Abstract
Background & objectives:
Oxidative stress contributes to severity of ulcerative colitis (UC) but the status of erythrocyte antioxidant defence remains unknown. The present study was aimed to study the role of oxidative stress and antioxidant levels in erythrocytes of UC patients from north India.
Methods:
A total of 81 adult UC patients and 85 age and sex matched apparently healthy controls were included in this study. Levels of lipid peroxidation (LPO), reduced glutathione (GSH), catalase and superoxide dismutase (SOD) were measured in erythrocytes.
Results:
Mean age of UC patients was 43.5 yr (range 18-64 yr) while in the control group this was 45.3 yr (range 20-64 yr). LPO, catalase and SOD levels in UC patients were significantly increased (P< 0.05) compared to healthy controls, while GSH levels in UC patients were significantly decreased (P< 0.05) compared to healthy controls Ulcerative colitis activity score (UCAI) was 157.4±27.6 in UC patients.
Interpretation & conclusions:
Increased levels of LPO, SOD, catalase and a decreased level of GSH represent that oxidative stress plays a significant role in pathophysiology of UC. Further, the levels of LPO, GSH, catalase and SOD remained same during different UCAI.
Keywords: Catalase, inflammatory bowel disease, lipid peroxidation (LPO), oxidative stress, reduced glutathione (GSH), superoxide dismutase (SOD), ulcerative colitis
Ulcerative colitis (UC) and Crohn's disease are the types of inflammatory bowel diseases (IBD). UC mainly affects the lining of the large intestine (colon) and rectum. This disease may affect any age group, but it is most prevalent in individuals in the age groups 15-30 and 50-70 yr1. Incidence rate of UC in north India has been reported to be 6.02 cases per 100,000 inhabitants2. Although the aetiology of this inflammatory disorder remains essentially unknown, there have been significant advances in identifying genetic and environmental factors that contribute to its pathogenesis3. Stress and certain food items may trigger symptoms of this disease. It is currently thought that loss of tolerance against indigenous enteric flora is the fundamental event in the pathogenesis of UC4. Amongst the immune-regulatory factors, oxidative stress is one of the major contributing factors involved in the development of the disease and may be secondary to inflammation5. Substantial evidence suggests that chronic intestinal inflammation is associated with enhanced production of reactive oxygen and nitrogen species (ROS/RNS). The overproduction of ROS/RNS and the consequent oxidative stress and redox modulation by antioxidants, e.g. superoxide dismutase (SOD), catalase, reduced glutathione (GSH) have been demonstrated to play a critical role in the pathophysiology of UC in both experimental animals and human subjects6,7. SOD convert the superoxide anion into the easily diffusible and stable metabolite hydrogen peroxide (H2O2) and then catalase acts on H2O2 and neutralizes it into water.
The microbiota is critical for maintaining intestinal homeostasis through activation of innate immune toll like receptors8 and could also play a causal role in the impaired mucosal integrity and repair seen in UC patients. In diseases like IBD, diet is implicated as a contributing factor by having direct effects on host metabolism and/or immune responses. However, recent evidence suggests that diet also influences the composition of the microbiome9. Therefore, it can be assumed that environmental factors including diet can affect the pathogenesis of this disease. Though studies related to oxidative stress in UC have been done in different populations5,6,7, no such study has been reported from India. Therefore, this study was planned to elucidate the role of oxidative stress and antioxidants levels in erythrocytes of UC patients from India.
Material & Methods
In this study, 81 consecutively selected adults patients with UC between age 18-70 yr attending Gastroenterology Clinic in Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were enrolled in this study. Diagnosis was based on clinical, radiological, endoscopy and histological findings. Eighty five apparently healthy age and sex matched individuals were also enrolled. They were relatives of the patients who gave their consent for participation in the study. The study was done from May 2009 to December 2011. Written informed consent was obtained from all subjects. Study protocol was approved by the PGIMER, Chandigarh.
Individual with age ≥ 18 and ≤ 70 yr; confirmed UC patients on the basis of clinical, radiological, endoscopy and histological findings; and UC patients who were in remission state and on maintenance therapy with 5-aminosalicylic acid, were included in the study. Patients with hepatic changes, acute infections, other chronic inflammatory diseases and pregnancy, and those who underwent colostomy were excluded.
After 12 h of fasting, venous blood samples (5 ml) were drawn from patients and control subjects. Blood was collected in EDTA containers. Blood collected in EDTA was used immediately for the preparation of erythrocyte hemolysate.
Erythrocytes were isolated from blood collected in EDTA by centrifugation. The erythrocytes were washed thrice with ice-cold normal saline and then lysed by adding ice-cold distilled water. The haemolysate was used for measurement of oxidative stress and antioxidant levels.
Laboratory evaluation: Oxidative stress was assayed by monitoring lipid peroxidation in all the groups by the method of Ohkawa et al10. Reduced glutathione (GSH) in whole blood was estimated by the method of Ellman11. Catalase activity (mmol/min/gHb) in erythrocyte haemolysate was estimated by monitoring the decrease in absorbance at 230 nm, resulting from decomposition of hydrogen peroxide for 10 min at 37ºC12. Superoxide dismutase (SOD) was measured by Kono method and represented in I.U13.
