Abstract
The combination of high-resolution three-dimensional medical imaging, increased computing power, and modern computational methods provide unprecedented capabilities for assessing the repair and healing of fractured bone. Fracture healing is a natural process that restores the mechanical integrity of bone and is greatly influenced by the prevailing mechanical environment. Mechanobiological theories have been proposed to provide greater insight into the relationships between mechanics (stress and strain) and biology. Computational approaches for modeling these relationships have evolved from simple tools to analyse fracture healing at a single point in time to current models that capture complex biological events such as angiogenesis, stochasticity in cellular activities, and cell-phenotype specific activities. The predictive capacity of these models has been established using corroborating physical experiments. For clinical application, mechanobiological models accounting for patient-to-patient variability hold the potential to predict fracture healing and thereby help clinicians to customize treatment. Advanced imaging tools permit patient-specific geometries to be used in such models. Refining the models to study the strain fields within a fracture gap and adapting the models for case-specific simulation may provide more accurate examination of the relationship between strain and fracture healing in actual patients. Medical imaging systems have significantly advanced the capability for less invasive visualization of injured musculoskeletal tissues, but all too often the consideration of these rich datasets has stopped at the level of subjective observation. Computational image analysis methods have not yet been applied to study fracture healing, but two comparable challenges which have been addressed in this general area are the evaluation of fracture severity and of fracture-associated soft tissue injury. CT-based methodologies developed to assess and quantify these factors are described and results presented to show the potential of these analysis methods.
Keywords: Fracture healing, computational techniques, mechanobioloy
Introduction
The combination of high-resolution three-dimensional (3D) medical imaging, increased computing power, and modern computational methods today provide unprecedented capabilities for assessing biological processes that include the repair and healing of fractured bone. While these capabilities have to date been underutilized, that is beginning to change. This paper discusses areas of computational techniques suitable for the assessment of fracture repair in which significant advances have been made.
Computational prediction of the likelihood of successful fracture healing for a given fracture’s mechanical and biological state may soon be possible. Patient-specific determination of the fixation construct optimizing the likelihood of uneventful fracture healing is likewise possible. Fully 3D assessment of the formation of fracture callus and progress towards mineralization is now conceivable using CT and/or ultrasound. Such assessments require secondary computational analysis of the source image data to extract meaningful measures. The use of such approaches for longitudinal assessments is made more plausible as lower radiation dose conebeam CT technology becomes more widely available.
Current mechanobiological theories and models of fracture healing
Fracture healing is a natural process that restores the mechanical integrity of bone. This regenerative process involves cell differentiation and tissue remodelling, both of which are influenced by the mechanical environment. However, it is not yet completely understood how differentiation pathways are related to mechanical factors. Several mechanobiological theories have been proposed to provide greater insight into these phenomena.
Much of the present-day understanding of the regulative effect of mechanical forces on tissue differentiation is based on research performed by Pauwels.1 He analysed the mechanical environment within a healing fracture callus and hypothesised that hydrostatic stress and shear strain are the stimuli that guide cells to differentiate into connective tissue, which will ultimately form bone when mature and stabilised. This theory has inspired many researchers during recent decades, while computational modelling has emerged as an alternative approach to investigate biological processes. Carter and co-workers (1988) expanded Pauwels´s theory by using a finite element (FE) model to explain how mechanical loading guides cell differentiation in a fracture callus.2 Their theory related high hydrostatic stresses with cartilage ossification, and octahedral shear stress and strain with stimulation of fibrous tissue. Unlike Pauwels, they included direct bone formation corresponding with intramembranous ossification under low stresses and strains. The model was able to predict realistic tissue patterns consistent with biological observations, but there was no quantification of the magnitude of the mechanical stimuli with tissue formation.2
Claes and Heigele extended the theory of Carter et al. by defining certain thresholds for the local stress and strain magnitudes to determine whether endochondral or intramembranous ossification takes place.3 Their quantitative mechano-regulation theory was based on the observation that bone formation occurs mainly near calcified surfaces and that both intramembranous and endochondral ossification exist concurrently in fracture healing. Histological images from in vivo experiments were used to show that their FE model properly predicted tissue differentiation in the callus at three stages of the healing event.3
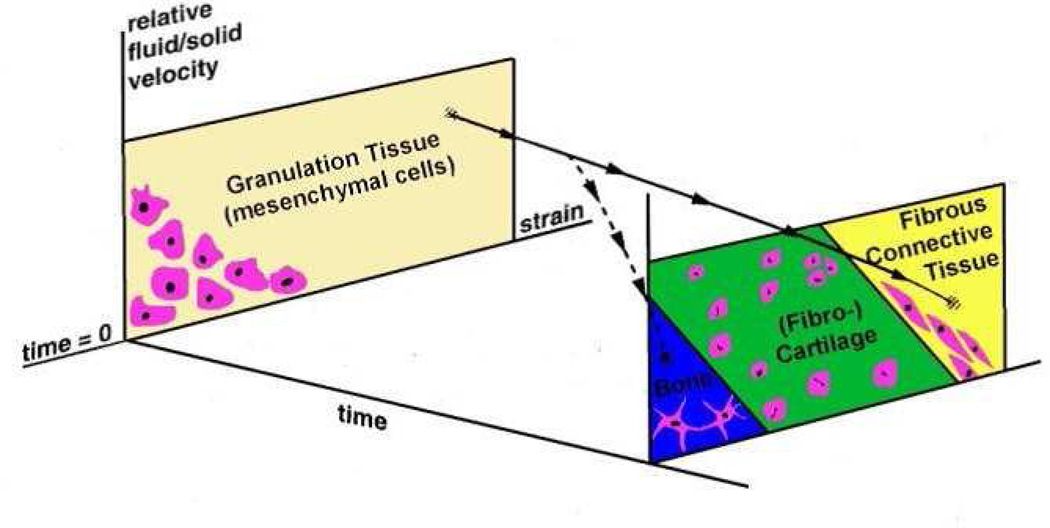
Biological tissues are composed of a solid phase and an interstitial fluid phase. Therefore, two-phase models are required to investigate internal stresses in the fluid or at the fluid/solid interface where many cell phenotypes are present. Prendergast et al. presented a poroelastic FE model of a bone implant interface to predict tissue differentiation. The tissue phenotype was regulated by the applied biophysical stimuli; shear strain on the solid collagenous phase and relative velocity on the interstitial fluid phase (Figure 1).4 This new approach was supported by in vivo results and it was found that high and intermediate levels of biophysical stimuli govern fibrous tissue and cartilage formation, respectively, and low levels of stimulus are responsible for bone differentiation. Isaksson et al. compared the mechanobiological models of Carter, Claes and Heigele, and Prendergast in a fracture healing study, and they concluded that the concept based on strain and fluid velocity as stimuli correlated best with experimental results.5 The algorithms mentioned above predicted tissue phenotype only at specific time points, being unable to simulate tissue differentiation over the complete regeneration period. As new mechano-regulatory concepts emerged, mechano-biological computations developed into computer simulations and were able to simulate chronological tissue differentiation by employing the algorithm of Prendergast et al. in an iterative FE simulations.6
Figure 1.
