Abstract
Cerebellar glioblastoma multiforme (GBM) is a rare tumor that accounts for only 1% of all cases of GBM and its giant cell variant is even much rarely encountered in adults. A case of cerebellar giant cell GBM managed at our institution reporting its clinical presentation, radiological and histological findings, and treatment instituted is described. In conjunction, a literature review, including particular issues, clinical data, advances in imaging studies, pathological characteristics, treatment options, and the behavior of such malignant tumor is presented. It is very important for the neurosurgeon to make the differential diagnosis between the cerebellar GBM, and other diseases such as metastasis, anaplastic astrocytomas, and cerebellar infarct because their treatment modalities, prognosis, and outcome are different.
Keywords: Cerebellar glioblastoma multiforme, giant cell glioblastoma multiforme, posterior fossa glioma
Introduction
Glioblastoma is the most frequent tumor among all primary intracranial tumors with a frequency of approximately 15-50%. It can be seen in all age groups; however, patients are generally older than 50 years. When compared with childhood tumors, localization in the posterior fossa in adults is rare.[1] Cerebellar glioblastoma multiforme (GBM) is a rare tumor that accounts for only 1% of all cases of GBM and its giant cell variant is even much rarely encountered in adults.[2]
Case Report
A 51-year-old woman complained of headache with vomiting and gait ataxia of 1 month duration. She also noted diminution of her vision during the course of illness. She denied any difficulties in chewing or swallowing. She had 6/36 visual acuity and mild papilledema bilaterally. Her gait was ataxic in nature and had positive cerebellar signs. Hermental status and other cranial nerve examinations were normal. Magnetic resonance imaging scan showed a left cerebellar intra-axial mass lesion near cerebellopontine angle with T1-weighted hypointensity and T2-weighted hyperintensity with mild perifocal edema and mass effect [Figure 1]. On contrast study, the lesion was enhancing homogeneously with area of unenhanced cystic component in it. Magnetic resonance (MR) spectroscopy showed choline peak with reduced N-acetyl aspartate (NAA) and choline: NAA ratio of 5.74. Lactate peak [Figure 2] was also observed at some places. Metastatic tumor, malignant lymphoma, and GBM were considered for radiological differential diagnosis. Workups for common metastatic lesions from breast, lung, colon, and secondary central nervous system lymphoma were found to be negative.
Figure 1.
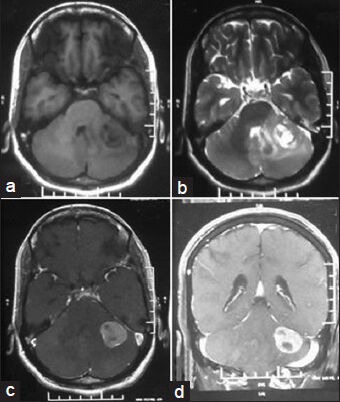
Magnetic resonance imaging scan showing a heterogeneous intra axial mass in left cerebellar hemisphere with T1 hypointensity, (a) T2 hyperintensity, (b) with contrast enhancement, (c) and coronal section showing same tumoral aspect (d)
Figure 2.
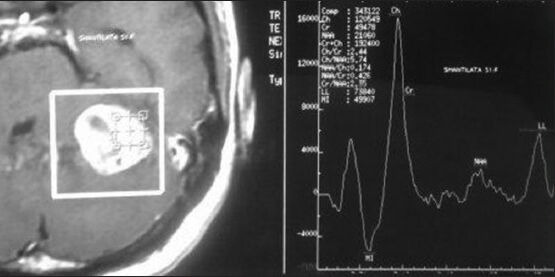
MR spectroscopy showing choline peak with decrease in creatine and N-acetyl aspartate peak with at places lactate peak compatible with GBM pattern
Through a left retromastoid suboccipital craniectomy a greyish-pink, soft, noncapsulated, vascular tumor in the left cerebellar hemisphere was excised in piecemeal near totally [Figure 3]. Diagnosis of giant cell variant of GBM was made on histopathological section [Figure 4a–c]. Then the patient was referred for radiotherapy. At follow up after 2 months, the patient has been doing well without any cranial nerve deficit.