The activity index (AI) calculated by qualified gastroenterologist for UC was expressed as follows: AI = 60 x blood stool + 13 x bowel movements + 0.5 x erythrocyte sedimentation rate - 4 x haemoglobin - 15 x albumin + 200. Index below 150, between 150 and 220, and above 220 nearly corresponded to mild, moderate, and severe disease, respectively14.
Statistical analysis: Statistical significance for LPO, GSH, catalase and SOD levels between UC patients and healthy controls, was evaluated using an unpaired two-tailed Student's ‘t’ test. ANOVA was used to compare UCAI with LPO, GSH, catalase and SOD. All statistical analyses were performed by using SPSS version 16.0 for Windows (SPSS, inc., Chicago, IL, USA).
Results
Mean age of UC patients was 43.5±19.3 yr while in control group it was 45.3±20.5 yr. There were 49 males in UC patients and 51 males in control subjects. The age range in UC patients was 18-64 yr while in controls 20-64 yr. The age and sex distribution among UC patients and controls was found to be comparable.
The levels of LPO, catalase and SOD were found to be significantly increased in UC patients compared to control (P< 0.05). The glutathione levels were significantly decreased (P< 0.05) in UC patients compared to controls (Table).
Table.
Levels of lipid peroxidase (LPO), reduced glutathione (GSH), catalase and superoxide dismutase (SOD) in patients with ulcerative colitis (UC) and controls
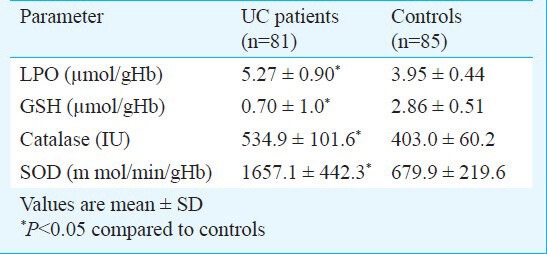
Mean ulcerative colitis activity index (UCAI) score was 157.4 ± 27.6 in UC patients. Comparison of oxidative stress and antioxidant enzyme levels with UCAI in UC patients has shown that levels of oxidative stress (LPO) and antioxidant status (GSH, catalase, SOD) were comparable at different UCAI scores.
Discussion
UC is a persistent inflammatory disease which is multifactorial in nature15. The colon is more responsive to oxidative damage because of the relatively short amount of antioxidants accessible in the mucosa. The accumulation of ROS could cause damage to specific genes involved in cell growth or differentiation or could cause changes in antioxidant enzyme levels16. Oxidative stress has been well documented in UC patients with increased ROS levels and decreased antioxidant levels in the inflamed mucosa, which ultimately contribute to chronic tissue damage17. In the present study, oxidative stress was assessed in the erythrocytes. Erythrocytes (RBCs) are more exposed to oxidative stress than other cell types due to an abundance of heme iron and oxygen, which can generate superoxides, H2O2 and lipid peroxides. The hydroperoxides themselves are not very reactive but are readily converted in the presence of free iron and electron donor molecules to hydroxyl and alkoxyl radicals, which indiscriminately inflict damage on biomolecules. Therefore, RBCs are believed to have a very effective defence mechanism against peroxides18.
Increased ROS which are produced through the oxidative burst associated with the inflammatory process in the cell may lead to severe damage to macromolecules19. Levels of LPO were significantly increased in the present study which was indicative of increased oxidative stress in UC patients as compared to controls. Similar finding have been reported from Turkish patients of UC showing increased activity of myeloperoxidase20. SOD, GSH and catalase are dominating intracellular antioxidants present or released into the circulation following disease related erythrocyte catastrophe21, their plasma/serum activities are changed by several orders of magnitude than corresponding erythrocyte levels22.
Decline in level of reduced glutathione has often been considered to be indicative of increased oxidative stress23. Thus, low level of GSH observed in UC patients may be due to increased oxidative stress in these patients. The results of the present study were similar to that reported earlier24.
In the present study, increased levels of catalase were seen in the UC patients compared to controls as has been reported earlier25. Catalase reduced the oxidative stress by converting toxic H2O2 to water.
In UC patients with onset of inflammation, levels of free radicals like superoxide anion (O-) are increased and can cause both morphological and functional damage in the cell25. The cells protect themselves against oxidative damage by enzymatic and non-enzymatic antioxidant system. Superoxide dismutase is the primary enzymatic antioxidant defence system in the cell26. Increase in SOD activity observed in UC patients indicated that antioxidant defence system was functional in these patients. Similar findings of increased SOD has been reported in experimentally induced UC in albino rats27.
No significant association was observed between the level of LPO, GSH, catalase and SOD when compared with UC activity index. It shows that the level of antioxidant and oxidative stress molecules are independent of UCAI. The reason for this may be that most of the patients were in remission state.
