Abstract
Evidence from experimental models has suggested that acute activation of brain stress and anxiety pathways impacts subsequent behaviors that are mediated or modulated by limbic circuitry. There have been limited investigations of prior or chronic activation of these pathways on subsequent limbic-mediated behaviors. In this study, we tested whether recurrent administration of the anxiogenic compound yohimbine (YOH) could have post-injection effects on brain activation, stress hormones, and performance in sucrose self-administration and startle response paradigms. Rats received six injections across two weeks of either 2 mg/kg YOH or saline. Behavioral evaluation confirmed the continued efficacy of the YOH regimen, and increased adrenal corticosterone (CORT) was observed. Several days following YOH or SAL administration, cFos, CORT and adrenocorticotropin hormone (ACTH), and behavioral performance were measured. cFos was elevated post-YOH in the hippocampus; ventral tegmental area/zona inserta; and central and medial nuclei of the amygdala. This activation is consistent with a sustained effect of YOH to activate fear and anxiety circuitry in the CNS. CORT but not ACTH was elevated in the YOH-rats following startle testing. Self-administration and startle tests suggested an increase of non-specific activity in the post-YOH rats; there was no increase in sucrose self-administration or startle response per se. Our findings suggest that recurrent YOH administration may prove a useful and reliable model for simulating recurrent stress/anxiety, and that enhancements to the paradigm such as higher or more frequent dosing of YOH could yield stronger or more extensive behavioral effects.
Keywords: rats, glucocorticoids, cFos, stress
INTRODUCTION
Chronic stress leads to both indirect behavioral and direct metabolic consequences that contribute to obesity and risk for cardiovascular disease. For example, an extensive literature argues persuasively for strong effects of stress on aspects of food intake behaviors, which can be substantially modulated by lifestage and lifestyle factors. In rats, chronic stress paradigms are associated with increased ‘comfort food’ intake, and this in turn modulates the hypothalamic-pituitary-adrenal (HPA) axis [1,2]. In humans, chronic intermittent stressors [3,4] lead to changes of food preferences and choices of high-fat, high sugar-containing snacks. Anxiety disorders are also associated with altered food intake behavior and energy homeostasis. The extreme anxiety syndrome, post-traumatic stress disorder (PTSD), has been associated with increased obesity [5], and may be a risk factor for metabolic syndrome [6].
Self-administration of rewarding substances is facilitated by stress and its underlying CNS and neuroendocrine mediators. Studies from Piazza, LeMoal, and colleagues have demonstrated the ability of glucocorticoids to facilitate drug-taking activity in rodents [7,8]. Extensive research has shown that stress, acute corticotropin-releasing factor (CRF), and acute CNS noradrenergic activation lead to enhanced reinstatement or relapse, for, e.g., alcohol [9] and cocaine [10]. Shaham and colleagues have developed a model of acute yohimbine (YOH) administration immediately prior to a self-administration session as a surrogate for an acute stress/anxiety experience, which increases drug- and sucrose-seeking and reinstatement [11,12]. YOH is an alpha2 adrenoceptor antagonist which stimulates endogenous norepinephrine release in the CNS, arousal, perceptions of anxiety in humans, and anxiety- or fear-related behaviors in animals [13-16]. Motivation for sucrose, and sucrose-seeking, activate many of the identical pathways that are activated by drug seeking and motivation [17,18]. Reinstatement of sucrose-taking by rats can be activated by a single acute YOH treatment [12]. In summary, stress (HPA axis activation) may increase sucrose self-administration, and acute anxiety may increase sucrose craving. A combination of these factors may result in strong drive to seek and consume sweets, and motivation for sweet food may result in excess intake of sugars (and concomitant fats). This behavior would contribute risk for impaired metabolic and cardiovascular health (e.g.,[19]).
Circumstances of traumatic stress result in activation not only of the HPA axis, but CNS arousal mechanisms, including catecholaminergic pathways and CRF-ergic neurons that are independent of HPA axis circuitry, for example within the amygdala, a key CNS locus for emotional memory, and fear (see [20] for discussion). In circumstances of recurrent traumatic stress, this circuitry can become sensitized and contributes to the development or experience of PTSD [15]. In animal studies, the amygdala, nucleus accumbens, bed nucleus of the stria terminalis, hippocampus, ventromedial hypothalamus (VMH), and some prefrontal cortex regions have been identified as key CNS components of fear circuitry. Noradrenergic activity in the amygdala and other markers of enhanced CNS noradrenergic activation are elevated in PTSD [13,14,21,22].
Whereas numerous studies confirm the acute or immediate effects of activation of CNS stress/anxiety pathways on behaviors sensitive to CNS noradrenergic activity or HPA axis activation, findings about the consequences of prior experience of stress/anxiety are only beginning to emerge. In the present study we tested whether a recurrent YOH administration paradigm could serve as a model of chronic stress. We characterized behavioral responses to injections, and measured food intake, body weight, and stress hormones during the YOH treatment period. We further measured cFos as a marker of brain region activation; stress hormones; and sucrose self-administration and startle responses in the week following the YOH regimen.