Mechanoregulatory model presented by Prendergast et al.4 that describes the hypothesis that tissue differentiation is controlled by two biophysical stimuli, shear strain on the solid collagenous phase and relative velocity on the interstitial fluid phase.
Later, Lacroix et al. adapted the Prendergast model to describe fracture healing in a time-dependent fashion adding diffusion equations to model progenitor cell dispersal in the callus.7 This theory was able to predict tissue differentiation and bone resorption under different gap sizes and loads, and it highlights the importance of cell activities on healing patterns and rates. Diffusion is not the mechanism of cell migration and proliferation, but despite this, Lacroix’s theory paved the way for many researchers to include cell activities in their mechano-regulatory algorithms. Subsequent mechano-biological models combined FE analysis with lattice models to include cell activities. Perez and Prendergast developed a 2D FE lattice model representing both, cells and extracellular matrix in which individual cell activity was guided by a “random walk”.8 Byrne adapted this model into 3D and later implemented it in fracture healing of a human tibia under realistic muscle loading, predicting healing beyond the reparative phase.9 Another lattice approach was presented by Checa and Prendergast, incorporating angiogenesis in the modulation of cell phenotype, raising the question of whether mechanoregulatory theories must be coupled with bioregulatory networks.10
Computer models to assess fracture healing
Mechanobiological computer models have evolved during the last two decades from simple tools only able to analyse fracture healing at a single point in time to current models that can predict tissue differentiation and remodelling over time. The predictive capacity of these models is measured by the corroborating experiments used in their validation. A model is deemed more valid as more tests are used to either corroborate or refute the model.11
Recent models can capture many complex biological events that are involved during tissue regeneration, such as angiogenesis, stochasticity in cellular activities, and cell-phenotype specific activities. A priori, it is reasonable to assume that inclusion of a higher degree of complexity in a model will yield increasing accuracy. However, as the complexity of a model increases, more data from experiments are needed for corroboration. Jacobs and Kelly suggested that this fact could lead to a paradox of validation; the information needed for validation may obviate the model in first instance.12 Although, as long as the field develops, the predictive power of a model can go beyond the data used for its validation, eliminating the paradox of validation. Thus, mechano-biological models can provide insight into the mechanical regulation of tissue differentiation that standard experiments are incapable of, becoming a new technique to test scientific hypotheses.
Mechanobiological researchers have made efforts developing sophisticated models to achieve greater explanatory power. Generally, these models have been corroborated without incorporating animal variability; therefore, their ability to predict tissue differentiation in animals or specimens for which they have not been validated, is limited. Variability exists in the biological system in different levels such as at the organ and cellular levels. To account for cellular variability nondeterministic simulations following the lattice approach have been proposed to consider stochastic cell activities.9, 10 Nevertheless, more emphasis should be placed on understanding the main sources of variability in mechanobiology, instead of incorporating randomness to the parameters of the model.
The potential use of in silico models in clinical assessment
The vast majority of computational studies of fracture healing have involved animal models or used previously published bone healing data. As a whole, this research has demonstrated that fracture healing can be modeled using measures of strain and fluid flow at the fracture site and suggests that the mechanical environment can be controlled to affect bone healing outcomes. For clinical application, well-corroborated mechanoregulatory models accounting for patient-to-patient variability hold the potential to predict and assess fracture healing and help clinicians to customize treatment. Regarding patient-specific features in mechanobiological computer models, advanced imaging tools permit capture of patient-specific geometries to create an FE model for each patient. Furthermore, CT and X-ray information can be included in these FE models to add patient-specific material properties. Simulations could include other specifics as well, such as the weight or gender of the patient, perhaps even variability in physical activity levels.
Clinician-inspired studies have employed FE analysis to evaluate the mechanical properties of fixation constructs. The majority of these have focused on the concentration of stress within implants and comparisons of construct strength.13–16 There is also the potential for the use of FE analysis in translational research. Combining FE results with clinical outcomes has allowed for recommendations regarding implant design and surgical technique to reduce wear and dislocation with total hip arthroplasty.17 The ultimate goal of using FE to assess fracture healing is similar; to develop a research tool capable of guiding technological advances and improvements in clinical decision-making. An accurate FE model of clinical healing could allow advancements in our understanding of the mechanoregulation of bone healing as well as provide useful data regarding the mechanical strength of a healing fracture.
