p-SCN-Bz-DOTA molecules attached to a single molecule of antibody was determined using natural/cold LuCl3. Further, 450μl of purified Rituximab (BioSim)-DOTA-SCN solution (225μg Rituximab (BioSim) -1.5 nmol) was incubated with 10MBq 177LuCl3 in the first tube, 10MBq 177LuCl3 and 10 μl natLuCl3 (3 nmol) in the second tube and 10MBq 177LuCl3 and 20 μl natLuCl3 (6 nmol) in the third tube. All the three tubes were incubated for 30 min at 37°C in thermomixer. After 25 min, 25 μl of 0.05M EDTA was added in each tube and was further incubated for 5 min. The samples from the tubes were spotted on the TLC silica gel strips (stationary phase) and the strips were developed in the 20mM citric acid: 10 per cent ACN (mobile phase). The strips cut at a Rf of 0.5 were assayed for radioactivity and the amount of intact chelate was determined. Using the counts, percentage of 177Lu coupled to the mAb and equivalent to mAb was calculated.
Radiolabelling of the immunoconjugate with 177Lu: The sterile solution of 177LuCl3 was buffered with 0.5M NH4OAc (pH-5.5) to 177Lu-acetate; 1480 - 1850 MBq 177Lu-acetate was subsequently added to the immunoconjugate and pH was maintained at 5-5.5. The reaction mixture was incubated at 37°C for 30 min in the thermomixer. At 25 min, 50 μl of 0.05M EDTA was added to the sample and incubated for another 5 min. Purification of radiolabelled immunoconjugate for free 177Lu and 177Lu-DOTA impurities was done by size exclusion chromatography on a PD10 column (in ascorbic acid). Fractions were collected and radioactivity of each fraction was measured. The presence of protein in each fraction was determined using fast protein assay method14,15. Folin-Colciteau reagent (20 µl) was added in each fraction14,15. The fractions were analysed subjectively for the intensity of cyan colour and objectively for the amount of radioactivity. The fraction containing most intense blue colour and maximum radioactivity was selected for further quality control procedures.
Radiochemical purity determination: Radiochemical purity controls were performed using TLC and size exclusion HPLC. TLC of the purified radioimmunoconjugate (RIC) was performed using Silica gel strips (ITLC- SG, Pall Corporation, Ann Arbor, USA) and 20 mM citric acid : 10 per cent ACN as the solvent. The radiochemical purity (RCP) and retention factor (Rf) were assessed by calculating the area under the curve using the “Winscan” software Eckert and Ziegler. HPLC analyses were performed for the product synthesised and similar control experiments were set up for 177LuCl3, 177Lu-DOTA, using a TSK-Gel G3000SWXL size-exclusion column 7.8×300 mm, pre-equilibrated with 0.05M phosphate buffer, pH 7.4, at a flow rate of 0.5 ml/min. The column eluent was passed through a UV detector (detection wavelength 280 nm and 254 nm) and then through a radioactivity detector.
In vitro stability: In vitro stability studies for radioimmunoconjugate were performed by three methods:
(i) Periodic stability testing - Stability of 177Lu-DOTA-SCN-Rituximab (BioSim) was determined by storing the final solution at 4°C for 6 days and performing frequent TLC analysis to determine the radiochemical purity using the procedure described above. TLC analysis was performed to monitor any degradation or presence of other impurities.
(ii) Stability testing of radiolabelled compound in human serum - Human serum (1 ml) samples from healthy volunteers and lymphoma patients were incubated with 37MBq of RIC at 37°C and TLC analysis was performed at regular intervals for six days to check for any dissociation of the complex.
(iii) DTPA challenge - 177Lu-DOTA-SCN-Rituximab (BioSim) solution was incubated with different concentrations (25, 50, 100 mM) of DTPA for 120 h at 37°C and regular TLC analysis was performed to determine the stability of the complex.
