Abstract
The potent cytolethal distending toxin produced by Haemophilus ducreyi is a putative virulence factor in the pathogenesis of chancroid. We studied its action on eukaryotic cells, with the long-term goal of understanding the pathophysiology of the disease. Intoxication of cultured human epithelial-like cells, human keratinocytes, and hamster fibroblasts was irreversible, and appeared as a gradual distention of three- to fivefold the size of control cells. Organized actin assemblies appeared concomitantly with cell enlargement, promoted by a mechanism that probably does not involve small GTPases of the Rho protein family. Intoxicated cells did not proliferate. Similar to cells treated with other cytolethal distending toxins, these cells accumulated in the G2 phase of the cell cycle, demonstrating an increased level of the tyrosine phosphorylated (inactive) form of the cyclin-dependent kinase p34cdc2. DNA synthesis was not affected until several hours after this increase, suggesting that the toxin acts directly on some kinase/phosphatase in the signaling network controlling the p34cdc2 activity. We propose that this toxin has an important role both in the generation of chancroid ulcers and in their slow healing. The toxin may also be an interesting new tool for molecular studies of the eukaryotic cell- cycle machinery.
Introduction
Haemophilus ducreyi is a Gram-negative, facultatively anaerobic coccobacillus that causes chancroid (soft chancre). This sexually transmitted disease develops with characteristic mucocutaneous, shallow ulcers on the external genitals. The ulcers are painful and show a pronounced retardation of healing. The disease is endemic in many developing countries, where an association between genital ulcers and transmission of HIV has been demonstrated (1–4). Information is limited concerning the pathogenic mechanisms responsible for the development of ulcers in chancroid. A few bacterial components potentially involved in destruction of cells and tissues have been identified. The lipo-oligosaccharide caused a dermal lesion in animal models (5, 6), but the concentrations needed were unphysiologically high. The 122-kDa cell-associated hemolysin was able to kill human foreskin fibroblasts in vitro (7, 8), but a hemolysin-negative isogenic mutant was equally active as the wild type in causing pustules in human skin (9).
A separate, secreted cytotoxin of H. ducreyi was shown to kill cultured epithelial-like cells of human origin (10). Immunization of rabbits with live bacteria or bacterial products elicited toxin-neutralizing serum antibodies (11). The toxin produced by different H. ducreyi strains was immunologically similar. A neutralizing monoclonal antibody (MAB M4D4) was prepared and used for immunoaffinity purification (12), yielding a cytotoxic 20-kDa protein that shared the same or similar epitope for all toxic strains (12). This H. ducreyi toxin induced cell enlargement followed by cell death (10). The effect is similar to that of the cytolethal distending toxins (CDTs) encoded by a cluster of three linked genes (cdtA,B,C) that have been detected in Escherichia coli (CDT-I, CDT-II, CDT-III) (13–15), Shigella (16), Campylobacter (17), and Actinobacillus actinomycetemcomitans (18).
Recently, the H. ducreyi cytotoxin was shown to be encoded by a similar cluster of three linked genes (19). Henceforth in this paper, it will be referred to as the H. ducreyi cytolethal distending toxin (HdCDT). The three proteins have calculated molecular masses of 25, 30, and 20 kDa and are 38%, 51%, and 24% identical with the products of the E. coli genes encoding CDT-I (13). HdCDT is even more related to the Actinobacillus CDT: the identities between the predicted amino acid sequences are 91%, 97%, and 94% (18). To our knowledge, HdCDT is the only one of the CDTs known to date that has been purified. The NH2-terminal amino acid sequence of the purified HdCDT (12) showed 100% homology with the NH2-terminal sequence of the cloned 20-kDa cdtC protein product (19). The HdCDT-neutralizing MAB M4D4 (12) bound to a His-fusion involving the 20-kDa cdtC protein product (19). These findings suggest that the active site of the toxin is localized in the C protein (19). Nonetheless, all three H. ducreyi cdt genes had to be expressed in recombinant E. coli strains to produce cytotoxic culture supernatants (19). Similar findings have been reported for the E. coli CDTs, but the roles of the cdtA and cdtB protein products are not yet clear (13, 15).
The E. coli, Campylobacter, and Actinobacillus crude CDTs have all been shown to induce cell-cycle arrest in the G2 phase (15, 18, 20, 21). A requirement for the transition of cells from G2 into mitosis is that the cyclin-dependent kinase p34cdc2 is activated. The final step in that process is a dephosphorylation in Thr-14 and Tyr-15 of p34cdc2 (22). A hyperphosphorylation of p34cdc2 has been reported to occur concomitantly with the cell-cycle arrest induced by CDTs (20, 21). Here we report that the purified HdCDT also induces G2 arrest and inactivation of p34cdc2. The tyrosine phosphorylation of p34cdc2 was the earliest detectable toxin-induced effect, occurring before HEp-2 cell enlargement and promotion of the actin cytoskeleton (ACSK). HdCDT did not act by adenosine diphosphate (ADP) ribosylation or glucosylation, and the ACSK effect appeared not to involve small guanosine triphosphatases (GTPases) of the Rho protein family. HdCDT is likely to play an important role in the development, persistence, and slow healing of the ulcerative lesions characteristic of chancroid.
Methods
Materials.
HdCDT was purified by immunoaffinity chromatography as described (12). The protein concentration was 122 μg/ml. Toxin B from Clostridium difficile (TcdB) and Lethal Toxin from Clostridium sordellii (TcsL) were kindly supplied by C. von Eichel-Streiber (University of Mainz, Mainz, Germany). Clostridium botulinum exoenzyme C3 was from Upstate Biotechnology Inc. (Lake Placid, New York, USA). UDP-[14C]glucose (specific activity 318 mCi/mmol) and [32P]NAD (specific activity 800 Ci/mmol) were from Du Pont Nen (Dreieich, Germany). Methyl-[3H]thymidine (specific activity 5.0 Ci/mmol) and [γ-32P]ATP (specific activity 30 Ci/mmol) were from Amersham Sweden AB (Solna, Sweden). All other reagents were of analytical grade and obtained from local commercial sources.
