Abstract
Background & objectives:
Atrial fibrillation (AF) is a common complication after acute myocardial infarction (AMI) and associated with increased morbidity and mortality. Previous studies identified high white and red blood cell count as potential risk factors for new onset AF. The objective of this retrospective, nested case-control study was to examine the association of different parameters of the blood count with the development of new onset of AF after AMI.
Methods:
A total of 66 consecutive patients with new onset AF after AMI and 132 sex and age matched controls were enrolled into the study and analyzed whether parameters of the blood count, including leukocytes, platelets, haemoglobin, haematocrit or erythrocyte count, are associated with the occurrence of AF after AMI. All AMI patients had undergone coronary angiography.
Results:
Patients with post-AMI AF displayed significantly higher levels of haemoglobin (14.2 g/dl, IQR 12.4-15 vs. 12.9 g/dl, IQR 11.7-13.8; P< 0.001), haematocrit (41.7 %, IQR 36.6-44.3 vs. 38.7 %, IQR 34.7-41.5; P 0.0015), and erythrocyte count (4.6 T/l, IQR 4.1-5 vs. 4.2 T/l, IQR 3.9-4.65; P< 0.001). In the unadjusted and adjusted logistic regression analysis, the blood parameters most strongly associated with the outcome were serum haemoglobin (crude OR 2.20, 95% CI 1.40- 3.47, P 0.001; adjusted OR 3.82, 95% CI 1.71- 8.54, P 0.001) and erythrocyte count (crude OR 2.10, 95% CI 1.36-3.22, P 0.001; adjusted OR 3.79, 95% CI 1.73- 8.33, P 0.001), whereas haematocrit did not reach statistical significance.
Interpretation & conclusions:
This study shows a significant independent association between serum haemoglobin, haematocrit, erythrocyte count and occurrence of AF after AMI. However, the pathophysiologic mechanism underlying these associations and its potential clinical applicability need to be further elucidated.
Keywords: Atrial fibrillation, myocardial infarction, red blood cell count
Atrial fibrillation (AF) is a common complication of acute myocardial infarction (AMI)1. New onset AF is associated with increased long-term morbidity as well as in-hospital and long-term mortality2,3,4,5,6,7,8. Although AF post AMI is a well described adverse prognostic marker, data about predisposing factors for the development of AF post AMI are scarce. So far, symptomatic heart failure, elevated heart rate at the time of hospital admission and advanced age have been detected as significant clinical predictors for new onset AF in patients with AMI2,6,9,10. Besides these clinical risk predictors, there is limited information about blood parameter associated with the development of AF.
Previous studies analyzing risk factors of new onset AF predominantly focused on patients after cardiac surgery and revealed a significant association of high white blood cell count (WBC)11,12,13,14,15,16 and elevated red blood cell count (RBC)16,17 with a greater risk of AF. Recently, Rienstra et al18. demonstrated a significant relationship between WBC and the incidence of new onset AF in a community-based study population derived from the Framingham Heart Study. However, data on RBC count and AF are scarce. Smaller studies identified an association between elevated haematocrit levels and AF in patients with polyuria19 and mitral stenosis20, but the impact of the blood count on the development of new onset AF in patients with AMI has not been investigated yet. Therefore, the objective of this nested case-control study was to examine the association of different parameters of the blood count with the development of new onset of atrial fibrillation after acute myocardial infarction.
Material & Methods
We retrospectively evaluated consecutive patients admitted to the Vienna General Hospital, a university affiliated tertiary care center, Austria, with a diagnosis of AMI (ICD-10: I21) between January 2003 and December 2011. Due to the observation that there is no difference in the incidence of new onset AF in AMI patients with and without ST-segment elevation,21 all patients with diagnosed AMI were enrolled in the present study. Patients with new onset AF (ICD-10: I48.1 and I48.10) within 48 h after hospital admission were subjected to further analyses.
All AMI patients admitted to the Cardiology department are monitored with a 4 lead ECG for at least the first 48 h. Additionally, post-AMI AF episodes had to be documented with a 12-lead ECG. Patients without post-AMI AF served as controls, whereas patients with known episodes of AF prior to AMI were excluded from this study. Diagnosis of AMI was consistent with the consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction22. Identifying suitable patients for this study was performed using an electronic clinical data repository. Two independent physicians confirmed suitability for study inclusion of all identified patients by chart review.