Immunoreactivity: The immunoreactivity check of 177Lu-labelled biosimilar mAb was done by the method described by Lindmo et al16 with RAMOS cell suspension (procured from American Type Culture Collection, USA). The cell suspension (2.0 × 107 cells; 3.5 ml) was washed twice with 15ml BSA/PBS by centrifuging the cells at 250 g for 5 min. The cells were then reconstituted to a total volume of 3.5 ml with BSA/PBS solution. The binding assay was performed in triplicate with five cell concentrations (0.5×107, 0.25×107, 1.3×106, 6.3×105, 3.13×105 cells/ml) and a control for non-specific binding using a cell concentration of 3.13×105 cells/ml. The non specific binding was determined by saturation of the binding sites by addition of 2 μl cold Rituximab (BioSim) at a concentration of 5 mg/ml to the control tube. 177Lu-DOTA-Rituximab (BioSim) was diluted to a concentration of 10-15 ng/ml and 0.5 ml (5-7.5 ng) of the labelled antibody was added to all the tubes. The tubes were placed in the head over head rotator for overnight at 4°C. After incubation, cell pellets were separated from the supernatants by centrifugation at 250 g for 5 min, and 0.5 ml of the supernatant was removed from the tubes. The tubes with the pellets and supernatant were separately counted in gamma well counter (Biodex, USA) to calculate the bound activity. The immunoreactive fraction of the RIC was determined using a double inverse plot of total applied radioactivity over specific bound radioactivity against the reciprocal of the number of cells. This plot gives a straight line where the reciprocal of the y- intercept on the ordinate equals immunoreactive fraction.
Apyrogenicity: Testing for pyrogenicity was performed on single-patient doses. The level of pyrogenicity in 177Lu-DOTA-SCN-Rituximab (BioSim) sample was evaluated by the Pyrogen Plus limulus amoebocyte lysate kit (Charles River, Boston, MA, USA).
Biodistribution studies in patients: The patients were administered an intravenous (iv) infusion of “cold” Rituximab (BioSim) calculated on the basis of 375 mg/m2 under close supervision in day care facility1. Within 4 h of completing the “cold” antibody infusion, 50 mCi (1850 MBq) of 177Lu-DOTA-SCN-Rituximab (BioSim) was administered as slow iv infusion. Serial imaging was done for the patients on a dual head gamma camera GE, Millenium VG, Milwaukee, USA and whole body scans were acquired at the speed of 15 cm/h. Regions of interest (ROI) were drawn manually over the source organs. ROIs data were quantified by using geometric mean of anterior and posterior whole body scan with geometric based background subtraction method. As a result of geometric mean and background correction, time dependent per cent injected activity (% IA) for various organs was calculated.
Results
An average 1-1.5 molecule of p-SCN-Bz-DOTA could be randomly conjugated to one Rituximab (Biosim) molecule when the chelator to antibody ratio was 1:10. This concentration was not found to be sufficient for prompt labelling with Lu-177. At the molar ratio of 1:50, the stoichiometry of 4.25 ± 1.04 DOTA-SCN molecules attached to each antibody molecule was observed. The final radioimmunoconjugate was a clear solution with no particulate matter or milky appearance. The pH of the final product was 5- 5.5.
Radiochemical purity analyses: Labelling yield of 177Lu-DOTA-SCN-Rituximab (BioSim) ranged from 78-80 per cent. TLC analysis showed radiochemical purity of > 95 per cent after purification with PD10 column. In the present chromatography system, the radiolabelled antibody retained at the origin whereas free 177Lu and 177Lu-DOTA migrated with the solvent front. The retention factor (Rf) of 177Lu-DOTA-SCN-Rituximab (BioSim) was determined to be 0.01 – 0.15 (Fig. 1A) whereas Rf of free 177Lu and 177Lu-DOTA was 0.4 – 0.5 (Fig. 1B). Due to high molecular mass, the retention time of the radiolabelled antibody was less whereas the low molecular weight 177Lu and 177Lu-DOTA species were eluted later when the sample was loaded on the gel filtration HPLC column. Single 177Lu-DOTA-SCN-Rituximab(BioSim) peak was obtained at 15–15.5 mins in the UV detector (Fig. 2A) which corresponded to the peak in the radioactive detector (Fig. 2B). This confirmed the successful labelling of 177Lu to the DOTA-SCN-Rituximab (BioSim) conjugate. 177Lu-DOTA and free 177Lu were eluted at 25.5 – 26 min (UV detector) and 26 – 26.5 min (radioactive detector). The product had high specific activity of ~540 mCi/mg as the labelling procedure was performed on the same day of 177Lu delivery.
Fig. 1.