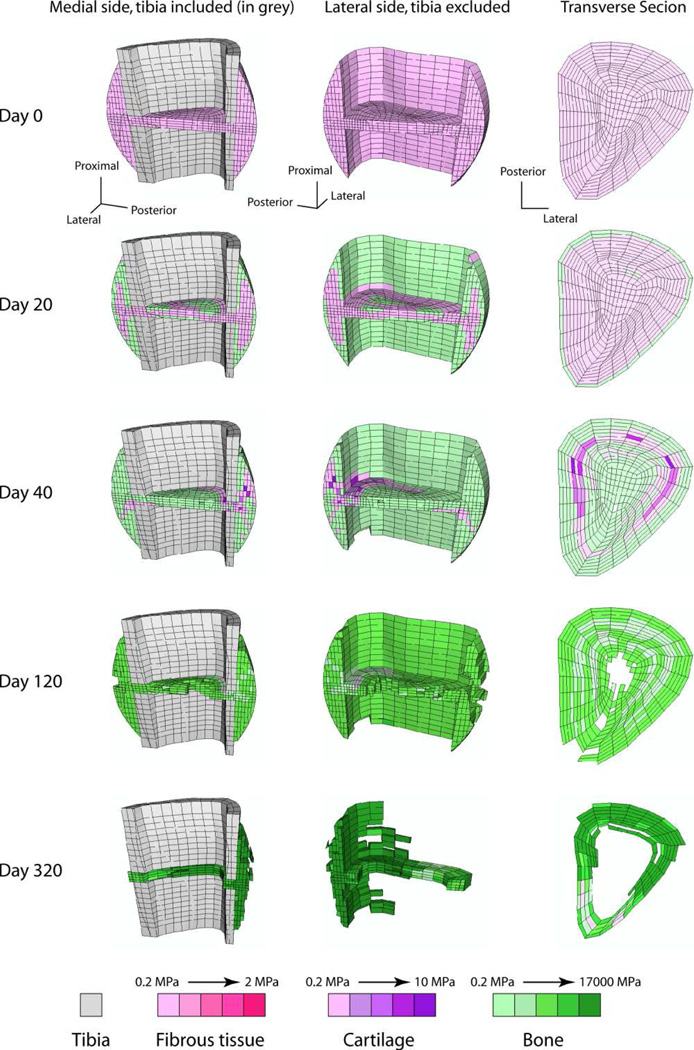
Recent FE-based studies of fracture healing have taken an increasingly translational approach to studying the effect of mechanical factors on fracture healing. Personalised FE modeling aims to employ mechano-regulatory algorithms to predict tissue differentiation for a specific patient. The mechanical stimuli during fracture healing come from the physical activity of the patient and are determined by the method of fixation. As discussed before, Byrne et al. simulated 3D fracture healing in a tibia under realistic muscle conditions and with an external fixator based on the combination of mechano-regulation of cell activities in a lattice approach with FE modelling.9 It successfully predicted the progression of tissue differentiation patterns in the callus of a 3D realistic anatomical fracture over time, which agreed with histological observations (Figure 2). Additionally, fracture healing was simulated beyond the reparative phase. Despite the limitations of this model, it demonstrates the potential of patient-specific in silico modeling to aid clinicians in choosing the optimal fixation construct, predicting the outcome of fracture healing and monitoring its progression.
Figure 2.
Cross-sectional view of the predicted healing patterns on a human tibia fracture under realistic muscle loading and an external fixator applied. The simulation was based in a combination of mechanoregulation of cell activities in a lattice approach with finite element modelling.9
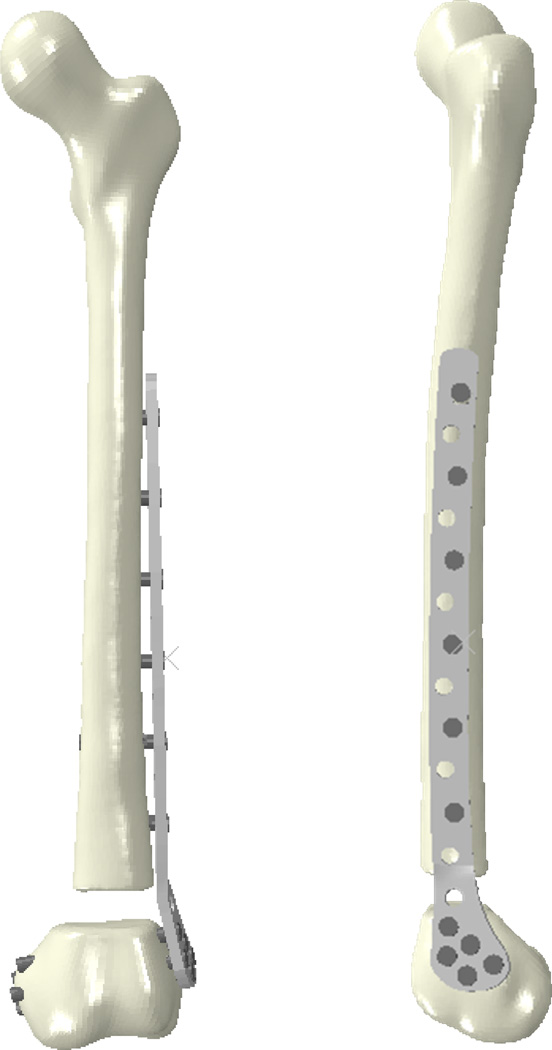
In other work, far cortical locking screw constructs have been modelled using FE analysis. That work has demonstrated increased parallel motion at the fracture site, which in turn has been associated with accelerated bone healing in an ovine osteotomy model.18, 19 Case-specific FE analysis was recently utilized in a clinical series of 64 supracondylar femur fractures treated with locking plate fixation to study the effects of varying constructs on 3D fracture gap motion and its relationship to fracture healing (Figure 3). FE-predicted vertical motion was found to promote callus formation while translational motion (shear) inhibited callus.20 These results corroborate a previous FE study that concluded the use of uni-axial interfragmentary strain was inadequate for predicting tissue differentiation.21
Figure 3.
Finite element model simulating a distal femur fracture treated with a locking plate construct. Screw position and plate/screw material was varied to study the effect of these construct parameters on 3D fracture gap motion.
Despite the fact that the concept of mechanoregulation of bone healing has been studied for over 40 years, an immense amount remains unknown. Although both animal and clinical studies have demonstrated a positive relationship between compressive motion and callus formation and an inhibitory effect of shear, the strain that optimizes fracture healing for a given fracture remains elusive.19, 20, 22, 23 Refining FE models to study the 3D strain-field within the fracture gap and adapting these models for clinical case-specific simulations holds the potential to provide more accurate examination of the relationship between strain and fracture healing in actual patients.
Barriers to in silico assessment of clinical fracture healing
While there is great promise in this area, there are barriers to the advancement of in silico assessment of clinical fracture healing. There is no consensus regarding the optimal radiographic criteria for predicting the final healing of fractures or when these criteria should be employed. The available literature reports varying definitions of radiographic union that do not allow findings to be directly compared.24 With limited objective data defining how healing progresses over time, and how this process varies for fractures which achieve union in comparison to those which go on to nonunion, criteria used to predict healing are the subject of significant ongoing debate.