Post-AMI AF patients were matched in a 1:2 ratio with AMI patients without AF. Matching was performed on the basis of age, gender and year of hospitalization. Classical cardiovascular risk factors were recorded according to guidelines. Patients were classified as diabetics if they received medication for diabetes. Hypertension was assumed to be present in patients, if their blood pressure was above 140/90 mm Hg in at least two measurements or if they were already on antihypertensive medication. Fresh blood samples were analyzed according to local laboratory standard procedure. Left ventricular ejection fraction (LVEF) was calculated using the Simpson method according to the American Society of Echocardiography/European Association of Echocardiography (ASE/EAE) guidelines23 and categorized into normal (> 55%), mildly reduced (45-54%), moderately reduced (30-44%) and severely reduced (< 30%). Left atrial (LA) size was measured at the end-ventricular systole in an apical 4-chamber view according to the ASE/EAE guidelines23. The antegrade radiocontrast flow of the infarct-related artery was assessed on the final coronary angiogram by the operator with the use of thrombolysis in myocardial infarction (TIMI) criteria.24 The study protocol was approved by the institutional review board of the Medical University of Vienna.
Statistical analysis: Discrete data were presented as counts and percentages and compared using chi-square test. Continuous data were presented as medians and inter-quartile ranges (IQR) and compared using the Mann-Whitney test. Conditional logistic regression analysis was performed to calculate odds ratios (OR) associated with an increase of one IQR of laboratory measurement. Multivariate confounder-adjusted odds ratios were obtained by including those variables that were judged to be of clinical relevance and was adjusted for the following established cardiovascular risk factors: gender, age, diabetes, hypertension, and hyperlipidaemia. Potential collinearity in the multivariate model was tested using the variance inflation factor. The correctness of model-fit of the logistic regression analysis was measured using Hosmer-Lemeshow goodness-of-fit-test25. First-degree interaction was investigated using interaction terms between the independent variables and potential confounders. Effect modification was judged to be present at a false discovery rate of 