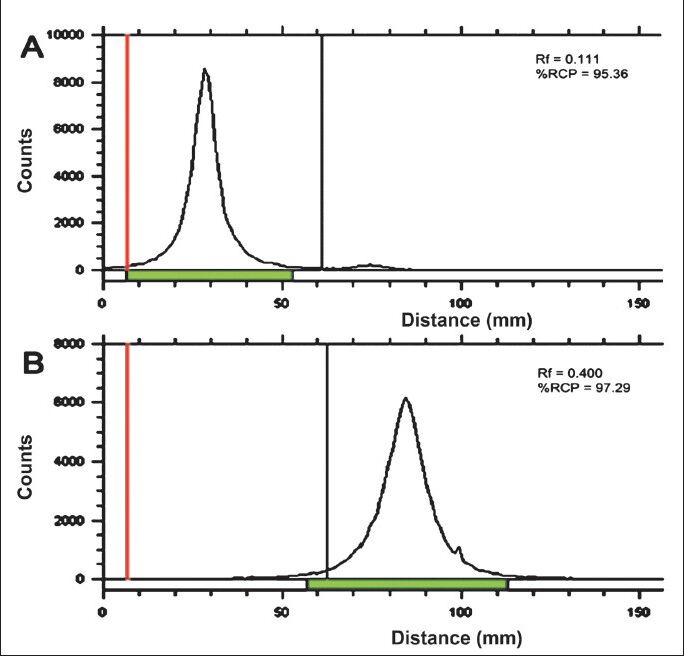
Thin layer chromatography (TLC) of the purified radioimmunoconjugate (RIC) showing the retention factor (Rf) = 0.01 - 0.15 (A). The free 177Lu and 177Lu-DOTA had the Rf = 0.4 - 0.5 (B).
Fig. 2.
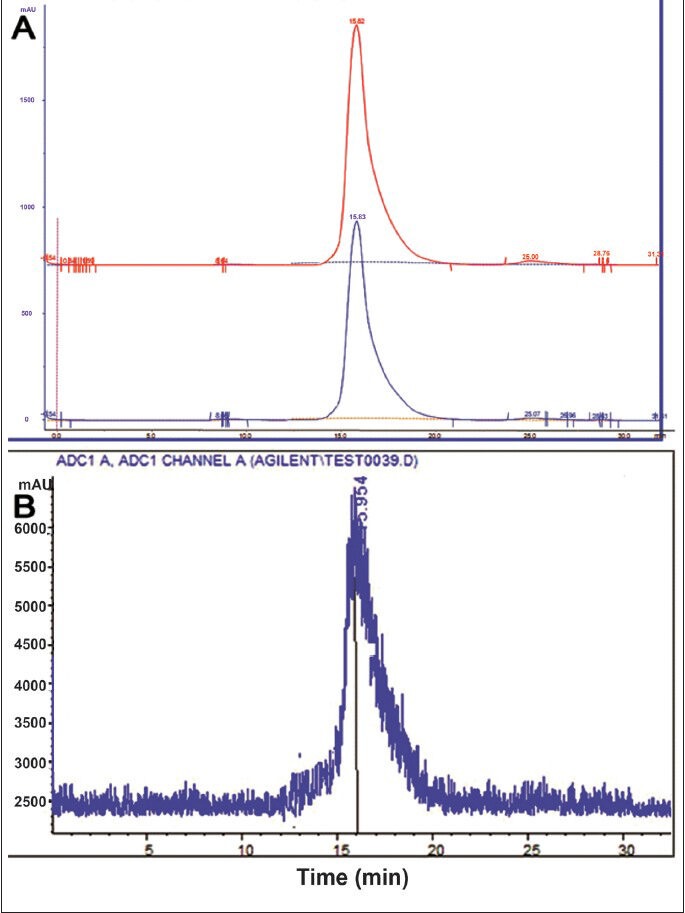
HPLC UV profile of DOTA-SCN-Rituximab (BioSim) immunoconjugate showed the peak at 15-16 min (A). The radiolabelled immunoconjugate peak also corresponded at the same time (B).
Stability: In vitro stability of the 177Lu-DOTA-SCN-Rituximab (BioSim) when tested by TLC by periodic sampling showed that metal ion was intact with the immunoconjugate under physiological conditions. Stability was found to be > 95 per cent at multiple time points upto 120 h (Fig. 3). After incubation of radiolabelled antibody with freshly prepared serum, 95-98 per cent of radioactivity was bound to the antibody upto sixth day with no evidence for either degradation or transchelation of 177Lu to other serum proteins. No significant difference was found in the percentage dissociation of 177Lu-immunoconjugate in the serum of healthy subjects and diseased patients (Fig. 4). 177Lu-DOTA-SCN-Rituximab (BioSim) was stable under DTPA challenge demonstrating > 95 per cent bound radioactivity even after 120 h of incubation. (Fig. 5).
Fig. 3.
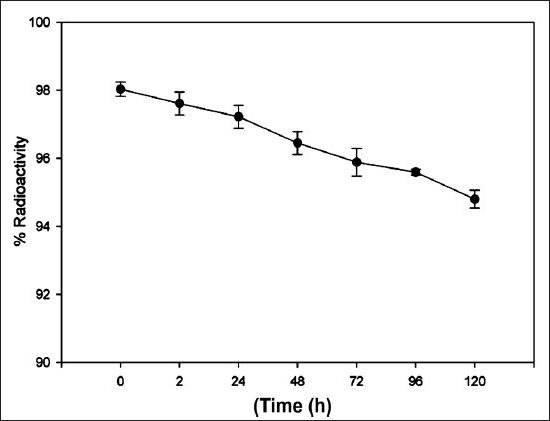
The in vitro stability profile of the 177Lu-DOTA-SCN-Rituximab (BioSim) assessed by periodic sampling. Values are mean ± SD (n = 3).