Figure 3.
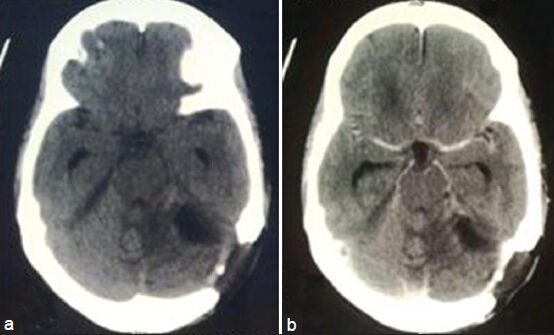
Post-op CT scan plain, (a) and contrast, (b) showing near total resection of the tumor
Figure 4.
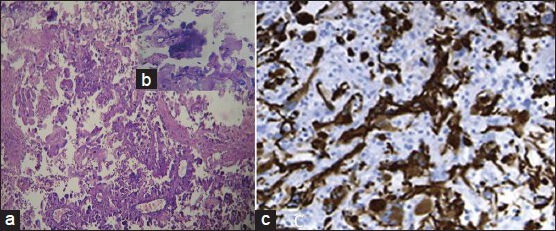
Photomicrographs of Giant-cell N-acetyl aspartate showing numerous bizarre multinucleated giant cells, (a) H and E ×200, (b) H and E ×400 and, (c) Immunohistochemistry showing glial fibrillary acidic protein positivity)
Discussion
Glioblastoma is the most frequent tumor among all primary intracranial tumors with a frequency of about 15%-50%. It can be seen in all age groups; however, patients are generally older than 50 years.[1] When compared with childhood tumors, localization in the posterior fossa in adults is rare. Giant cell variant of GBM is even much rarely encountered in adults.[2]
The giant cell GBM is a histological variant of glioblastoma presenting a prevalence of bizarre, multinucleated (more than 20 nuclei) giant (up to 400 μm diameter) cells. The giant cell glioblastoma was originally termed “Monstrocellular brain tumor” by Schmincke in early twentieth century due to its stromal reticulin network but it was first called giant cell glioma by Meyer in 1913. The astrocytic nature of the tumor was firmly established through the consistent Glial fibrillary acidic protein (GFAP) expression, so it belongs to World Health Organization grade IV tumor of astrocytic origin.[3] Prominent nucleoli, typical and atypical mitotic figures, irregularly shaped chromatin fragments, and necrosis, namely, pseudopalisading or large ischemic forms are characteristic.[4] The accumulation of tumor cells around blood vessels may originate pseudorosette formation. An in vitro study showed that some giant multinucleated cells might have originated from conversion of number of small tumor cells of astrocytic origin.[4] Immunohistochemistry studies show staining for GFAP, vimentin, S-100 protein, and α1-antichymotrypsin. Numerous mitoses, atypical mitosis, and necrosis with pseudopalisading differentiate it from pleomorphic xanthoastrocytoma. More mitotic figures with high leveling index will differentiate it from anaplastic astrocytoma.[4]
Cerebellar GBM is a rare tumor that accounts for only 1% of all cases of GBM and its giant cell variant is even much rarely encountered in adults. Giant cell GBM predominates in cerebral hemispheres mainly temporal and parietal lobes but cerebellum, lateral ventricles, and spinal cord are rare sites.[4] The mean age at clinical presentation is fourth decades of life and affects males more frequently than females (M:F ratio is 1:6).[3,4]
Glioblastomas appear as heterogenous masses on MR imaging due to the formation of necrosis and/or cysts. On T1-weighted images, the cystic part looks hypointense and solid part looks isointense; however, on T2-weighted images they are heterogeneously hyperintense. MR spectroscopy is a useful method in differentiating these tumors from other diseases. In GBM choline: Creatine ratio is more than 3:1 and NAA peak is reduced.[1] Decrease in NAA is related to neuron loss due to tumor, decrease in creatine due to metabolic changes, and increase in choline is due to increase in membrane synthesis and cells. Diffusion-weighted imaging (DWI) is also used in the differential diagnosis of rim-enhancing cerebellar mass lesions.[1] Cystic or solid components of brain tumors display high apparent diffusion coefficient (ADC) values and low signals in DWI with a high b value. Cystic or necrotic components of these tumors exhibit low ADC values on ADC maps, and low signal on high b-value DWIs. To the contrary, while cavity content of abscesses is seen as high signal intensities in diffusion sequence images, ADC values are low. In a recent study, it was found that the intensely enhancing solid components of hemangioblastomas demonstrated low signals in DWI together with high ADC values.[1]