Cell culture and toxin treatment.
Human larynx carcinoma cells (HEp-2; American Type Culture Collection [ATCC] No. CCL-23), Chinese hamster lung fibroblasts (Don; ATCC No. CCL-16), human cervix carcinoma cells (HeLa; ATCC No. CCL-2), and a human keratinocyte line (HaCaT; kindly provided by N.E. Fusenig, Heidelberg, Germany) (23) were cultivated in Eagle's MEM supplemented with 10% FBS, 5 mM L-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ ml) in a humid atmosphere containing 5% CO2. Mouse Swiss fibroblasts (3T3; ATCC No. CCL-92) were cultivated in DMEM with supplements as already described here. HEp-2 or HeLa cells in logarithmic growth phase (in 96-well or 24-well plates) were cooled on ice for 15 min before addition of ice-cold toxin (122 ng/ml). After 15-min exposure, the cells were washed three times with cold HBSS. Eagle's MEM was added and the cells were further incubated at 37°C. Don and 3T3 fibroblasts were exposed to 122 ng/ml HdCDT continuously at 37°C. HaCaT cells suspended in culture medium were treated with HdCDT for 2 h at 37°C followed by toxin-free growth medium as described previously (10). The cytopathic effect (CPE) was neutralizable by preincubation of the toxin (30 min at 37°C) with the MAB M4D4 (12).
Staining of F-actin with FITC-phalloidin and measurement of cell area.
HdCDT-treated cells were stained with FITC-phalloidin and visualized by fluorescence microscopy as previously described (24). Ten randomly chosen fields were photographed and the negatives scanned (Personal Densitometer SI; Molecular Dynamics, Sunnyvale, California, USA). The cell areas were quantified using ImageQuant software (Molecular Dynamics).
Assay of small GTPases.
Small GTPases in cell lysates were labeled with the glucosyltransferase toxins TcdB and TcsL in the presence of UDP-[14C]glucose. The GTPases were separated by SDS-PAGE and visualized by PhosphorImager (Molecular Dynamics) analysis as described (25). Cell lysates were sonicated three times for 3 s, centrifuged (3,500 g × 10 min), and the supernatants were subjected to high-speed centrifugation (52,000 g × 1 h) at 8°C. The supernatants from the second centrifugation were used as cytosol. The pellets, resuspended in 200 μl of lysis buffer, were used as the membrane fraction.
Assay of ADP-ribosyltransferase activity and glucosyltransferase activity.
ADP-ribosyltransferase activity was assayed as described (24) by incubating HdCDT (12.2g/ml) with fresh cell lysate and [32P]NAD for 60 min, followed by SDS-PAGE of the proteins and detection of radioactivity. The C. botulinum ADP-ribosyltransferase C3 served as positive control. Glucosyltransferase activity was assayed as described (25), with TcdB as positive control.
Assay of DNA synthesis.
DNA synthesis was assayed by two methods: (a) Toxin-treated HEp-2 cells were incubated at 37°C in medium for 6, 12, and 24 h followed by fresh medium with 10 μg/ml bromodeoxyuridine (BrdU; Sigma Chemical Co., St. Louis, Missouri, USA) for 12 h. Cells were fixed and permeabilized for immunofluorescence (24) and incubated (90 min) in a humid chamber with monoclonal anti-BrdU (Clone BU-33; Sigma Chemical Co.) diluted 1:1,000 in PBS containing 0.5 mg/ml DNAse I (Sigma Chemical Co.) and 0.1 % BSA. After two rinses with PBS and incubation (60 min at 22°C) with tetrarhodamine isothiocyanate (TRITC)-rabbit anti–mouse conjugate (diluted 1:250), BrdU-incorporating cells were visualized by fluorescence microscopy and counted in 10 randomly chosen fields. (b) Cells growing in 24-well plates were exposed to toxin (quadruplicate samples) and postincubated in fresh medium as already described here. At the indicated times, the cells were incubated for 30 min with [3H]thymidine (1 μCi/ml), and then rapidly removed by trypsinization and treated for 30 min with trichloroacetic acid (final concentration 10%). The macromolecular fraction was precipitated by centrifugation (13,000 g × 10 min), washed in 10% trichloroacetic acid, dissolved in scintillation liquid (OptiPhase HiSafe; Fisons Chemicals, Loughborough, United Kingdom), and counted on a liquid scintillation counter (LKB Wallac, Loughborough, United Kingdom).
Cell cycle analysis by flow cytometry.
Cells were treated with 122 ng/ml HdCDT, rinsed with HBSS, scraped off (Cell Scraper; Nalge Nunc International, Naperville, Illinois, USA), and centrifuged (3,500 g × 7 min). The pellet was resuspended in 1.5 ml HBSS and fixed at 0°C for 10 min with 3 ml ethanol (70%). The cells were pelleted and stained for 15 min with 1 ml propidium iodide (PI) solution (0.05 mg/ml; RNAse, 0.02 mg/ml; NP40, 0.3%; sodium citrate, 1 mg/ml). Flow cytometry analysis of the DNA content was performed with a FACSort flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). The data from 103 cells were collected and analyzed using CellQuest software (Becton Dickinson).
Western blot.
Cells were lysed in 300 μl of SDS electrophoresis sample buffer (26) and samples were boiled for 10 min. The proteins were separated by 10% SDS-PAGE, transferred to Protran Nitrocellulose (Schleicher & Schuell, Dassel Germany) and probed with antiphosphotyrosine (Upstate Biotechnology) or anti-cdc2 (Transduction Laboratories, Lexington, Kentucky, USA) antibodies. Blots were developed with a Chemiluminescence Western Blotting Kit (Boehringer Mannheim, Mannheim, Germany) using a peroxidase-labeled secondary antibody. The amount of protein in the lysates was determined by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, California, USA) using BSA as a standard.