Fig. 4.
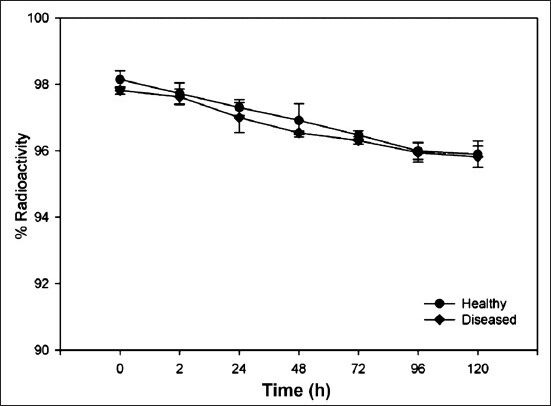
Radio-immunoconjugate stability profile in human serum from healthy volunteer (n=3) and lymphoma patients (n=3). Values are mean ± SD.
Fig. 5.
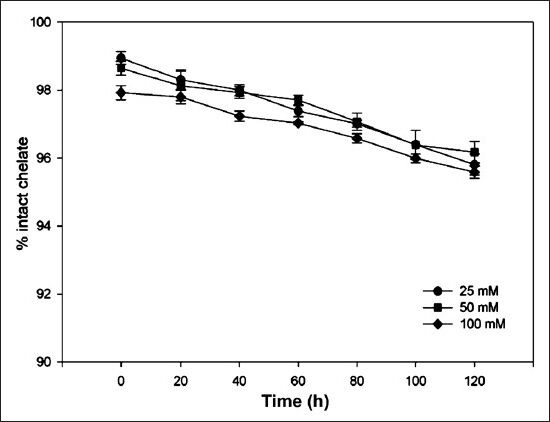
177Lu-DOTA-SCN-Rituximab (BioSim) stability profile at different concentrations of Di-ethyltriaminepenta-acetic acid (DTPA) (25, 50, 100 mM). Values are mean ± SD (n=3).
Immunoreactivity: The immunoreactivity of the mAb-conjugate showed high and specific binding ability to target cells. The cell pellets in the control tube for non-specific binding showed less than 2 per cent of the total radioactivity. This demonstrated the specific binding of 177Lu-DOTA-SCN-Rituximab(BioSim) to CD20 receptors on RAMOS cells. The results of immunoreactivity of radiolabelled rituximab are shown in Fig. 6. The binding of the immunoconjugate increased in a parabolic pattern with increasing cell concentration. The plot showed that 70 per cent of the immunoreactivity was retained by the RAMOS cells at highest cell concentration (Fig. 6A). Fig. 6B shows a double inverse plot of total/bound (T/B) as a function of inverse cell concentration 1/cells. The data showed a linear relationship between total/bound (T/B) and inverse of cell concentration described by equation y =0.3643x + 0.8957. The immunoreactive fraction was found to be r = R20.9952 = 0.7984 where R = 0.8957. The regression equation so derived was corrected for non-specific binding percentage before plotting the values.
Fig. 6.