MATERIALS AND METHODS
Subjects
Subjects were male Albino rats from Simonsen (Gilroy, CA). Rats were maintained on chow (Laboratory Rodent Diet 5001, LabDiet) ad libitum. They were maintained on a 12:12 h light-dark cycle with lights on at 6 AM. Rats were brought in at 6 weeks of age, immediately post-weaning, and were housed for acclimation until 8 weeks of age. At this age, the YOH protocol was begun (8-10 wk). In two cohorts of rats, three days after the last YOH injection, rats were euthanized for measurement of brain activation via quantitative immunocytochemistry for cFos (see below). One cohort of rats was pre-implanted with chronic IV catheters for conscious blood sampling and measurement of stress hormones at multiple time points (adrenocorticotrophic hormone [ACTH] and corticosterone [CORT], see below); this cohort of rats was taken through a startle response protocol three days after the last YOH injection, as an indicator of generalized arousal (see below). One cohort of rats was taken through the self-administration training and testing protocol, beginning three days after the last YOH injection. The timing of these measurements was picked to evaluate rats post-YOH treatment for prolonged effects of the chronic YOH. All procedures performed on the rats followed the NIH guidelines for animal care, and were approved by the Animal Care and Use Subcommittee of the Research and Development Committee at the VA Puget Sound Health Care System (MIRB# 01481).
YOH administration and behavioral observation
Rats were weighed and randomized for intraperitoneal saline (SAL) or (YOH) injections given on Monday, Wednesday, and Friday for two weeks (six injections total). The dose of YOH, 2 mg/kg, was based upon its efficacy in both stress and drug self-administration paradigms, and can be considered a mid-range dose based upon dose response data from studies in rats [11]. In the morning shortly after ‘lights on’, rats were transported to a procedure room separate from their housing room, and placed in clean cages for a 3h period following injection of SAL or YOH. Two observers noted dominant activities of the rats at 30 min intervals. Rats had a few chow pellets in the cages and water bottles. Rats were described as: asleep, quiet, grooming, eating, digging in shavings, active/exploring/moving, lying on belly. All rats from the different cohorts (described above) had this behavioral characterization carried out and a compilation of the behavioral characterization is summarized in RESULTS. Behaviors were collapsed into ‘Asleep’; ‘Quiet’ which included limited movement or grooming; ‘Eating’; and ‘Exploring’ which included digging, exploration, and vertical and active horizontal movements (e.g., curling up to sleep would not be considered ‘Active’). The behavioral characterization was carried out for each of the six injection sessions, to determine whether behavioral accommodation to the YOH injection occurred (i.e., an index of habituation to YOH).
Sucrose self-administration
Procedures were based upon our published methodology [23,24]. All self-administration sessions were carried out between 0700 and 1200 hr. The protocol included initial autoshaping followed by five fixed ratio (FR) sessions. Rats were trained to self-administer 5% sucrose (0.5 ml reward) delivered into a liquid drop receptacle. The operant boxes, controlled by a Med Associates (Georgia, VT) system, had two levers, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. The sucrose solution was delivered into a liquid drop receptacle for oral consumption (Med Associates). After auto-shaping, self-administration was conducted during one-h sessions for five days under a continuous reinforcement schedule (FR1: each lever press was reinforced), with a maximum possible of 50 sucrose rewards delivered per session. Each session began with the insertion of the active lever and the illumination of a white houselight that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background)+light (7.5 W white light above the active lever) discrete compound cue accompanied each reward delivery, followed by a 20-sec time out after each sucrose delivery.
cFos Immunocytochemistry (ICC) and Quantitation
Fluorescence ICC was used to identify and quantitate Fos-positive neuronal cell bodies in response to a history of YOH vs. SAL injections, according to our established methodology [23,24], as a marker of brain regional activation. On the Monday following the injection series (i.e., three days after the last injection), rats were moved into the experiment room at the same time as for previous injections, and put in the same cages in which they had received injections. After 90 minutes in their cages (with no injection), rats were deeply anesthetized with isoflurane inhalation and perfused with 0.9%NaCl followed with cold 4% paraformaldehyde solution. Thus cFos expression would reflect the activation of the CNS at the onset of the behavioral task, rather than being the result of the animals experiencing the task. Brains were removed and post-fixed in paraformaldehyde several days, then subsequently placed in 20% sucrose-PBS, then 30% sucrose-PBS solution. Brains were sectioned on a cryostat (Leica CM 3050S cryostat) for immunohistochemistry. Slide-mounted 12 μm whole-brain coronal sections were washed three times in phosphate buffered saline (PBS, OXOID, Hampshire, England). Sections were washed for 20 min with 100% ethanol/DI water (50%, v/v) followed by a PBS wash, then blocked for 1 hour at room temperature in PBS containing 5% normal goat or donkey serum. Sections were then washed multiple times in PBS and incubated overnight at 4°C in primary antibody solutions made up in PBS. Sections were washed three times in PBS and then incubated in the dark at room temperature in secondary antibody solution made up in PBS for 1 hour. Sections were subsequently washed again in PBS, and mounted and cover-slipped in Vectashield hard set mounting medium (Vector; Burlingame, CA) mounting medium. Digital images of sections were acquired using a Nikon Eclipse E-800 fluorescence microscope connected to a Qimaging Retiga digital capture camera using NIS Elements (Nikon) software.