0.05. P-values < 0.05 were used to indicate statistical significance. Power calculation showed that the 1:2 matched study design allowed us to detect a risk factor with an odds ratio of 2.0 or above with a power > 80 per cent at a significant level of 0.05. The STATA software package (Stata Statistical Software: Release 11, StataCorp LP, College Station, TX, USA) was used for all analysis.
Results
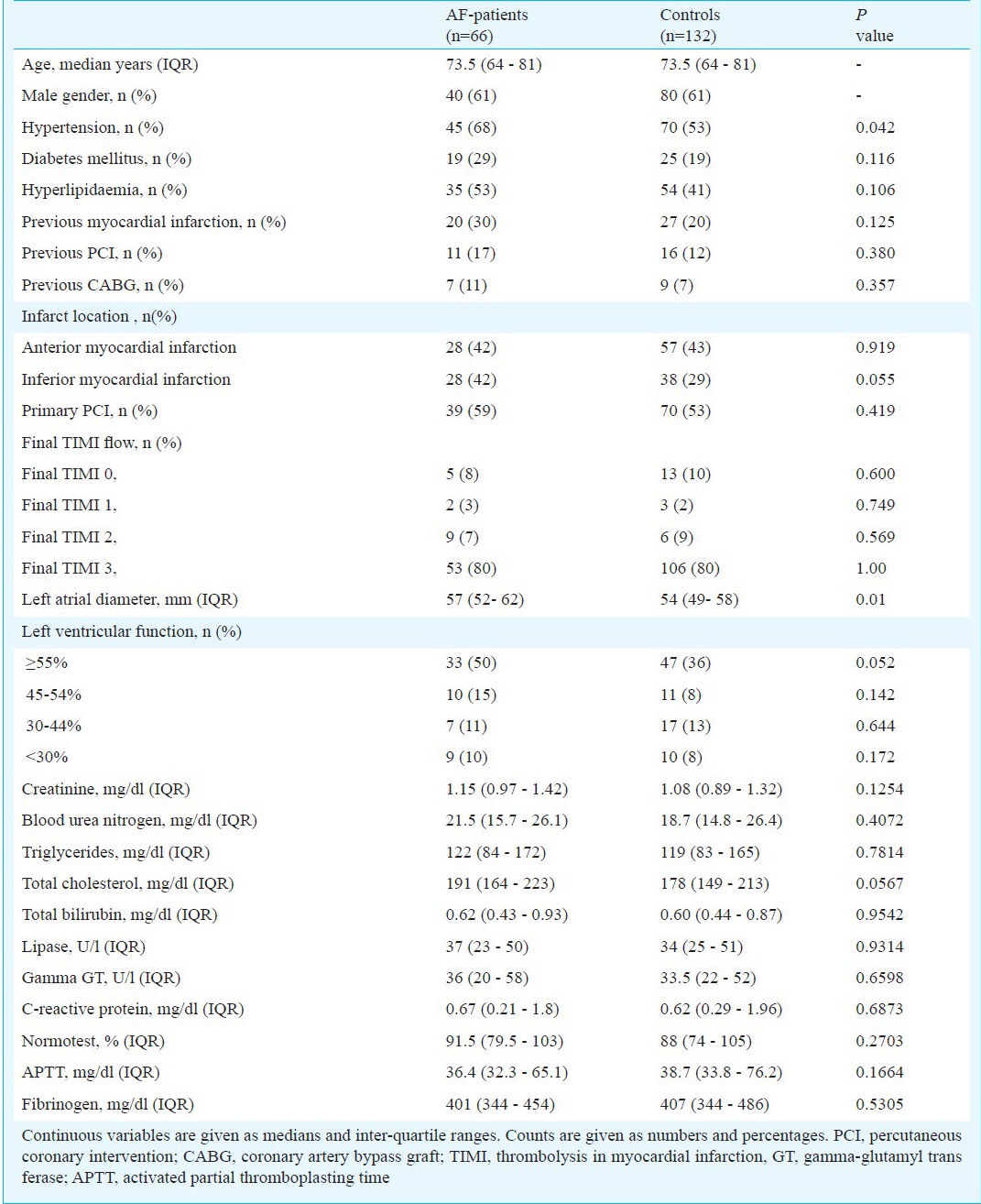
Of all patients admitted with AMI (n= 3324) between 2003 and 2011 to the Vienna General hospital, 66 fulfilled the inclusion criteria. The average age of patients with post-infarction AF was 73.5 yr (age range: 64- 81 yr), 61 per cent of them were male. Fifteen per cent of patients remained in atrial fibrillation at the time of hospital discharge. Further, 132 controls matched on age, gender, and admission year were included. All AMI patients had undergone coronary angiography. Detailed baseline characteristics are displayed in Table I.
Table I.
Baseline characteristics in atrial fibrillation (AF) patients and controls
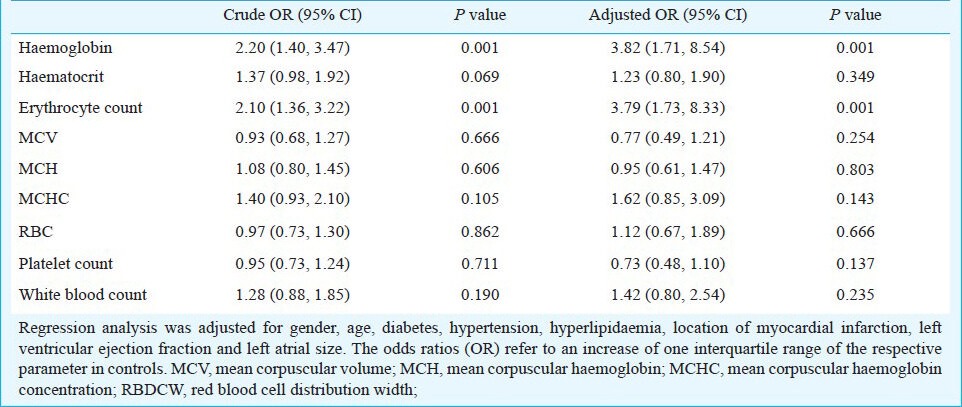
Blood count and post-infarction atrial fibrillation: Patients with post-AMI AF displayed significantly higher levels of haemoglobin (14.2 g/dl, IQR 12.4- 15 vs. 12.9 g/dl, IQR 11.7- 13.8; P< 0.001), haematocrit (41.7 %, IQR 36.6- 44.3 vs. 38.7 %, IQR 34.7- 41.5; P 0.0015), and erythrocyte count (4.6 T/l, IQR 4.1- 5 vs. 4.2 T/l, IQR 3.9- 4.65; P < 0.001). No significant difference was detected between cases and controls for leukocyte count, red cell distribution width, and thrombocytes (P 0.138, 0.123, and 0.287, respectively, Table II). As shown in Table III, the blood parameters most strongly associated with post-AMI AF in the adjusted and unadjusted model were serum haemoglobin (crude OR 2.20, 95% CI 1.40- 3.47, P 0.001; adjusted OR 3.82, 95% CI 1.71- 8.54, P 0.001) and erythrocyte count (crude OR 2.10, 95% CI 1.36- 3.22, P 0.001; adjusted OR 3.79, 95% CI 1.73- 8.33, P 0.001), whereas haematocrit did not reach significance in the logistic regression analysis. No significant interactions was seen between the aforementioned variables and hypertension, site of AMI, left atrial size (LA) size, left ventricular ejection fraction, previous MCI, and previous interventions (data not shown). Calibration of the logistic regression model was adequate using Hosmer Lemeshow goodness-of-fit-test.