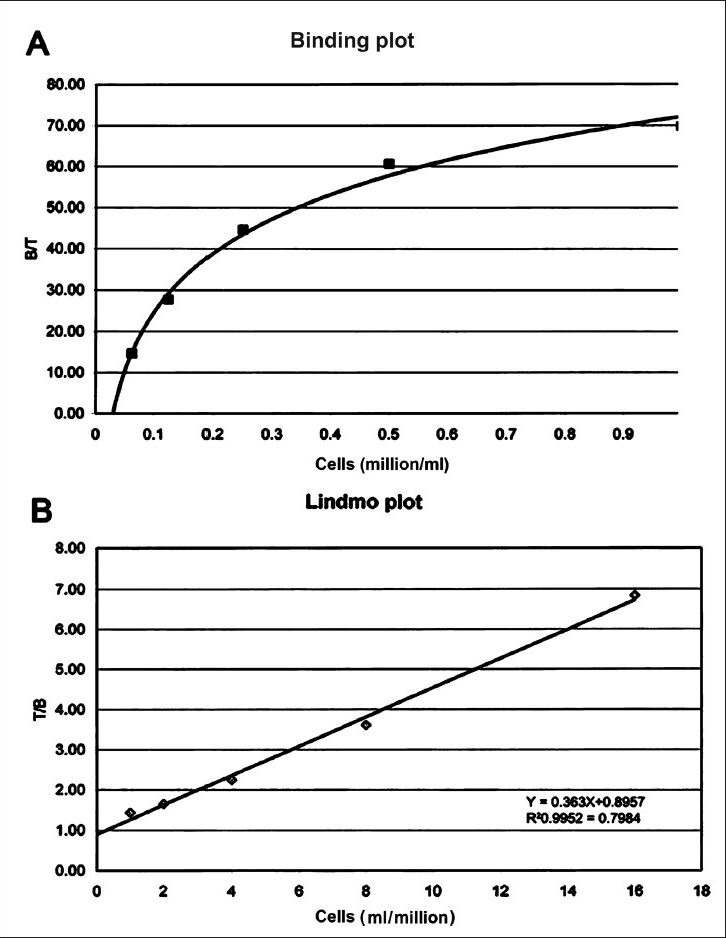
Binding plot (A) of the ratio of specifically bound radiolabelled antibody to the total applied radioactivity (B/T) as a function of cell concentration (X-axis). The cell concentration is expressed as cells (million/ml). Lindmo plot (B) showing total/bound activity as a function of inverse of cell concentration expressed as 1/cells (ml/million).
Bacterial endotoxin test: The radiolabelled doses of 177Lu-immunoconjugate were proven to be pyrogen free. Bacterial endotoxin level in the sample was < 0.1 EU/ml (permissible limit < 0.5 EU/ml)17.
Biodistribution studies: Whole body scans were acquired at 4, 24, 48, 96 and 144 h on a dual head gamma camera. The physiological uptake of 177Lu-DOTA-SCN-Rituximab was seen in liver, spleen, heart and kidneys (Fig. 7). The maximum uptake of RIC in liver was 22.0 ± 8.0 per cent observed at 96 h post injection. The peak uptakes of RIC in kidneys (3.8 ± 0.8%) and spleen (2.5 ± 1.3%) were observed at 48 h and in heart (3.5 ±1.5%) at 24 h post injection.
Fig. 7.
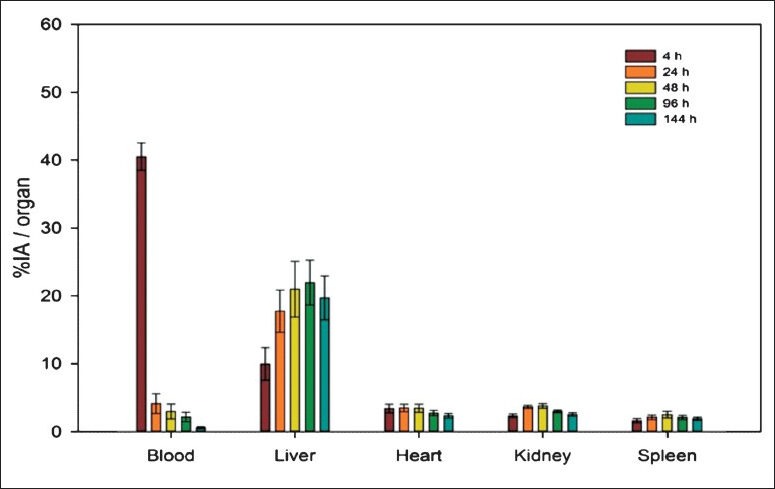
Bar diagram with error bars (mean ± SD) showing percentage injected activity (% IA) for blood, liver, heart, kidneys and spleen in the patients (n=3).
Discussion
The radiosensitivity of NHL has made them attractive targets for RIT18,19,20,21. Several mAbs directed against different antigens on the surface of B-lymphoma cells have been used in the treatment of NHL. RIT using anti-CD20 antibodies appears to be a viable alternative for the treatment of B-cell NHL patients who do not benefit completely from chemotherapy, radiotherapy or biotherapy22.
The CD20 cell surface antigen is a 33–37 kDa phosphoprotein with four transmembrane regions, a 44-amino acid extracellular loop and cytoplasmic N and C termini. It is known to regulate an early step (or steps) in the activation process for cell cycle initiation and differentiation and also functions as a calcium ion channel. The expression of CD20 antigen is lineage restricted to healthy and malignant B-cell and expressed by 95 per cent of B-cell NHLs. Also, the antigen is characteristically absent in haematopoietic stem cells and does not circulate as a free antigen in the plasma. The ligand-receptor complex is neither internalized nor shed from the plasma membrane following ligation23,24,25,26. These properties make it an ideal target for RIT.