Among radiographic criteria for healing, bridging callus has been found in basic science and clinical studies to be a relatively reliable radiographic sign (kappa 0.79–0.83) that predicts mechanical stability of a healing fracture better than other measures such as callus area and quality.25–30 However, there is little evidence regarding the minimum number of bridging cortices required for a fracture to be considered healed. Previous studies, including those satisfying FDA standards, have utilized bridging of three cortices as the radiographic criterion required to document a healed fracture.31 Alternatively, radiographic union has been defined as bridging of at least two cortices.32 Re-fracture following external fixator removal has been associated with bridging of less than three cortices in a pediatric population, but there is little evidence to suggest this is a universal concept, particularly in the setting of internal fixation.33
A significant limitation in predicting the final healing outcome of fractures is a lack of chronological 3D data for both normal and abnormal fracture healing (union, delayed union, and non-union). Most studies examine radiographic “snapshots” of fractures at varying times after injury. Recently presented evidence collected by examining all radiographs of 176 tibia fractures treated with intramedullary nailing from injury to their final healing outcome suggests that predicting clinical fracture healing can be highly accurate when radiographic fracture callus assessments are performed at the appropriate time post-injury. The presence of any radiographic bridging callus by four months postoperatively was highly sensitive and specific in predicting the final healing outcome.34 Given the delayed mineralization of callus during fracture healing and the limitations of plain radiographic assessments, 3D imaging may offer an even earlier and more accurate prediction of the final healing outcome as has been suggested for modalities such as ultrasound.32 Additionally, FE analysis offers the potential to demonstrate how imaging findings and patient symptoms correspond with increasing mechanical stability at the fracture site.
Toward more comprehensive image-based measures of fracture healing
Modern medical imaging systems have significantly advanced the capability for less invasive visualization of injured musculoskeletal tissues, but all too often the consideration of these rich datasets has stopped at the level of subjective observation. While undeniably helpful in understanding the general scope of injury, this approach provides but limited understanding of the injury itself.
CT scans are routinely acquired in evaluating problematic extremity fractures, and computational image analysis provides a framework for extracting objective metrics that characterize the injury. Central to such analysis is the identification of regions of interest in the image data. Subsequent analysis of the CT data can focus on direct intensity measures or on spatial measures of the local distribution of intensities (i.e., the image texture). The former approach readily captures regions of contrasting intensities, while the latter can capture more subtle differences in appearance. This is relevant in the context of fracture healing because the underlying biological process involves ordered progression from less to more mineralized tissues, and the greatest contrast in CT intensities will most often be between soft and hard tissues. More subtle gradations in tissue constituents can be difficult to identify on CT. Computational image analysis methods have not yet been applied to study fracture healing, but two comparable challenges addressed in this general area are the evaluation of fracture severity and of fracture-associated soft tissue injury.
Example 1: CT-based measures of fracture severity
Clinicians have long understood intuitively that the severity of a peri-articular fracture can be characterized by the amount of energy expended in fracturing the bone, and that the fracture severity is a key prognosticator for subsequent outcomes. Despite this intuitive appreciation, however, there has historically been no means to objectively quantify the fracture severity based on fracture energy. Recent progress in this area has built on fracture mechanics theory,35 which formally relates the fracture-liberated (de novo) surface area in a brittle solid, such as bone, to the energy of the fracture. CT provides a source from which to extract the de novo surface area, using computational image analysis.
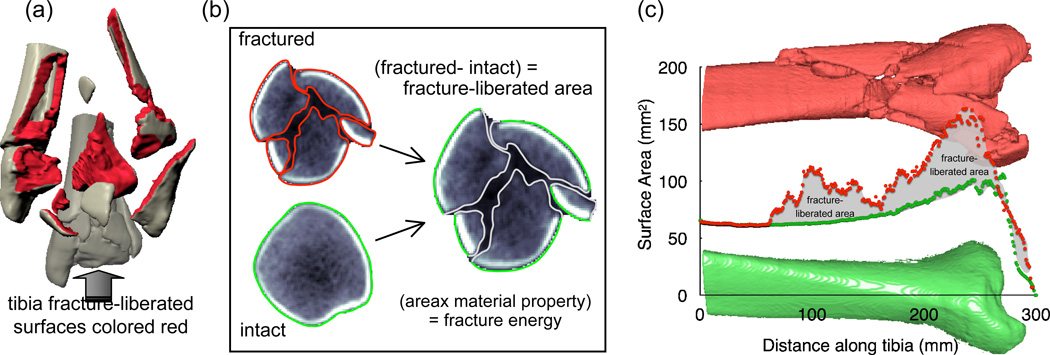
In order to identify and measure the fracture-liberated surface area from CT scan data, the free edges of bone must be extracted from the images on a slice-by-slice basis (Figure 4). The CT intensities along those edges enable density weighting of the surface area to accommodate variation in the properties from site to site, as well as between patients. By appropriately weighting the surface area according to the local bone density, an accurate measure of the fracture energy can be obtained. Furthermore, fracture fragmentation can be classified based upon its proximity to the articular surface, thereby prioritizing articular comminution in any severity assessment.
Figure 4.
(a) The fracture severity assessment methodology focuses on identifying and measuring the fracture-liberated surface area. (b) CT slices taken from a fracture case show the computationally identified tibia bone boundaries. (c) The bone surface areas (contralateral intact and fractured, from the same patient) plotted along the length of the distal tibia show the final basis for fracture energy calculation.