Two-dimensional gel electrophoresis.
Cells were lysed in sample buffer (26), boiled for 10 min, cooled on ice for 5 min, and treated for 8 min at 22°C with a 1:20 diluted solution of DNAse I (1 mg/ml), RNAse (0.25 mg/ml), 0.5 M Tris, 0.1 M MgCl2 (pH 7.0). Lysates were incubated 20 min at 22°C with acetone (final concentration 80%) and centrifuged (12,000 g × 10 min). The pellet was washed once with acetone, centrifuged, dried for 5 min, and resuspended in 50 μl of buffer (9.9 M urea; 4% Triton X-100; 2.2% ampholytes; 100 mM 2-mercaptoethanol). After isoelectric focusing in 11-cm Immobiline dry strips (pH 3–10; Pharmacia Biotech AB, Sollentuna, Sweden), the proteins were separated in the second dimension by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti-phosphotyrosine or anti-cdc2 antibodies.
Assay of cell proliferation and cell death.
Cells were treated with HdCDT for 15 min on ice or continuously at 37°C. At different time points, quadruplicates of control and treated cells were removed by trypsinization and counted. Cell death was assayed by uptake of trypan blue (0.025%).
Immunoprecipitation of p34cdc2.
HEp-2 cells in 75-cm2 flasks were treated with HdCDT for 15 min on ice followed by medium for 24 h. Control cells were treated with nocodazole (100 nM) for 16 h or untreated. The p34cdc2 was immunoprecipitated using the Immunoprecipitation Kit (Protein G) (Boehringer Mannheim) with 4.5 μg of cdc2 antibody and incubation for 5 h with the protein G-agarose beads. Samples were analyzed by Western blot with anti-phosphotyrosine antibodies.
Assay of histone H1 kinase activity.
p34cdc2 in HEp-2 cell lysates (1,500 μg protein) was immunoprecipitated. The p34cdc2/cyclin B complexes were incubated 10 min at 37°C with 2.5 μCi of [γ-32P], 10 μg of histone H1 (Boehringer Mannheim), 100 mM NaCl, 10% Triton X-100, 50 μM ATP, and 10 mM MgCl2. Then, 10% SDS-PAGE radiolabeled bands were analyzed using a PhosphorImager SF (Molecular Dynamics).
Results
Sensitivity of different cell lines to HdCDT and optimal conditions for toxin treatment.
A 15-minute exposure of precooled HEp-2 or HeLa cells to ice-cold HdCDT, followed by fresh toxin-free growth medium, sufficed to induce a morphological change in 12–24 hours, later resulting in cell death. Also the human keratinocyte line HaCat was sensitive (Fig. 1), although in this case, a two-hour incubation with HdCDT at 37°C was needed before the postincubation in medium. A requisite for the CPE in Don fibroblasts was the continued presence of HdCDT in the medium. Mouse 3T3 fibroblasts, however, did not change morphology even when treated continuously with HdCDT. Most experiments presented in the following section were performed on HEp-2 cells, using the 15-minute binding step on ice with 122 ng/ml HdCDT. These standard conditions were optimal for obtaining a reproducible CPE, beginning 12–15 hours after toxin treatment.
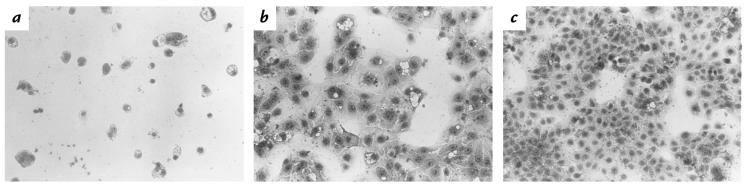
Figure 1.
Effect of HdCDT on HaCat keratinocytes. Growing cells were exposed to 122 ng/ml HdCDT for 2 h at 37°C. After postincubation in medium for 48 h (a) or 24 h (b), cells were fixed and stained with Giemsa. (c) Control cells, 48 h. ×100. HdCDT, Haemophilus ducreyi cytolethal distending toxin.
HdCDT-treated cells are enlarged, followed by shrinkage and deterioration.
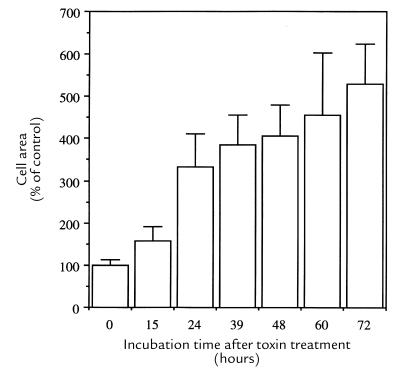
The first morphological change seen in toxin-sensitive cells was an enlargement. In HaCat cells, this was clear 24 hours after treatment with 122 ng/ml (Fig. 1b) and cells rounded up after 48 hours (Fig. 1a). In HEp-2 and HeLa cells, a distention was already obvious 15 hours after toxin binding and maximal in 72 hours. After 24 hours, HEp-2 cells were three times, and after 72 hours five times, larger than the average control cell (Fig. 2). About 48 hours after toxin exposure, cells began to round up and shrink. Such cells incorporated trypan blue, indicating a damaged plasma membrane. Don fibroblasts were maximally enlarged after 24 hours of continued HdCDT treatment (122 ng/ml) and were completely destroyed without rounding after a total exposure time of 48 hours (data not shown).
Figure 2.
Area of HdCDT-treated HEp-2 cells. Cells were treated with HdCDT (15 min on ice). After postincubation at 37°C for the times indicated, cells were stained with FITC-phalloidin and photographed. Cell areas were quantified using ImageQuant software, and the results were expressed as percentage + SD of untreated control cells.