Rituximab, an IgG1 monoclonal chimeric mouse/human antibody has the advantages of longer retention time, low toxicity, almost no development of human anti-mouse antibodies (HAMA) and very low human anti-chimeric antibodies upon repeated exposure albeit high specificity for CD20 antigen. These factors increase the likelihood of producing more effective responses than its murine counterparts. Biosimilar Rituximab (Reditux) has been approved for use in India since 2007. Reditux has been shown to have comparable safety and clinical efficacy profile as MabThera in treatment of NHL27. In the present study we designed a protocol for radiolabelling of Rituximab (BioSim) with 177Lu and assessed the biodistribution profile of the same in NHL patients.
In the existing literature, there are two different approaches for labelling the radioisotope to the antibody. In the first approach, an immunoconjugate of antibody and bifunctional chelator-DOTA is synthesized which is then labelled to the radioisotope. The other process involves conjugation of the radioisotope to DOTA followed by labelling to the antibody. In the present study, the former approach of preparing immunoconjugate, Rituximab (BioSim)-DOTA-SCN, followed by radiolabelling with Lu-177 was used.
Reditux available in solution form (10 mg/ml) was used without further concentration in the present study. Forrer et al10 had concentrated the Rituximab solution by centrifuging 3 - 5 times followed by purification of the DOTA-Rituximab immunoconjugate. The centrifugation process was omitted in our study due to the possible risk of decrease in the specificity for CD20 antigen by formation of oligomers during centrifugation.
The bifunctional chelating agent used in the study was para-isothiocyanate-benzyl-DOTA (p-SCN-Bz-DOTA), a modified form of DOTA. DTPA, DOTA-NHS and p-SCN-Bz-DOTA are the other bifunctional chelating agents that have been tried by various authors for labelling radionuclide and anti-CD20 antibody. Insufficient radiochemical purity was observed when 1–1.5 molecules of DOTA were found attached to single molecule of antibody. Modification of the experiment at the same pH 8-9 but higher concentration of DOTA (1:50) resulted in conjugation of an average of 4 molecules to every anti-CD20 antibody. The resultant radiochemical purity with (DOTA) 1-Rituximab (BioSim) was 80-85 per cent compared to 95 per cent when four molecules of DOTA were attached to single antibody. These results were comparable to existing literature10,11.
Forrer et al10 obtained a configuration of (DOTA) 4-Rituximab immunoconjugate at pH - 9.5 and rituximab concentration of 100 mg/ml. They reported the formation of (DOTA) 1-Rituximab at pH 9 and rituximab concentration of 10 mg/ml, which was insufficient for prompt labelling as well as for good therapeutic response. However, in our experiment at pH 8 - 9 and Rituximab (BioSim) concentration of 10 mg/ml, (DOTA) 4-Rituximab (BioSim) formation was observed by increasing the concentration of DOTA without changing Rituximab (BioSim) concentration.
Rigorous quality control tests were carried out to ensure the formation of RIC. TLC was performed to separate unlabelled Lutetium-177, 177Lu-DOTA and labelled 177Lu-DOTA-SCN-Rituximab (BioSim). In 20mM citric acid: 10 per cent ACN as mobile phase and Silica gel strips as stationary phase, 177Lu-labelled biosimilar mAb was retained at the origin whereas the undesirable impurities (177Lu and 177Lu-DOTA) migrated along the solvent. Audicio et al11 used two chromatography systems. First, ITLC-SG strip embedded in 5 per cent BSA as support and ethanol : NH4OH : H2O (2:1:5) as mobile phase which showed 177Lu-DOTA-anti-CD20 peak at the solvent front (Rf = 1), and second using ITLC-SG as support and sodium acetate solution (14%) as mobile phase where 177Lu-DOTA-anti-CD20 remained at the origin (Rf = 0). The advantage of the present chromatography system was that the impurities (Lu-177, 177Lu-DOTA) migrated along the solvent front whereas RIC was retained at the origin. Therefore, single chromatography system was found to be sufficient to separate the desirable from the undesirable species. HPLC was further carried out to increase the specificity of results. There was a concordance of appearance of peaks at 15 - 16 min under both UV detector and radioactive detector confirming the proper radiolabelling of the immunoconjugate.
The in vitro stability of the RIC was studied using periodic sampling, DTPA challenge and incubation in human serum for six days. The results revealed that RIC was stable without any significant degradation upto one physical half-life of radioisotope. These results were adequate to proceed with the biological evaluation of radiolabelled anti-CD20 and were also comparable to the results of other studies10,11.