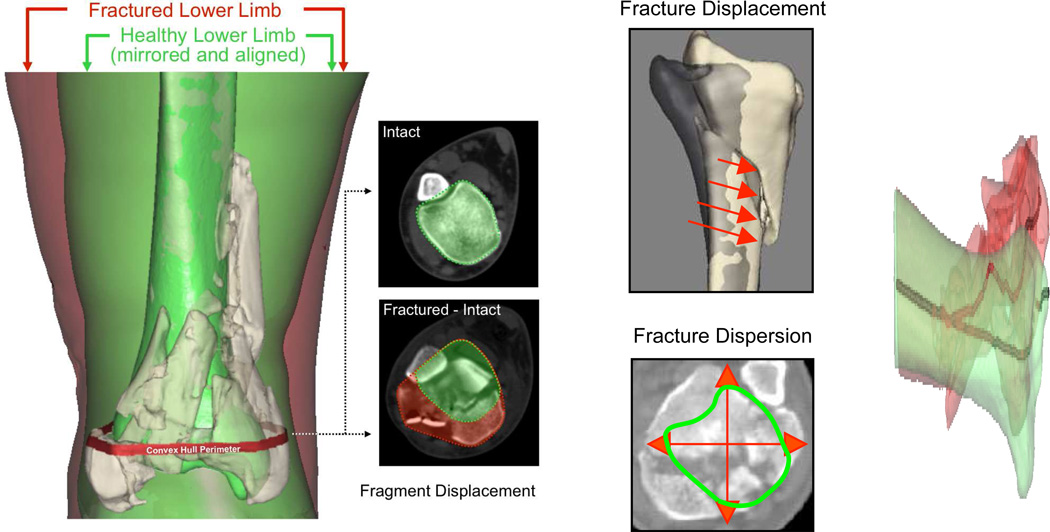
In addition to fracture energy, fragment displacement and dispersion are often used to characterize fractures. CT data also provide a basis for objectively assessing these factors. When fracture fragments are displaced, the bony regions in given cross-sections are generally translated away from their intact positions, the bone structure is disrupted, and fragments are dispersed relative to one another. The bone surfaces identified in fracture energy analysis may be used to quantify fragment displacement (Figure 5). Fragment displacement can be quantified by calculating the volume of tissues through which fracture fragments are collectively dispersed, relative to their pre-fracture position. The displaced tissue volume provides a metric of fragment dispersion that implicitly incorporates limb axial malalignment.
Figure 5.
Depiction of the fragment displacement/dispersion metric calculation. [Taken with permission from Anderson DD, Marsh JL, Brown TD. The pathomechanical etiology of post-traumatic osteoarthritis following intraarticular fractures.Iowa Orthop J. 31:1–20, 2011.]
CT-based measures of fracture severity, conceived in the laboratory, have been taken through the stage of validation36 and have since been applied to study patients with intra-articular fractures.37, 38 The aim of these studies was to correlate objective metrics of fracture severity with the clinical outcome. The studies involved a group of patients with unilateral tibial plafond fractures that were surgically repaired and subsequently followed for two years. CT-based metrics of fracture severity were correlated with two-year radiographic appearance of the ankles assessed by Kellgren-Lawrence (KL) grade.38
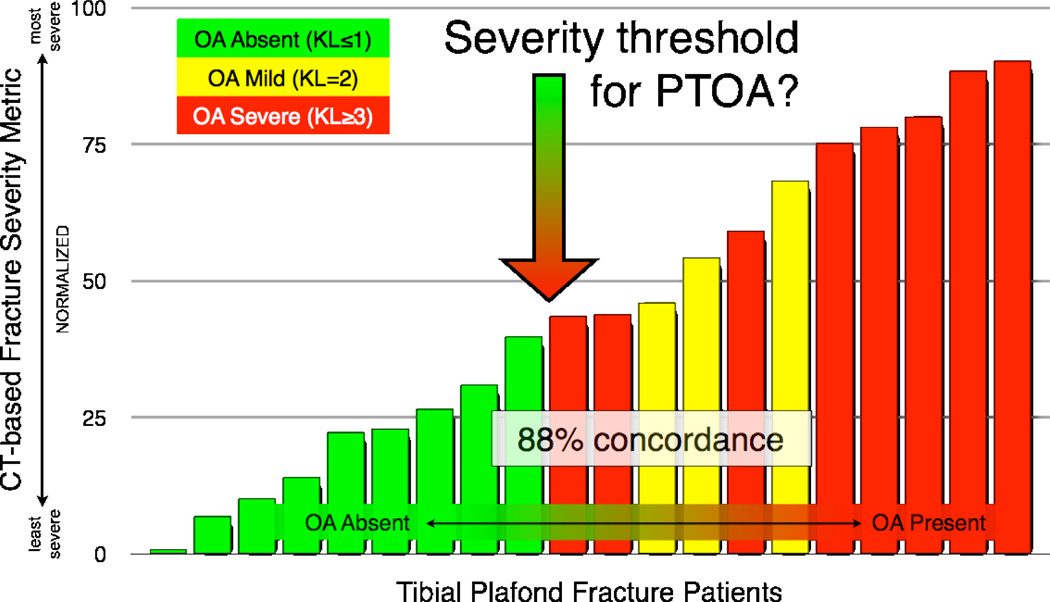
There was an 88% concordance found between the fracture severity metric and the KL grade. The computational image analysis results (Figure 6) also suggested a threshold of fracture severity that predicts whether a joint will develop post-traumatic osteoarthritis (OA). The existence of a fracture severity threshold has broad implications for the treatment of intra-articular fractures. In fractured joints identified as being most highly at risk to develop post-traumatic OA, the value of accurate surgical reduction should be especially carefully weighed against the backdrop of potential surgical complications. New interventions aimed at forestalling post-traumatic OA would certainly need to stratify patients according to their expected baseline risk for joint degeneration.
Figure 6.
The CT-based severity metric successfully discriminated between cases that developed PTOA and those that did not, in a threshold-like manner.