The ACSK is promoted in HdCDT-treated cells.
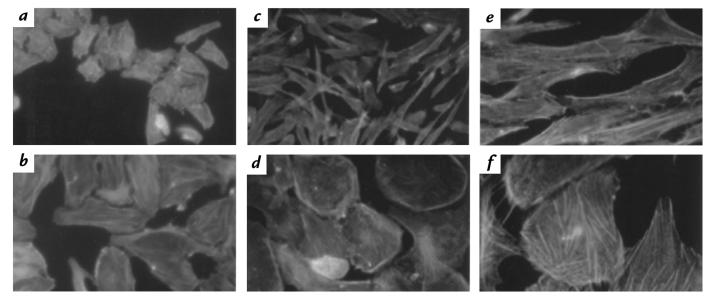
Another member of the CDT family was shown to induce actin rearrangements in Chinese hamster ovary (CHO) cells (27). Similar changes of F-actin occurred in HdCDT-treated cells. Prominent stress fibers developed and membrane ruffling was promoted in both HEp-2 and Don cells (Fig. 3, b, d, and f). Thus, the earliest morphological effect of HdCDT on these cells is a distention appearing concomitantly with a promoted ACSK.
Figure 3.
Effect of HdCDT on the ACSK of HEp-2 and Don cells. HEp-2 cells (a and b) were treated with toxin (15 min on ice) and postincubated at 37°C for 48 h. Don cells (c–f) were exposed to HdCDT for 24 h at 37°C. F-actin was stained with FITC-phalloidin as detailed in Methods. HEp-2 cells: control (a) and toxin treated (b); Don cells: controls (c, e) and toxin treated (d, f). ×400 (a–d); ×1000 (e and f). ACSK, actin cytoskeleton.
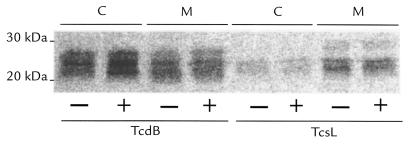
Given that small GTPases control both the ACSK (28) and cell proliferation (29), we asked whether HdCDT might affect such proteins. For detection of small GTPases, we used two glucosyltransferase toxins, TcdB and TcsL, produced by C. difficile and C. sordellii, respectively, and known to glucosylate small GTPases of the Rho and Ras subfamilies (24). Lysates made of HdCDT-treated and maximally enlarged HEp-2 cells were mixed with each glucosyltransferase in the presence of UDP-[14C]glucose (Fig. 4). Three observations suggested that HdCDT had no effect on small GTPases: (a) no alteration was detectable in the electrophoretic mobilities of any of the GTPases glucosylated by either TcdB or TcsL; (b) the extent of GTPase glucosylation by TcdB/TcsL was not altered in HdCDT-intoxicated cells; and (c) the distribution between the cytosolic and membrane fractions of GTPases glucosylated by these toxins was not changed.
Figure 4.
Effect of HdCDT on small GTP-binding proteins. HEp-2 cells were treated with HdCDT (15 min on ice) and postincubated at 37°C for 24 h. Lysates were prepared from these cells (+) and control cells (–). Cytosolic (C) and membrane (M) fractions were prepared by differential centrifugations and incubated (1 h at 37°C) with TcdB or TcsL in the presence of UDP-[14C]Glucose. Proteins were separated by 12.5% SDS-PAGE, and radiolabeled bands were detected by PhosphorImager analysis. TcdB, Toxin B from Clostridium difficile; TcsL, Lethal Toxin from Clostridium sordellii.
HdCDT lacks ADP-ribosyltransferase or glucosyltransferase activities.
Because ADP ribosylation is a common effect of many bacterial toxins, we investigated whether HdCDT would ADP ribosylate some cellular target protein. HEp-2 cell lysates were treated with HdCDT in the presence of [32P]NAD according to a protocol described previously (24) and using C. botulinum exoenzyme C3 as positive control. However, no ADP-ribosylated proteins were detectable in such treated cell lysates (data not shown). HdCDT was also devoid of glucosyltransferase activity as tested in a principally similar way (25), but using UDP-[14C]glucose as the cofactor and C. difficile TcdB as the positive control (data not shown). Thus, none of these two enzymatic activities can be responsible for the cytotoxic effect induced by HdCDT.
HdCDT inhibits cell proliferation.
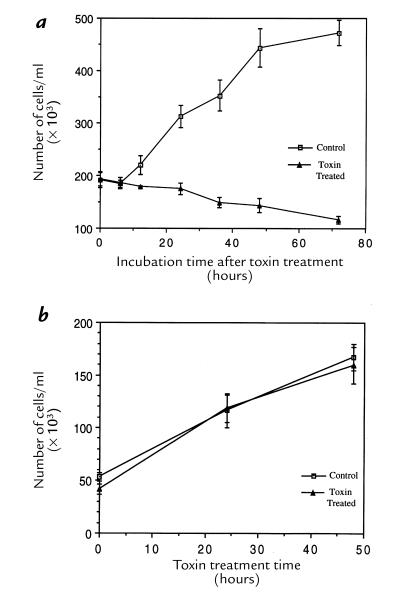
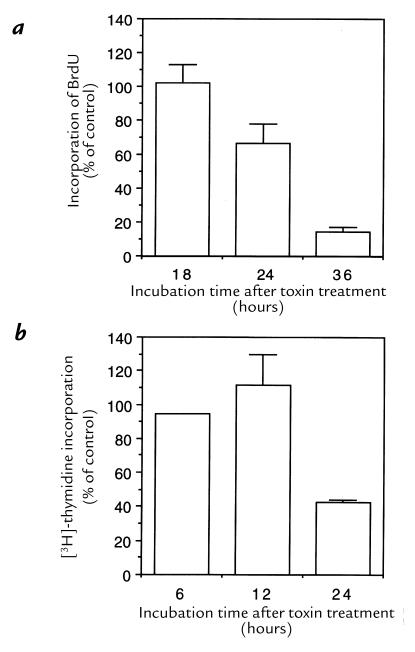
We determined the capacity of HdCDT-treated cells to proliferate by counting them at various stages of intoxication. After the brief standard exposure to HdCDT, the HEp-2 cells apparently did not divide at all (Fig. 5a). The same effect was induced in HaCat, HeLa, and Don cells (data not shown). By contrast, 3T3 fibroblasts proliferated normally despite the continuous presence of HdCDT in the medium (Fig. 5b), consistent with the finding that these cells were not morphologically altered by the toxin. The synthesis of DNA in HdCDT-exposed HEp-2 cells was not affected up to 18 hours after treatment, and incorporation of both BrdU and [3H]thymidine was decreased 24–36 hours after toxin exposure (Fig. 6). Thus, the division of cells responding to HdCDT with a CPE was irreversibly inhibited, but a blocked DNA synthesis was not the primary cause of this antiproliferative effect.