The RIC did not show significant non-specific binding to the RAMOS cells. Regarding the immunoreactivity, the RAMOS cells showed preserved affinity of approximately 70 per cent at cell concentration of 0.5 × 106 cells/ml, which was also comparable with existing studies. The result of double-inverse plot was a straight-line equation y = 0.3643x + 0.8957, where y was inverse of binding ratio and x was inverse of cell concentration.
The method of labelling DOTA-SCN-Rituximab (BioSim) with Lu-177 was found to be novel and simple. The product was synthesized in a relatively shorter time duration and the results of this modified method were comparable with that reported by others10,11.
The RIC prepared by this method was injected in the patients of relapsed and refractory lymphoma for the assessment of biodistribution. There was a rapid clearance of RIC from intravascular compartment in the first 24 h with progressively increasing uptake in the body organs. Hepatobiliary route was the predominant pathway of elimination of the RIC as evident by high liver uptake. The liver demonstrated a gradual increase in uptake of RIC from 10 per cent at 4 h to a peak uptake of 22 per cent at 4th day post administration followed by a gradual decrease thereafter. Spleen and kidneys showed a relatively similar pattern of biodistribution with peak uptake of approximately 3-4 per cent at 2nd day. There was no abnormally increased tracer uptake of RIC in skeleton in the post-therapy scans upto 6th day of imaging reflecting absence of unconjugated 177Lu in the final product as well as in vivo stability of the compound. The biodistribution pattern noticed was comparable to that in the published literature10,28. However, biodistribution of RIC was studied in a limited number of subjects and, therefore, cannot be generalized to all patients due to a great degree of individual variation, which was a limitation of this study.
In conclusion, our results show that anti-CD20 antibody-Rituximab (BioSim) can be labelled with Lutetium-177 by a shorter and simple method with quality control using single chromatography system. Further experimentation in clinical trials with this novel RIC is warranted considering the in vitro results of radiochemical purity, in vitro stability and immunoreactivity of 177Lu-DOTA-anti CD20 antibody- Rituximab (BioSim).
References
- 1.Hagenbeek A, Lewington V. Report of a European Consensus Workshop to develop recommendations for the optimal use of 90Y-Ibritumomab tiuxetan (Zevalin) in lymphoma. Ann Oncol. 2005;16:786–92. doi: 10.1093/annonc/mdi148. [DOI] [PubMed] [Google Scholar]
- 2.Dias CR, Jeger S, Osso JA, Jr, Müller C, Pasquale C, Hohn A, et al. Radiolabeling of rituximab with 188Re and 99m Tc using the tricarbonyl technology. Nucl Med Biol. 2011;38:19–28. doi: 10.1016/j.nucmedbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Jacene HA, Filice R, Kasecamp W, Wahl RL. Comparison of 90Y-Ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med. 2007;48:1767–76. doi: 10.2967/jnumed.107.043489. [DOI] [PubMed] [Google Scholar]
- 4.Jacene HA, Filice R, Kasecamp W, Wahl RL. 18F-FDG PET/CT for monitoring the response of lymphoma to radioimmunotherapy. J Nucl Med. 2009;50:8–17. doi: 10.2967/jnumed.108.055376. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman GA, White CA, Witzig TE, Gordon LI, Emmanouilides C, Raubitschek A, et al. Radioimmunotherapy of relapsed non-Hodgkin's lymphoma with zevalin,: a 90Y labelled anti-CD20 monoclonal antibody. Clin Cancer Res. 1999;5(Suppl 10):3281s–6s. [PubMed] [Google Scholar]
- 6.Bernhardt P, Benjegard SA, Kolby L, Johanson V, Nilsson O, Ahlman H, et al. Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int J Radiat Oncol Biol Phys. 2001;51:514–24. doi: 10.1016/s0360-3016(01)01663-7. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt P, Forssell-Aronsson E, Jacobsson L, Skarnemark G. Low-energy electron emitters for targeted radiotherapy of small tumours. Acta Oncol. 2001;40:602–8. doi: 10.1080/028418601750444141. [DOI] [PubMed] [Google Scholar]
- 8.Stillebroer AB, Zegers CM, Boerman OC, Oosterwijk E, Mulders PF, O’Donoghue JA, et al. Dosimetric analysis of 177Lu-cG250 radioimmunotherapy in renal cell carcinoma patients: correlation with myelotoxicity and pretherapeutic absorbed dose predictions based on 111In-cG250 imaging. J Nucl Med. 2012;53:82–9. doi: 10.2967/jnumed.111.094896. [DOI] [PubMed] [Google Scholar]