Example 2: CT-based measures of fracture-associated soft tissue injury severity
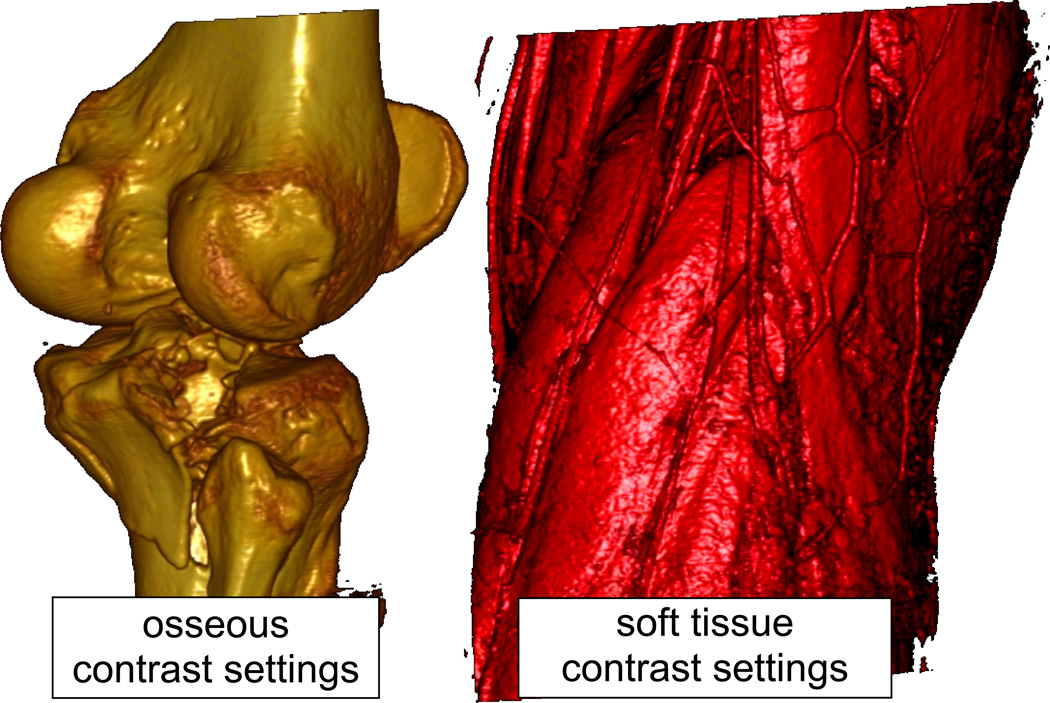
The severity of soft tissue injury is often the primary factor dictating the timing and type of surgical intervention to stabilize a severe fracture. Soft tissue structure is often overlooked in orthopaedic CT images, since the display contrast is focused on osseous structures. When the contrast is suitably adjusted, however, even standard clinical CT scanners depict soft tissues with vivid clarity (Figure 7). The normal anatomy of muscle and tendon, subcutaneous tissues, and fascia exhibit readily identifiable, fairly homogeneous structures in CT images. Previous work has documented an abundance of information about atraumatic soft tissue conditions embedded within CT scan data.39, 40
Figure 7.
Volume renderings from CT scans of a tibial plateau fracture case, showing the bones (left) and the surrounding soft tissues (right – same view). Note the exquisite detail with which even individual vessels are depicted on the image tuned for soft tissue contrast.
A CT based methodology was developed to assess and quantify fracture-associated soft tissue injury. A group of twenty-one patients with tibial plafond fractures were studied.41 Cases were specifically chosen to represent the spectrum of injury from mild partial articular fractures to severely comminuted fractures involving the entire tibial plafond.
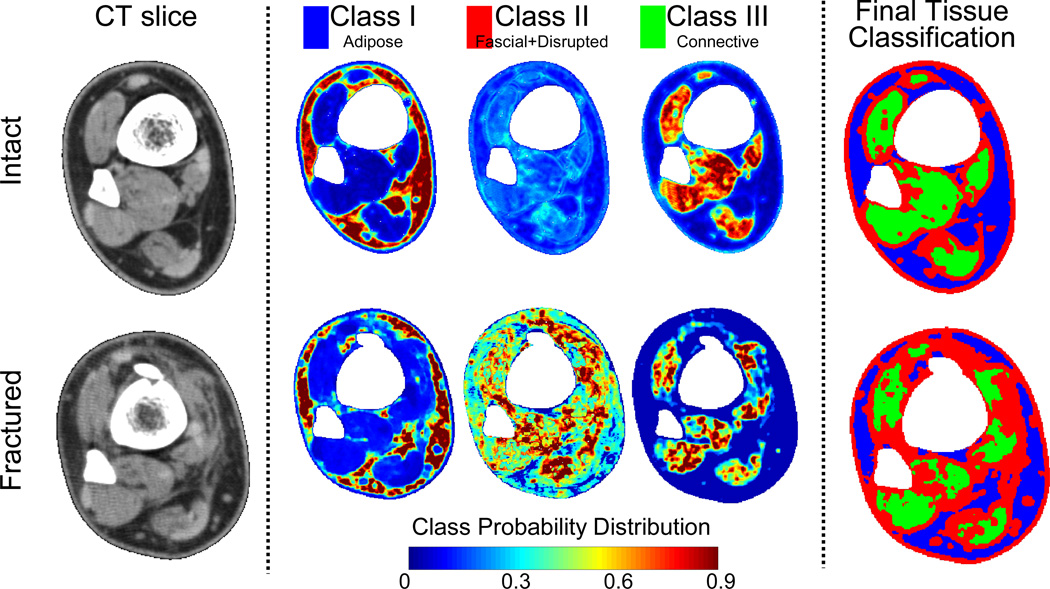
Following trauma, the CT appearance of soft tissues is altered, with decreased muscle attenuation (muscle appears darker), blurred tissue demarcations, increased subcutaneous tissue attenuation (appears lighter), and an overall edematous volumetric increase. These structural changes observed in CT scans can be quantified with measurable certainty using statistical texture modeling and probability theory.42 This approach reduces a given “texture” within an image to a characteristic local spatial population of intensities. The algorithm developed to perform these probabilistic textural assessments begins with supervised training data. Multivariate statistics performed on these samplings of CT intensity data provide probability density functions for the primary tissue classes (Figure 8), which are then used to calculate the likelihood that a given image region comes from a specific tissue. To assess the extent of injury, the probabilities associated with fascia/damaged regions (tissue Class II in Figure 8) were summed across the limbs, and the difference of these cumulative sums (fractured minus intact) was calculated, with the presumption that the Class II region in intact tibias was exclusively fascial tissue.
Figure 8.
CT sections (leftmost) from a fractured distal tibia (lower row) and its intact contralateral (upper row) typify changes with soft tissue trauma. Texture-based classification into three tissue classes yields probability density distributions (range of 0 to 1), with red (P≥0.9) pixels being the most likely matches to a given class.