Figure 5.
Effect of HdCDT on cell proliferation. (a) HEp-2 cells were treated with HdCDT (15 min on ice), postincubated at 37°C for the times indicated, trypsinized, and counted. (b) 3T3 cells were treated with HdCDT at 37°C continuously until trypsinization and counting at the times indicated. Each point represents the mean of quadruplicate samples ± SD. This is a representative experiment of two experiments, each of which was conducted with quadriplicate samples.
Figure 6.
DNA synthesis in HdCDT-treated cells. (a) Incorporation of BrdU. HEp-2 cells treated with HdCDT for 15 min on ice were postincubated for 18, 24, and 36 h in medium at 37°C. BrdU (10 μg) was present during the final 12 h, and its incorporation was visualized by staining with anti-BrdU. (b) Incorporation of [3H]thymidine. HEp-2 cells treated with HdCDT as described above were postincubated for 6, 12, and 24 h. [3H]thymidine (1 μCi/ml) was present during the final 30 min, and its incorporation in DNA was determined as detailed in Methods. Mean values + SD of three different experiments. BrdU, bromodeoxyuridine.
HdCDT-treated cells are arrested in G2 phase.
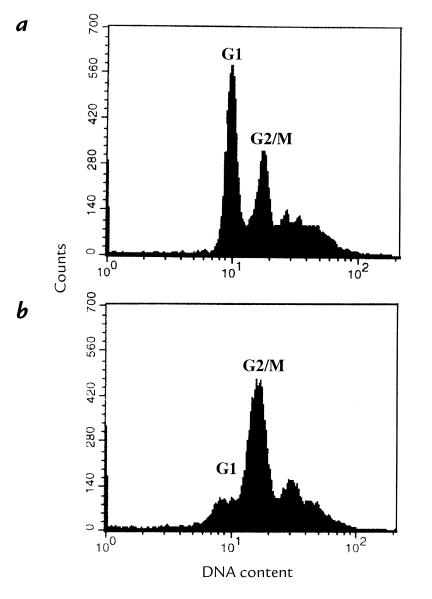
Flow cytometric analysis of HdCDT-treated HEp-2 cells indicated a block in the G2/M phase (Fig. 7), similar as described for other CDTs (15, 20). The nuclei were enlarged and no mitotic figures were seen, suggesting that the cells were blocked in the G2 rather than the M phase.
Figure 7.
Flow cytometric analysis of HdCDT-treated HEp-2 cells. DNA was stained with propidium iodide. (a) Control cells. (b) Cells exposed to HdCDT (15 min on ice) and postincubated for 36 h.
p34cdc2 in HdCDT-treated cells remains tyrosine phosphorylated and inactive.
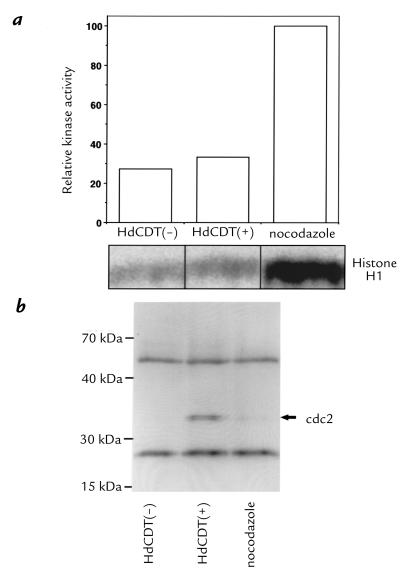
A hyperphosphorylation of the p34cdc2 kinase was demonstrated in cells treated with other CDTs (20, 21). We probed the proteins in HdCDT-treated cells by Western blot with anti-phosphotyrosine antibodies. Indeed p34cdc2 was significantly more phosphorylated than in control lysates (data not shown). The p34cdc2 protein was immunoprecipitated and its kinase activity was measured. In toxin-treated cells, the p34cdc2 kinase activity was about 20% of that in cells synchronized into the G2/M phase by treatment with nocodazole (Fig. 8a). Immunoprecipitated p34cdc2 was visualized with anti-phosphotyrosine antibody (Fig. 8b). As expected, the p34cdc2 in nocodazole-treated cells showing a high kinase activity was not tyrosine phosphorylated, whereas that in the toxin-treated cells was (Fig. 8b). Although the p34cdc2 in untreated control cells was also not phosphorylated, its activity (Fig. 8a) was as low as in the HdCDT-treated cells, because most of these cells were in interphase; i.e., the p34cdc2/cyclin complex was not yet formed.
Figure 8.