Table II.
Distribution of blood count parameters in AF patients and controls
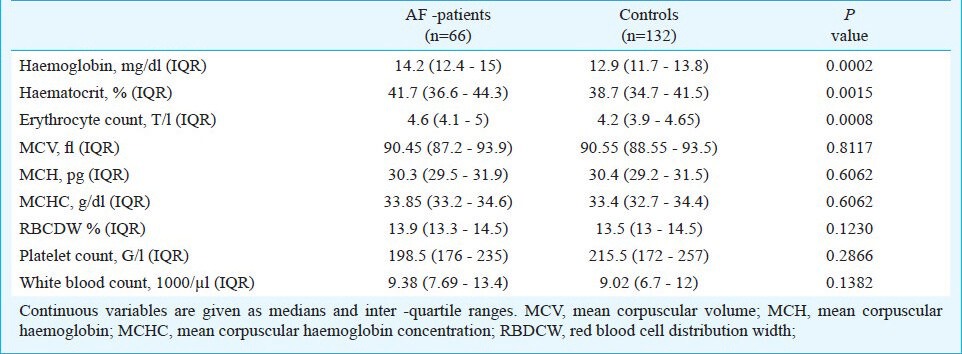
Table III.
Conditional logistic regression analysis of blood count parameters
Discussion
This study establishes a novel independent association between baseline parameters of the RBC count, including haemoglobin, haematocrit and erythrocyte count, at hospital admission and new onset AF after AMI. Furthermore, WBC was not associated with AF in AMI patients.
The post-operative occurrence of AF following cardiac surgery has been found to be associated with an increase of RBC and blood transfusion rate16,17. In line with these findings, Peverill et al20. revealed a positive correlation of haematocrit and AF in a study population of patients with mitral stenosis. Therefore, our study extends the aforementioned results by identifying an association between elevated parameters of the RBC count and the development of new onset AF in an AMI study population. Although the underlying mechanism remained unknown and a causal-effect relationship could not be proved, we identified a strong and independent association that might be of clinical relevance.
In contrast to previous studies investigating the occurrence of AF after cardiac surgery11,14, we could not detect a significantly elevated WBC in patients with new onset AF after AMI.
The main limitation of the current study was its retrospective nested case-control study design and, therefore, all potential limitations of retrospective studies applied. Although all clinical and laboratory data were recorded prospectively in an electronic clinical data repository, the data were retrospectively collected by chart review. Therefore, some potential confounders (e.g. duration of AF episode, time course of blood count) might not be available in our study population. Additionally, the observational design of the study allowed us to identify an association between parameters of the RBC count and occurrence of AF after AMI, but we were not able to indicate a causal relation. Therefore, further prospective studies are warranted to confirm these results and to establish a causal relationship. Nevertheless, since the prevalence of new onset AF is low, the nested case-control study design is a valid epidemiologic choice. Hypertension was significantly more common and LA size was significantly larger in patients with new onset AF post AMI, which might contribute to the development of AF. However, our results were more pronounced after adjustment for hypertension and LA size in the multivariate model, and, therefore, suggest a strong and independent association between blood count parameters and new onset AF post AMI. The incidence of new onset AF was lower in our study compared to other study populations3,7,8,10,26,27,28,29. A potentialexplanation might be the fact that most studies do not differentiate new onset AF from previous or recurrent AF. Therefore, it is difficult to state precise statistical data about the accurate incidence of AF post AMI. Additionally, our study inclusion criteria were strict in order to assure a valid retrospective study selection, which could have influenced the incidence of the disease.
In conclusion, this study shows a significant independent association between serum haemoglobin, haematocrit, erythrocyte count and occurrence of atrial fibrillation after acute myocardial infarction. These results are intriguing since the aforementioned parameters are broadly available and inexpensive. With regard to the high mortality and morbidity from new onset atrial fibrillation2,3,4,5,6,7,8, the identification of susceptible patients is still an important health care issue. The pathophysiologic mechanisms underlying these associations and the potential clinical applicability need to be further elucidated.
Acknowledgment
The last author (GG) was supported by an Erwin Schrödinger Fellowship of the Austrian Science Fund (FWF J 3319-B-13).