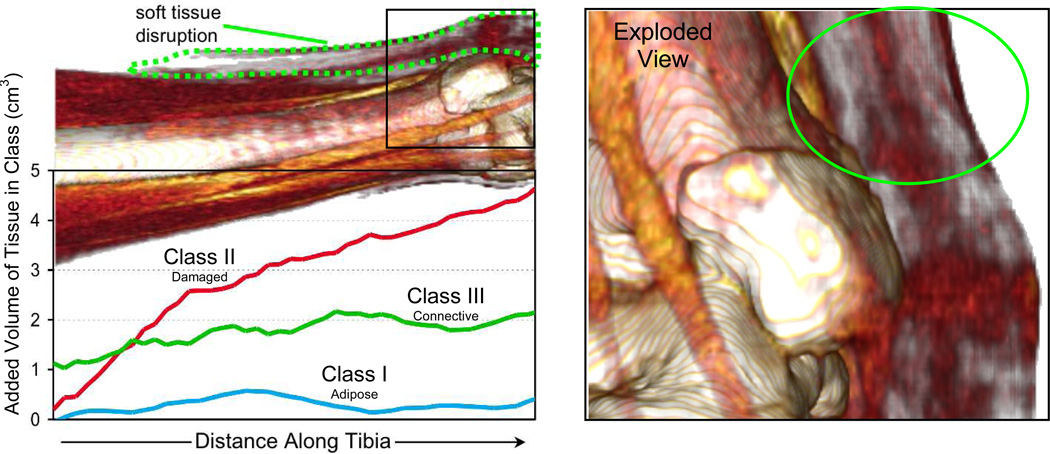
The resultant quantity, reflecting the overall structural disruption seen in the soft tissues, was compared to subjective clinical assessments of the soft tissue injury, made at the same time that CT scans had been obtained. An inspection of the different tissue classification volumes over the length of the distal tibia provides additional insight (Figure 9). Note virtually no change in the adipose tissue class (Class I) as a result of the injury, while Class III had a fairly constant change over the length of the tibia, an observation that appears to reflect diffuse edema. Tissue Class II (fascial/disrupted) volumes varied over the length of the distal tibia, evocative of the distribution of soft tissue injury. Comparison of the computed Class II probability-weighted volumes for the fractured and the intact contralateral tibias for two fracture cases with different degrees of associated soft tissue injury indicates the ability to discriminate between severity amongst fracture cases.
Figure 9.
Line plot of volumetric differences by class between fractured and intact limbs along the distal tibia. The exploded view to the right clearly shows soft tissue abnormality over the medial malleolus in this fairly benign fracture, with substantial soft tissue injury
Conclusion
Advances in the evaluation of fracture healing and improved understanding of the optimal mechanical environment for fracture healing provide new insight into the treatment of fractures. Our current understanding of fracture healing and how treatments can modify this process can be further developed with the application of computational techniques such as those described in this article. The clinical applicability of these concepts will rely upon multidisciplinary collaboration. Combining image analysis techniques, case-specific finite element simulations, and clinical outcomes has great potential for improving our understanding of the fracture healing process and our ability to select treatments which optimize the outcome for patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pauwels F. Biomechanics of fracture healing. In: Manquet P, Furlong R, editors. Biomechanics of the Locomotor Apparatus (translated from the 1965 German edition) Berlin: Springer; 1980. pp. 106–120. [Google Scholar]
- 2.Carter DR, Blenman PR, Beaupre GS. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res. 1988;6:736–748. doi: 10.1002/jor.1100060517. [DOI] [PubMed] [Google Scholar]
- 3.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 4.Prendergast PJ, Huiskes R, Soballe K. ESB Research Award 1996. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997;30:539–548. doi: 10.1016/s0021-9290(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 5.Isaksson H, Comas O, van Donkelaar CC, Mediavilla J, Wilson W, Huiskes R, et al. Bone regeneration during distraction osteogenesis: mechano-regulation by shear strain and fluid velocity. J Biomech. 2007;40:2002–2011. doi: 10.1016/j.jbiomech.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Huiskes R, Van Driel WD, Prendergast PJ, Soballe K. A biomechanical regulatory model for periprosthetic fibrous-tissue differentiation. J Mater Sci Mater Med. 1997;8:785–788. doi: 10.1023/a:1018520914512. [DOI] [PubMed] [Google Scholar]
- 7.Lacroix D, Prendergast PJ. A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech. 2002;35:1163–1171. doi: 10.1016/s0021-9290(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 8.Perez MA, Prendergast PJ. Random-walk models of cell dispersal included in mechanobiological simulations of tissue differentiation. J Biomech. 2007;40:2244–2253. doi: 10.1016/j.jbiomech.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Byrne DP, Lacroix D, Prendergast PJ. Simulation of fracture healing in the tibia: mechanoregulation of cell activity using a lattice modeling approach. J Orthop Res. 2011;29:1496–1503. doi: 10.1002/jor.21362. [DOI] [PubMed] [Google Scholar]
- 10.Checa S, Prendergast PJ. A mechanobiological model for tissue differentiation that includes angiogenesis: a lattice-based modeling approach. Ann Biomed Eng. 2009;37:129–145. doi: 10.1007/s10439-008-9594-9. [DOI] [PubMed] [Google Scholar]
- 11.Khayyeri H, Isaksson H, Prendergast PJ. Corroboration of computational models for mechanoregulated stem cell differentiation. Comput Methods Biomech Biomed Engin. 2013 doi: 10.1080/10255842.2013.774381. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs CR, Kelly DJ. Cell mechanics: the role of simulation. In: Fernandes PR, Bártolo PJ, editors. Advances in modeling in tissue engineering Computational methods in applied sciences. Springer; 2011. pp. 1–14. [Google Scholar]
- 13.Cegonino J, Garcia Aznar JM, Doblare M, Palanca D, Seral B, Seral F. A comparative analysis of different treatments for distal femur fractures using the finite element method. Comput Methods Biomech Biomed Engin. 2004;7:245–256. doi: 10.1080/10255840412331307182. [DOI] [PubMed] [Google Scholar]
- 14.Cheung G, Zalzal P, Bhandari M, Spelt JK, Papini M. Finite element analysis of a femoral retrograde intramedullary nail subject to gait loading. Med Eng Phys. 2004;26:93–108. doi: 10.1016/j.medengphy.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SJ, Wyss UP, Pichora DR. Finite element stress analysis of a hybrid fracture fixation plate. Med Eng Phys. 1996;18:241–250. doi: 10.1016/1350-4533(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Sim E, Freimuller W, Reiter TJ. Finite element analysis of the stress distributions in the proximal end of the femur after stabilization of a pertrochanteric model fracture: a comparison of two implants. Injury. 1995;26:445–449. doi: 10.1016/0020-1383(95)00079-o. [DOI] [PubMed] [Google Scholar]