Protein kinase activity of immunoprecipitated p34cdc2 from HdCDT-treated cells. (a) HEp-2 cells were exposed to toxin (15 min on ice), postincubated for 24 h, and lysed. The p34cdc2 was immunoprecipitated and its kinase activity toward histone H1 was determined. Controls: lysates from nocodazole-treated cells and from nontreated interphase cells. (b) Western blot of the immunoprecipitated p34cdc2 with anti-phosphotyrosine antibodies. The two bands flanking the p34cdc2 represent the heavy and light chains of the antibody used for immunoprecipitation that have reacted with the secondary antibody.
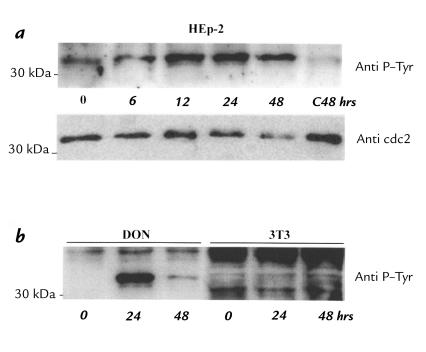
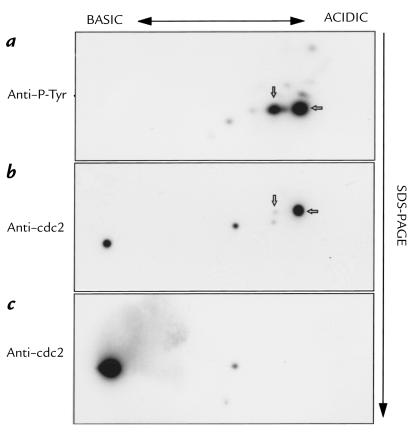
The phosphorylation of p34cdc2 was already increased six hours after toxin exposure, as judged both by the labeling intensity of the protein and the fact that it migrated somewhat more slowly than that in control cell lysates (Fig. 9a). By contrast, the HdCDT-insensitive 3T3 fibroblasts showed no change in tyrosine phosphorylation of p34cdc2 (Fig. 9b), indicating that the observed phosphorylation was indeed related to the cytotoxicity. A two-dimensional separation of the proteins in HEp-2 cell lysates identified two proteins reacting with anti-p34cdc2 antibodies in control cells (Fig. 10c). In lysates from HdCDT-treated cells, three new spots appeared in the more acidic part of the gel (Fig. 10b). Two of these were significantly tyrosine phosphorylated and migrated more slowly than the unphosphorylated forms (Fig. 10a). We conclude that HdCDT treatment prevents the exit of cells from the G2 phase, because their p34cdc2 remains inactive owing to tyrosine phosphorylation.
Figure 9.
Tyrosine phosphorylation of p34cdc2 in HdCDT-treated cells. (a) HEp-2 cells were exposed to the toxin (15 min on ice) and postincubated at 37°C. After the times indicated, cells were processed for Western blot with anti-phosphotyrosine or anti-cdc2 antibodies. The same sample was used for both immunoblots. (b) Don and 3T3 cells were treated with HdCDT at 37°C for 24 and 48 h and probed with anti-phosphotyrosine antibodies.
Figure 10.
Two-dimensional gel electrophoresis of p34cdc2 in HdCDT-treated cells. HEp-2 cells were exposed to the toxin (15 min on ice), postincubated for 48 h, and lysed. Proteins in the cell lysates were separated in two dimensions (see Methods) and analyzed by Western blot as for Fig. 9.
Discussion
Our long-term aim is to understand the pathophysiology behind the H. ducreyi infection by investigating the molecular actions of putative virulence factors of this bacterium (30). Here we show that the CDT purified from H. ducreyi, like other members of the CDT family (15, 20, 21), irreversibly blocks mammalian cell proliferation by inducing cell-cycle arrest in G2.
Sensitive cells and receptor.
We reported previously (10) that HdCDT was able to intoxicate human epithelioid cell lines. We now show that the spectrum of toxin-sensitive cells is broader, including a human keratinocyte line as well as hamster lung fibroblasts. In addition, we have recently observed that normal human monocytes, T and B cells, as well as normal human epidermal keratinocytes and fetal human fibroblasts, are sensitive to HdCDT (Svensson, L., and Lagergård, T., manuscript submitted for publication). The intoxication of HEp-2 and HeLa cells was irreversible after a short toxin exposure on ice, allowing binding but not internalization of the toxin. We conclude that these cells probably expose a high-affinity receptor for HdCDT. The receptor may exist in lower numbers or in a low-affinity form in Don fibroblasts , which were intoxicated only upon continued toxin exposure at 37°C, suggesting they could not bind HdCDT rapidly at low temperature. Although we do not know why 3T3 cells resist intoxication by HdCDT, we speculate that the putative toxin-specific receptor might be completely absent from 3T3 cells. HEp-2 cells will constitute a suitable model system in future identification of the cell-surface receptor for HdCDT.
Cell distention and effects on the ACSK.
The effect of E. coli CDT on the ACSK of CHO cells was speculated to result from a toxin attack on Rho proteins (27). We believe that the ACSK-promoting effect, by HdCDT at least, may be mediated by some signaling system other than the Rho proteins, because none of our experiments supported their direct involvement. The ACSK is controlled by Rho, Rac, and Cdc42 (28), which are targets of TcdB, whereas Ras, Rap, Ral, and Rac are attacked by TcsL (31, 32). Both TcdB and TcsL were able to fully glucosylate all their GTPase substrates in lysates from HdCDT-treated HEp-2 cells, and all these GTPases had unchanged electrophoretic mobilities. We previously detected a mobility change of ADP-ribosylated Rho by glucosylation with TcdB (Fig. 8a in ref. 25). Moreover, Rho in lysates from cells pretreated with TcdB could not be ADP ribosylated (25, 33). By analogy, our present observations suggest that HdCDT does not covalently modify the effector region of any of the GTPases attacked by TcdB and TcsL. In view of the similarities in action between the known CDTs, these observations may be valid also for the other toxins in this family.