- 9.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labelled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 10.Forrer F, Chen J, Fani M, Powell P, Lohri A, Muller-Brand J, et al. In vitro characterization of 177Lu-radiolabelled chimeric anti-CD20 monoclonal antibody and a preliminary dosimetry study. Eur J Nucl Med Mol Imaging. 2009;36:1443–52. doi: 10.1007/s00259-009-1120-2. [DOI] [PubMed] [Google Scholar]
- 11.Audicio PF, Castellano G, Tassano MR, Rezzano ME, Fernandez M, Riva E, et al. [177Lu]DOTA-anti-CD20: labeling and pre-clinical studies. Appl Radiat Isot. 2011;69:924–8. doi: 10.1016/j.apradiso.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Stopar TG, Mlinaric-Rascan I, Fettich J, Hojker S, Mather SJ. 99m Tc- rituximab radiolabelled by photo-activation: a new non-Hodgkin's lymphoma imaging agent. Eur J Nucl Med Mol Imaging. 2006;33:53–9. doi: 10.1007/s00259-005-1838-4. [DOI] [PubMed] [Google Scholar]
- 13.Torres-García E, Ferro-Flores G, Arteaga de Murphy C, Correa-González L, Pichardo-Romero PA. Biokinetics and dosimetry of 188Re-anti-CD20 in patients with non-Hodgkin's lymphoma: preliminary experience. Arch Med Res. 2008;39:100–9. doi: 10.1016/j.arcmed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Jalilian AR, Mirsadeghi L, Yari-kamrani Y, Rowshanfarzad P, Kamali-dehghan M, Sabet M. Development of [64 Cu ]-DOTA-anti-CD20 for targeted therapy. Radioanal Nucl Chem. 2007;274:563–8. [Google Scholar]
- 15.Bahrami-Samani A, Ghannadi-Maragheh M, Jalilian AR, Yousefnia H, Garousi H, Moradkhani S. Development of 153Sm-DTPA-rituximab for radioimmunotherapy. Nukleonika. 2009;54:271–7. [Google Scholar]
- 16.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 17.The United States Pharmacopeia Supplement 4a. 1984 [Google Scholar]
- 18.Press OW, Eary JF, Badger CC, Martin PJ, Applebaum FR, Levy R, et al. Treatment of refractory non-Hodgkin's lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7:1027–38. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg DM, Horowitz JA, Sharkey RM, Hall TC, Murthy S, Goldenberg H, et al. Targeting, dosimetry, and radioimmunotherapy of B-cell lymphomas with iodine-l31-labeled LL2 monoclonal antibody. J Clin Oncol. 1991;9:548–64. doi: 10.1200/JCO.1991.9.4.548. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski MS, Fig LM, Zasadny KR, Koral KF, DelRosario RB, Francis IR, et al. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labelled anti-CD37 antibody in B-cell lymphoma. J Clin Oncol. 1992;10:1696–711. doi: 10.1200/JCO.1992.10.11.1696. [DOI] [PubMed] [Google Scholar]
- 21.Press OW, Eary JF, Appelbaum FR, Martin PJ, Badger CC, Nelp WB, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329:1219–24. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 22.Juweid M, Sharkey RM, Markowitz A, Behr T, Swayne LC, Dunn R, et al. Treatment of non-Hodgkin's lymphoma with radiolabeled murine, chimeric, or humanized LL2, an anti-CD22 monoclonal antibody. Cancer Res. 1995;55(Suppl 23):5899s–907s. [PubMed] [Google Scholar]
- 23.Emmanouilides C. Current status of radioimmunotherapy for non-Hodgkin lymphoma. Haema. 2003;6:314–27. [Google Scholar]
- 24.Kaminski MS, Zasadny KR, Francis IR, Milik AW, Ross CW, Moon SD, et al. Radioimmunotherapy of B-cell lymphoma with (131I)anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329:459–65. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PW, Glennie MJ. Rituximab: mechanisms and applications. Br J Cancer. 2001;85:1619–23. doi: 10.1054/bjoc.2001.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avivi I, Robinson S, Goldstone A. Clinical use of rituximab in hematological malignancies. Br J Cancer. 2003;89:1389–94. doi: 10.1038/sj.bjc.6601187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy P, S, Sengar M, Menon H, Bagal B, Khattry N, Shridhar E, et al. A retrospective single centre analysis of safety, toxicity and efficacy of rituximab (original) and its biosimilar in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy (Abstract) Ann Oncol. 2012;23(Suppl 9):1074. [Google Scholar]
- 28.Kameswaran M, Pandey U, Samuel G, Sarma HD, Venkatesh M. Therapy. THER 02 (ORAL): Preparation and evaluation of 90Y rituximab for its potential as a radioimmunotherapeutic agent for non Hodgkin's lymphoma. Indian J Nucl Med. 2010;25:117–20. [Google Scholar]