Considering the different treatment modalities available, it is very important to make a proper differential diagnosis between infratemporal GBM and metastasis, hemangioblastoma, and also cerebellar infarct with contrast enhancement.[1,5,6] Considering malignant glial tumors, it would be always reasonable to attempt a gross-total tumor resection; however, the infiltrative nature of the GBM makes it difficult to achieve it many times. The use of radiation therapy after radical surgical resection is a well-established modality of treatment. Chemotherapy is not well established but is the only reasonable adjuvant treatment for very young children.[5,7] The biological behavior of cerebellar and supratentorial GBM is similar. On the other hand, it is possible that cerebellar GBM found in young patients has a better prognosis as in anaplastic astrocytomas, which have a longer survival time than supratentorial GBM. The median survival for cerebellar GBM is known to be approximately 19 months.[2]
Conclusion
Although GBM is the most frequent primary brain tumor in adults, its localization in the cerebellum is extremely rare. However, GBM must be considered in the differential diagnosis of aggressive mass lesions of the cerebellar hemisphere. Routine MRI findings and clinical symptoms are insufficient for an accurate diagnosis. Therefore, MR spectroscopy, DWI, and brain perfusion imaging technique may be used in prospective evaluation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Demir MK, Hakan T, Akinci O, Berkman Z. Primary cerebellar glioblastoma multiforme. Diagn Interv Radiol. 2005;11:83–6. [PubMed] [Google Scholar]
- 2.Hur H, Jung S, Jung TY, Kim IY. Cerebellar glioblastoma multiforme in an adult. J Korean Neurosurg Soc. 2008;43:194–7. doi: 10.3340/jkns.2008.43.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozak KR, Moody JS. Giant cell glioblastoma: A glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol. 2009;11:833–41. doi: 10.1215/15228517-2008-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobão CA, Barbosa AS, Nogueira J, Aversa A. Cerebellar glioblastoma multiforme in an adult. Arq Neuropsiquiatr. 2008;66:879–80. doi: 10.1590/s0004-282x2008000600020. [DOI] [PubMed] [Google Scholar]
- 5.Valle-Folgueral JM, Mascarenhas L, Costa JA, Vieira F, Soares-Fernandes J, Beleza P, et al. Giant cell glioblastoma: Review of the literature and illustrated case. Neurocirugia (Astur) 2008;19:343–9. doi: 10.1016/s1130-1473(08)70221-5. [DOI] [PubMed] [Google Scholar]
- 6.Vermani N, Mann HS, Brar RS, Bagai M. Cerebellar glioblastoma presenting with clinical and imaging features of posterior circulation stroke. J Postgrad Med. 2010;56:152–3. doi: 10.4103/0022-3859.65287. [DOI] [PubMed] [Google Scholar]
- 7.Mattos JP, Marenco HA, Campos JM, Faria AV, Queiroz LS, Borges G, et al. Cerebellar glioblastoma multiforme in an adult. Arq Neuropsiquiatr. 2006;64:132–5. doi: 10.1590/s0004-282x2006000100028. [DOI] [PubMed] [Google Scholar]