The ACSK promotion and enlargement of cells exposed to HdCDT or other CDTs resemble the effects of the cytotoxic necrotizing factor (CNF) from E. coli. CNF permanently activates Rho by deamidation of its Gln-63 (34, 35). This effect would not be detectable as a changed electrophoretic mobility of Rho. However, CNF-treated HEp-2 cells are resistant to the ACSK collapsing effect of TcdB (36), whereas the HdCDT-treated cells remained fully sensitive to TcdB (data not shown). Furthermore, multinucleation occurs in a majority of cells intoxicated by CNF (15) but was rarely seen in cells treated with HdCDT (this paper) or E. coli CDT (15). Thus, a similar effect on Rho as by CNF appears unlikely for HdCDT, as was also concluded for CDT (15). Finally, we considered the possibility that HdCDT could activate small GTPases indirectly by promoting their recruitment to the plasma membrane (28), but this hypothesis was also rejected (Fig. 4). Although we cannot exclude a very subtle HdCDT-induced novel type of small GTPase modification, undetectable by gel electrophoresis, a secondary ACSK-affecting signal from the nucleus appears more likely (see later here).
Effect on the cell-cycle machinery.
HdCDT-treated cells accumulated in the G2 phase, explaining why they could not proliferate at all, and consistent with the hypothesis that the cyclin-dependent kinase required for G2-to-M transition might be a target of the toxin. In cells cycling normally, this p34cdc2 kinase is activated when a phosphatase (Cdc25) removes the inhibitory phosphates from Thr-14 and Tyr-15 (22). In HdCDT-intoxicated cells, we observed an increased tyrosine phosphorylation of p34cdc2. It was also shown to have a significantly lower kinase activity than the p34cdc2 in nocodazole-treated positive control cells (Fig. 8). In the study demonstrating that the three CDT variants of E. coli prevent p34cdc2 dephosphorylation in HeLa cells (20), this effect was determined 24 hours after toxin exposure. The HdCDT effect was already detectable within six hours after toxin exposure (Fig. 9), hours before any significant signs of cell distention and ACSK promotion. The same phenotypic effects on the cell cycle have been recently documented in cells infected with HIV or transfected with vpr, one of its regulatory genes (37–40).
The currently known mechanisms for G2 arrest point to several possible explanations for a p34cdc2 hyperphosphorylation. It could be due to (a) damage of DNA or inhibition of DNA synthesis, evoking the G2 checkpoint response (41, 42); (b) hyperactivation of the kinases responsible for phosphorylation of p34cdc2 at Thr-14 and Tyr-15 (22); (c) a lack of activation or an inactivation of the Cdc25 phosphatase, which dephosphorylates at these positions (43); or even (d) a direct inactivation of p34cdc2, which might result in its hyperphosphorylation (44). As the hyperphosphorylated p34cdc2 in HdCDT-treated cells appeared (Fig. 9) many hours before detectable failure to synthesize DNA (Fig. 6), an impaired DNA synthesis does not appear to be a primary effect of the toxin. Indeed, we found recently that the p34cdc2 in completely confluent monolayers of Don fibroblasts was also tyrosine phosphorylated upon HdCDT exposure, despite the fact that these cells were not dividing (data not shown). A primary slight damage to DNA, however, cannot be excluded at this stage. The mechanism of these effects by other CDTs and by vpr are also not known, but at least E. coli CDT (27) had no early effect on the synthesis of DNA, whereas results on vpr are contradictory (39, 40). The possibility that HdCDT acts directly on p34cdc2 or on some of the kinases/phosphatases in the signaling network controlling its activity should be explored.
The connection between the G2 arrest and the cell enlargement, with promoted ACSK induced by CDTs, is not presently understood. From the severalfold increase in cell area, it is clear that toxin-treated cells continue to grow after the G2 arrest. One (remote) possibility is that the CDTs could harbor two different cytotoxic activities, one affecting the cell-cycle machinery and another affecting the ACSK. A similar severalfold enlargement of cells arrested in G2 occurred upon transfection with the vpr gene (37, 38). In the light of this finding, it appears more plausible that the enlargement induced by HdCDT and other CDTs is linked with, but secondary to, the molecular events leading to G2 arrest.
Clinical considerations.
CDT encoded in E. coli by the Shigella cdt genes caused secretory diarrhea and necrosis of the colonic epithelium in suckling mice (45), as demonstrated with a CDT– isogenic mutant. However, the relevance of CDT in human infections is far from clear and is not a trivial problem to elucidate, because CDT-producing E. coli strains may elaborate several toxins and may cause both gastrointestinal and extraintestinal infections (13, 16, 46). HdCDT, on the other hand, is one of two described toxins produced by H. ducreyi. The hemolysin (7) is active mainly on foreskin fibroblasts and does not affect epithelial cells or human skin (9). In contrast, HdCDT kills epithelial cells, and this potent toxin is produced by a majority of H. ducreyi isolates (47). Serum antibodies neutralizing the HdCDT were detected at significantly higher levels in patients with culture-confirmed chancroid than in healthy blood donors, indicating production of the toxin during the disease (12). Moreover, different strains of H. ducreyi cause one and the same disease, characterized by destruction of epidermis, and to some degree, the dermis.
HdCDT could be involved in genital ulceration by multiple actions. Given that the toxin blocks cell proliferation, it is likely to exert the strongest effect on cells of the basal layer of the epidermis, which need to constantly proliferate. The early events in the pathogenesis of chancroid may then involve minor abrasions of the epidermis, adherence of infecting bacteria to epidermal cells, and bacterial multiplication and production of HdCDT (48). A small amount of HdCDT can destroy both keratinocytes and other epithelial cells, thus providing a highly nourishing environment for the continued growth of this fastidious bacterium, facilitating enlargement of the ulcer. H. ducreyi is relatively resistant to the serum bactericidal activity (49), a feature that also should contribute to the persistence of bacteria in the lesions. When the ulcer progresses more deeply, fibroblasts of the dermis can be attacked by the toxin. At this stage, the hemolysin could aggravate the lesion, causing progression in the dermis. This is consistent with observations that chancroid ulcers are well vascularized and bleed easily on manipulation (50). The irreversible inhibition of cell proliferation by HdCDT may also explain the retardation of healing that is characteristic of chancroid. In fact, before the era of antibiotic treatment, the giant chancre in some patients could persist for months (50).