In conclusion, this study indicated that patients with UC had increased oxidative stress and decreased reduced glutathione antioxidant. Consistent elevated levels of oxidative stress may be the reason for recurrence of active infection in these patients despite being on maintenance therapy. Therefore, they may be treated additionally with antioxidants. The major limitation of the study was that oxidative stress was not measured in colonic biopsies and compared with oxidative stress in erythrocytes.
Acknowledgment
Authors acknowledge the financial support given by the Indian Council of Medical Research, New Delhi.
References
- 1.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. JBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 2.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–90. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parray FQ, Wani ML, Malik AA, Wani SN, Bijli AH, Irshad I, et al. Ulcerative colitis: a challenge to surgeons. Int J Prev Med. 2012;3:749–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Hamouda HE, Zakaria SS, Ismail SA, Khedr MA, Mayah WW. p53 antibodies, metallothioneins, and oxidative stress markers in chronic ulcerative colitis with dysplasia. World J Gastroenterol. 2011;17:2417–23. doi: 10.3748/wjg.v17.i19.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–84. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karp SM, Koch TR. Oxidative stress and antioxidants in inflammatory bowel disease. Dis Mon. 2006;52:199–207. doi: 10.1016/j.disamonth.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res. 1978;19:1053–7. [PubMed] [Google Scholar]
- 11.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophy. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- 13.Kono Y. Generation of superoxide radical during auto oxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophysics. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 14.Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87:971–6. [PubMed] [Google Scholar]
- 15.Head KA, Jurenka JS. Inflammatory bowel disease Part 1: ulcerative colitis pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247–83. [PubMed] [Google Scholar]
- 16.Loft S, Poulsen HE. Markers of oxidative damage to DNA: antioxidants and molecular damage. Methods Enzymol. 1999;300:166–84. doi: 10.1016/s0076-6879(99)00124-x. [DOI] [PubMed] [Google Scholar]
- 17.Sturniolo GC, Mestriner C, Lecis PE, D’Odorico A, Venturi C, Irato P, et al. Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroenterol. 1998;33:644–9. doi: 10.1080/00365529850171936. [DOI] [PubMed] [Google Scholar]
- 18.Giulivi C, Davies KJ. A novel antioxidant role for hemoglobin. The comproportionation of ferrylhemoglobin with oxyhemoglobin. J Biol Chem. 1990;265:19453–60. [PubMed] [Google Scholar]
- 19.Ferguson LR. Chronic inflammation and mutagenesis. Mutat Res. 2010;690:3–11. doi: 10.1016/j.mrfmmm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Baskol M, Baskol G, Koçer D, Ozbakir O, Yucesoy M. Advanced oxidation protein products: a novel marker of oxidative stress in ulcerative colitis. J Clin Gastroenterol. 2008;42:687–91. doi: 10.1097/MCG.0b013e318074f91f. [DOI] [PubMed] [Google Scholar]
- 21.Tavazzi B, Di Pierro D, Bartolini M, Marino M, Distefano S, Galvano M, et al. Lipid peroxidation, tissue necrosis, and metabolic and mechanical recovery of isolated reperfused rat heart as a function of increasing ischemia. Free Radic Res. 1998;28:25–37. doi: 10.3109/10715769809097873. [DOI] [PubMed] [Google Scholar]
- 22.Snyder LM, Fortier NL, Leb L, McKenney J, Trainor J, Sheerin H, et al. The role of membrane protein sulfhydryl groups in hydrogen peroxide-mediated membrane damage in human erythrocytes. Biochim Biophys Acta. 1988;937:229–40. doi: 10.1016/0005-2736(88)90245-3. [DOI] [PubMed] [Google Scholar]
- 23.McLennan SV, Heffernan S, Wright L, Rae C, Fisher E, Yue DK, et al. Changes in hepatic glutathione metabolism in diabetes. Diabetes. 1991;40:344–8. doi: 10.2337/diab.40.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Nieto N, Torres MI, Fernández MI, Giron MD, Ríos A, Suárez MD, et al. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820–7. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- 25.Deaciuc IV, D’Souza NB, Sarphie TG, Schmidt J, Hill DB, McClain CJ. Effects of exogenous superoxide anion and nitric oxide on the scavenging function and electron microscopic appearance of the sinusoidal endothelium in the isolated, perfused rat liver. J Hepatol. 1999;30:213–22. doi: 10.1016/s0168-8278(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 26.Galeotti T, Masotti L, Borrello S, Casali E. Oxy-radical metabolism and control of tumour growth. Xenobiotica. 1991;21:1041–51. doi: 10.3109/00498259109039544. [DOI] [PubMed] [Google Scholar]
- 27.Kanodia L, Borgohain M, Das S. Effect of fruit extract of Fragaria vesca L. on experimentally induced inflammatory bowel disease in albino rats. Indian J Pharmacol. 2011;43:18–21. doi: 10.4103/0253-7613.75660. [DOI] [PMC free article] [PubMed] [Google Scholar]