References
- 1.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–45. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 2.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: The gusto-i experience. Global utilization of streptokinase and TPA for occluded coronary arteries. J Am Coll Cardiol. 1997;30:406–13. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 3.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, et al. Atrial fibrillation and death after myocardial infarction: A community study. Circulation. 2011;123:2094–100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: A systematic review and meta-analysis. Circulation. 2011;123:1587–93. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. Trace study group. Trandolapril cardiac evalution. Eur Heart J. 1999;20:748–54. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 6.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation. 2000;101:969–74. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 7.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: The gusto-iii experience. Am Heart J. 2000;140:878–85. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 8.Zusman O, Amit G, Gilutz H, Zahger D. The significance of new onset atrial fibrillation complicating acute myocardial infarction. Clin Res Cardiol. 2012;101:17–22. doi: 10.1007/s00392-011-0357-5. [DOI] [PubMed] [Google Scholar]
- 9.Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, et al. Previously known and newly diagnosed atrial fibrillation: A major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–8. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Kinjo K, Sato H, Ohnishi Y, Hishida E, Nakatani D, Mizuno H, et al. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2003;92:1150–4. doi: 10.1016/j.amjcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–8. doi: 10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Amar D, Goenka A, Zhang H, Park B, Thaler HT. Leukocytosis and increased risk of atrial fibrillation after general thoracic surgery. Ann Thorac Surg. 2006;82:1057–61. doi: 10.1016/j.athoracsur.2006.03.103. [DOI] [PubMed] [Google Scholar]
- 13.Fontes ML, Amar D, Kulak A, Koval K, Zhang H, Shi W, et al. Increased preoperative white blood cell count predicts postoperative atrial fibrillation after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2009;23:484–7. doi: 10.1053/j.jvca.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–91. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:51–6. doi: 10.1053/j.jvca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Sood N, Coleman CI, Kluger J, White CM, Padala A, Baker WL. The association among blood transfusions, white blood cell count, and the frequency of post-cardiothoracic surgery atrial fibrillation: A nested cohort study from the Atrial Fibrillation Suppression Trials i, ii, and iii. J Cardiothorac Vasc Anesth. 2009;23:22–7. doi: 10.1053/j.jvca.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Koch CG, Li L, Van Wagoner DR, Duncan AI, Gillinov AM, Blackstone EH. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann Thorac Surg. 2006;82:1747–56. doi: 10.1016/j.athoracsur.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Rienstra M, Sun JX, Magnani JW, Sinner MF, Lubitz SA, Sullivan LM, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study) Am J Cardiol. 2012;109:533–7. doi: 10.1016/j.amjcard.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imataka K, Nakaoka H, Kitahara Y, Fujii J, Ishibashi M, Yamaji T. Blood hematocrit changes during paroxysmal atrial fibrillation. Am J Cardiol. 1987;59:172–3. doi: 10.1016/s0002-9149(87)80099-1. [DOI] [PubMed] [Google Scholar]
- 20.Peverill RE, Harper RW, Smolich JJ. Inverse relation of haematocrit to cardiac index in mitral stenosis and atrial fibrillation. Int J Cardiol. 1999;71:149–55. doi: 10.1016/s0167-5273(99)00145-x. [DOI] [PubMed] [Google Scholar]
- 21.Laurent G, Dentan G, Moreau D, Zeller M, Laurent Y, Vincent-Martin M, et al. Atrial fibrillation during myocardial infarction with and without ST segment elevation. Arch Mal Coeur Vaiss. 2005;98:608–14. [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.[No authors listed]. The Thrombolysis in Myocardial Infarction (timi) Trial. Phase i findings. Timi study group. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. New York: John Wiley & Sons; 2000. Applied logistic regression. [Google Scholar]
- 26.Behar S, Zahavi Z, Goldbourt U, Reicher-Reiss H. Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. Sprint Study Group. Eur Heart J. 1992;13:45–50. doi: 10.1093/oxfordjournals.eurheartj.a060046. [DOI] [PubMed] [Google Scholar]
- 27.Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. Sprint and Thrombolytic Survey Groups. Circulation. 1998;97:965–70. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 28.Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, et al. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008;94:867–73. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 29.Bishara R, Telman G, Bahouth F, Lessick J, Aronson D. Transient atrial fibrillation and risk of stroke after acute myocardial infarction. Thromb Haemost. 2011;106:877–84. doi: 10.1160/TH11-05-0343. [DOI] [PubMed] [Google Scholar]