In conclusion, we have shown that HdCDT, the potent CDT produced by H. ducreyi, like other CDTs inhibits cell proliferation by G2 arrest because the p34cdc2 kinase remains tyrosine phosphorylated and therefore inactive. The enzymatic (?) mode of action of the CDTs is not known, but for HdCDT we have excluded ADP-ribosyltransferase and glucosyltransferase activities. The irreversible block of cell proliferation is consistent with the generation of ulcers as well as their slow healing. In all probability, HdCDT is the most important virulence factor in ulcer formation by H. ducreyi. Moreover, HdCDT and other CDTs may become interesting new tools for studies of the signal transduction events in the transition of mammalian cells from the G2 phase into mitosis.
Acknowledgments
We thank C. von Eichel-Streiber for large clostridial cytotoxins, and T. Frisan (Microbiology and Tumorbiology Center, Karolinska Institutet, Stockholm, Sweden) for advice concerning flow cytometry. This work was supported by the Swedish Medical Research Council (05969), Karolinska Institutet, and Swedish International Development Cooperation Agency, Department for Research Cooperation. X. Cortes-Bratti was supported by a fellowship from the Swedish Institute.
References
- 1.Plummer FA, et al. Epidemiology of chancroid and Haemophilus ducreyi in Nairobi, Kenya. Lancet. 1983;2:1293–1295. doi: 10.1016/s0140-6736(83)91161-3. [DOI] [PubMed] [Google Scholar]
- 2.Morse SA. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trees DL, Morse SA. Chancroid and Haemophilus ducreyi: an update. . Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schryver A De, Maheus A. Epidemiology of sexually transmitted diseases: the global picture. Bull World Health Organ. 1990;68:639–654. [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnari AA, et al. Role of lipo-oligosaccharide in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagergård T. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 7.Palmer L, Munson RS., Jr Cloning and characterization of the genes encoding the hemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 8.Totten P, Norn DV, Stamm WE. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer KL, et al. Evaluation of a isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 10.Purvén M, Lagergård T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagergård T, Purvén M. Neutralizing antibodies to Haemophilus ducreyi cytotoxin. Infect Immun. 1993;61:1589–1592. doi: 10.1128/iai.61.4.1589-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purvén M, Frisk A, Lönnroth I, Lagergård T. Purification and identification of Haemophilus ducreyi cytotoxin using a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott DA, Kaper JB. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxins. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérès SY, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microb. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 16.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 17.Pickett CL, et al. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugai M, et al. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cope LD, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comayras C, et al. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehouse CA, et al. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackman MR, Pines JN. Cyclins and the G2/M transition. Cancer Surv. 1997;29:47–73. [PubMed] [Google Scholar]
- 23.Boukamp P, et al. Normal keratinization in a spontaneously immortalised aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaves-Olarte E, et al. UDP-glucose deficiency in a mutant cell line protects against glucosyltransferase toxins from Clostridium difficile and Clostridium sordellii. J Biol Chem. 1996;271:6925–6932. doi: 10.1074/jbc.271.12.6925. [DOI] [PubMed] [Google Scholar]
- 25.Chaves-Olarte E, Weidmann M, Eichel-Streiber CV, Thelestam M. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J Clin Invest. 1997;100:1734–1741. doi: 10.1172/JCI119698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Aragon V, Chao K, Dreyfus LA. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 29.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 30.Frisk A, Ahmed HJ, Dyck E Van, Lagergård T. Antibodies specific to surface antigens are not effective in complement-mediating killing of Haemophilus ducreyi. Microb Pathog. 1998;25:67–75. doi: 10.1006/mpat.1998.0219. [DOI] [PubMed] [Google Scholar]
- 31.Eichel-Streiber Cv, Boquet P, Sauerborn M, Thelestam M. Large clostridial cytotoxins — a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 1996;4:375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann F, Rex G, Aktories K, Just I. The Ras-related protein Ral is monoglucosylated by Clostridium sordellii lethal toxin. Biochem Biophys Res Commun. 1996;227:77–81. doi: 10.1006/bbrc.1996.1470. [DOI] [PubMed] [Google Scholar]
- 33.Just I, et al. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 34.Schmidt G, et al. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 35.Flatau G, et al. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 36.Fiorentini C, et al. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J, et al. Human immunodeficiency virus type 1 viral protein R (vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartz SR, Rogel ME, Emerman M. Human immunodeficiency virus type 1 cell cycle control: vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poon B, et al. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1671. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 42.O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez Y, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 44.Poon RY, Chau MS, Yamashita K, Hunter T. The role of cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- 45.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 47.Purvén M, Falsen E, Lagergård T. Cytotoxin production in 100 strains of Haemophilus ducreyi from different geographic locations. FEMS Microbiol Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 48.Lagergård T, Purvén M, Frisk A. Evidence of Haemophilus ducreyi adherence to and destruction of human epithelial cells. Microb Pathog. 1993;14:417–431. doi: 10.1006/mpat.1993.1041. [DOI] [PubMed] [Google Scholar]
- 49.Lagergård T, Frisk A, Purvén M, Nilsson LÅ. Serum bactericidal activity and phagocytosis in host defence against Haemophilus ducreyi. Microb Pathog. 1995;18:37–51. [PubMed] [Google Scholar]
- 50.Sullivan M. Chancroid. American Journal of Syphilis. 1940;24:482–521. [Google Scholar]