- 17.Elkins JM, Stroud NJ, Rudert MJ, Tochigi Y, Pedersen DR, Ellis BJ, et al. The capsule's contribution to total hip construct stability--a finite element analysis. J Orthop Res. 2011;29:1642–1648. doi: 10.1002/jor.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(Suppl 1):S21–S28. doi: 10.1097/BOT.0b013e318207885b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottlang M, Lesser M, Koerber J, Doornink J, von Rechenberg B, Augat P, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92:1652–1660. doi: 10.2106/JBJS.I.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lack WD, Elkins J, Lujan T, Peindl R, Kellam J, Anderson DD, et al. 29th Annual Meeting of the Orhopaedic Trauma Association. Phoenix: AZ2013; Finite element analysis of the distal femur: Fracture motion predicts clinical callus. [Google Scholar]
- 21.DiGioia AM, 3rd, Cheal EJ, Hayes WC. Three-dimensional strain fields in a uniform osteotomy gap. J Biomech Eng. 1986;108:273–280. doi: 10.1115/1.3138614. [DOI] [PubMed] [Google Scholar]
- 22.Perren SM, Rahn BA. Biomechanics of fracture healing. Can J Surg. 1980;23:228–232. [PubMed] [Google Scholar]
- 23.Yamagishi M, Yoshimura Y. The biomechanics of fracture healing. J Bone Joint Surg Am. 1955;37-A:1035–1068. [PubMed] [Google Scholar]
- 24.Bhandari M, Guyatt GH, Tong D, Adili A, Shaughnessy SG. Reamed versus nonreamed intramedullary nailing of lower extremity long bone fractures: a systematic overview and meta-analysis. J Orthop Trauma. 2000;14:2–9. doi: 10.1097/00005131-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Hammer RR, Hammerby S, Lindholm B. Accuracy of radiologic assessment of tibial shaft fracture union in humans. Clin Orthop Relat Res. 1985:233–238. [PubMed] [Google Scholar]
- 26.Kooistra BW, Dijkman BG, Busse JW, Sprague S, Schemitsch EH, Bhandari M. The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma. 2010;24(Suppl 1):S81–S86. doi: 10.1097/BOT.0b013e3181ca3fd1. [DOI] [PubMed] [Google Scholar]
- 27.McClelland D, Thomas PB, Bancroft G, Moorcraft CI. Fracture healing assessment comparing stiffness measurements using radiographs. Clin Orthop Relat Res. 2007;457:214–219. doi: 10.1097/BLO.0b013e31802f80a8. [DOI] [PubMed] [Google Scholar]
- 28.Panjabi MM, Walter SD, Karuda M, White AA, Lawson JP. Correlations of radiographic analysis of healing fractures with strength: a statistical analysis of experimental osteotomies. J Orthop Res. 1985;3:212–218. doi: 10.1002/jor.1100030211. [DOI] [PubMed] [Google Scholar]
- 29.Whelan DB, Bhandari M, McKee MD, Guyatt GH, Kreder HJ, Stephen D, et al. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84:15–18. doi: 10.1302/0301-620x.84b1.11347. [DOI] [PubMed] [Google Scholar]
- 30.Whelan DB, Bhandari M, Stephen D, Kreder H, McKee MD, Zdero R, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68:629–632. doi: 10.1097/TA.0b013e3181a7c16d. [DOI] [PubMed] [Google Scholar]
- 31.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Moed BR, Subramanian S, van Holsbeeck M, Watson JT, Cramer KE, Karges DE, et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma. 1998;12:206–213. doi: 10.1097/00005131-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Skaggs DL, Leet AI, Money MD, Shaw BA, Hale JM, Tolo VT. Secondary fractures associated with external fixation in pediatric femur fractures. J Pediatr Orthop. 1999;19:582–586. [PubMed] [Google Scholar]
- 34.Lack WD, Starman J, Seymour R, Bosse M, Karunakar M, Sims S, et al. 29th Annual Meeting of the Orhopaedic Trauma Association. Phoenix: AZ2013; Any cortical bridging predicts healing of tibial shaft fractures. [DOI] [PubMed] [Google Scholar]
- 35.Beardsley CL, Bertsch CR, Marsh JL, Brown TD. Interfragmentary surface area as an index of comminution energy: proof of concept in a bone fracture surrogate. J Biomech. 2002;35:331–338. doi: 10.1016/s0021-9290(01)00214-7. [DOI] [PubMed] [Google Scholar]
- 36.Beardsley CL, Anderson DD, Marsh JL, Brown TD. Interfragmentary surface area as an index of comminution severity in cortical bone impact. J Orthop Res. 2005;23:686–690. doi: 10.1016/j.orthres.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson DD, Mosqueda T, Thomas T, Hermanson EL, Brown TD, Marsh JL. Quantifying tibial plafond fracture severity: absorbed energy and fragment displacement agree with clinical rank ordering. J Orthop Res. 2008;26:1046–1052. doi: 10.1002/jor.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas TP, Anderson DD, Mosqueda TV, Van Hofwegen CJ, Hillis SL, Marsh JL, et al. Objective CT-based metrics of articular fracture severity to assess risk for posttraumatic osteoarthritis. J Orthop Trauma. 2010;24:764–769. doi: 10.1097/BOT.0b013e3181d7a0aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauchamp NJ, Jr, Scott WW, Jr, Gottlieb LM, Fishman EK. CT evaluation of soft tissue and muscle infection and inflammation: a systematic compartmental approach. Skeletal Radiol. 1995;24:317–324. doi: 10.1007/BF00197058. [DOI] [PubMed] [Google Scholar]
- 40.Hadjis NS, Carr DH, Banks L, Pflug JJ. The role of CT in the diagnosis of primary lymphedema of the lower limb. Am J Roentgenol. 1985;144:361–364. doi: 10.2214/ajr.144.2.361. [DOI] [PubMed] [Google Scholar]
- 41.Thomas TP, Anderson DD, Marsh JL, Brown TD. 23rd Annual Meeting of the Orthopaedic Trauma Association. Boston, MA: U.S.A; 2007. CT-Based method for measuring soft tissue damage in highly comminuted fractures. [Google Scholar]
- 42.Cootes TF, Edwards GJ, Taylor CJ. Active appearance models. IEEE Trans Pattern Anal & Machine Intelligence. 2001;23:681–685. [Google Scholar]