Atlas-matched 12 μm sections were evaluated for cFos quantitation and comparison between SAL and YOH conditions, in matched sections and regions at 20x magnification, based upon the atlas of Paxinos and Watson [25]. NIS Elements software (Nikon) was utilized to capture an image of the desired area. An area was delineated for counting and threshold for positive cell counts was established. The identical area and background (threshold) were utilized for sections from the respective experimental groups, and software counting of positive cells (quantitation) was carried out in the same session for all experimental groups, to prevent between-session changes in background setting. For statistical analysis, counts were taken from an individual rat only if corresponding or complete sections through each area were available; data for a specific area were not taken from a rat if there was incomplete bilateral representation for that area.
Startle response
Startle measurements were carried out according to the method of Rasmussen and colleagues [26]. This protocol included pre-test acclimation and startle test day. For pre-test acclimation, rats had three habituation days prior to testing, consisting of placing the rat in the cylindrical enclosure for 5 min with the chamber illumination off and with constant 60 dB background white noise. Two startle habituation sessions were on Tuesday and Thursday of Week 2 of injections (i.e., after the fourth and after the fifth injection day) and then on the following Monday (three days after the last injection day). 24h later the rats were taken through the startle protocol.
The acoustic startle procedure was performed with an SR-LAB system (San Diego Instruments) which includes a ventilated sound-attenuated cabinet containing a Plexiglas cylindrical rat enclosure mounted on a piezoelectric accelerometer which detects movement (the startle). The force exerted on the accelerometer during movement is digitized and expressed by the SL-LAB system in millivolts. An integrated tweeter provides background white noise and startle stimuli. Startle response signals generated by the accelerometer are recorded as 65 consecutive 1-ms recordings, starting at the onset of each startle stimulus. Results are analysed as maximum peak amplitude. A 40-ms 100 dB white noise startle stimulus has been determined by the Rasmussen lab to provide robust but sub-maximal startle responses when administered during background 60 dB white noise. Sound levels are calibrated with a Radio Shack Digital Sound Level Meter (33-2055) placed in the center of the cylindrical rat enclosure. Testing in semi-randomized order was conducted 2-4 hr after onset of light (i.e., a.m.). Each rat had a 5-min acclimation period (comparable to the habituation sessions) before startle responses to 10 consecutive presentations of a 40-ms 100 dB white noise stimulus. There was a 30-sec interval between sequential presentations of the acoustic stimulus. Each rat had 10 sequential trials in the session. Accelerometer data were collected for each startle trial as well as for the 5-min ‘pre-startle’ time immediately prior to the startle stimulus series. Rats were euthanized 90 min after the beginning of the startle stimulus series.
ACTH and corticosterone (CORT) assays
ACTH and CORT were measured on several occasions throughout the study to determine acute or lasting effects of YOH treatment. Blood samples were obtained from chronically implanted IV cannulas to allow conscious, non-disruptive sampling. Intravenous cannulas were implanted about one week prior to study, as per our established methodology (e.g. [24]). Following recovery from surgery, baseline a.m. samples were obtained from all the rats three days prior to the YOH/SAL injection schedule. Samples were also obtained 2.5 hr after injection, on Injection Day 3 and Injection Day 5. Samples were also obtained 10 min after startle response testing. Finally, terminal blood was collected from rats at the time of euthanasia (isoflurane) and a terminal sample was measured as well. All blood samples were collected on aprotinin cocktail. Separated plasma was frozen at −80°C until assay with commercial radioimmunoassay kits that we have used previously: The Immunoradiometric (IRMA) Assay kit (I-125) (Scantibodies Laboratory, Inc., Santee, CA), and The ImmunoChem Double Antibody Corticosterone I-125 RIA Kit from MP Biomedicals, LLC. Orangeburg, NY.
Statistical Analysis
Behavioral observation data acquired during the injection sessions were scored as 0 or 1 (absent or present) at 30 minute intervals of each session. These were analyzed using Two Factor with Replication Analysis of Variance (ANOVA) for comparisons within injection session, between sessions, and between treatments as described in the RESULTS. Body weight, food intake, and cFos data were also analyzed with single factor ANOVA. Other specific comparisons utilized one-tailed T-test, testing the pre-hoc hypothesis that YOH treatment would increase the parameter of interest (ACTH, CORT, post-hoc behavioral parameter, cFos immunoreactivity). Significance level was p≤ 0.05.
RESULTS
Behavioral Response to YOH injections
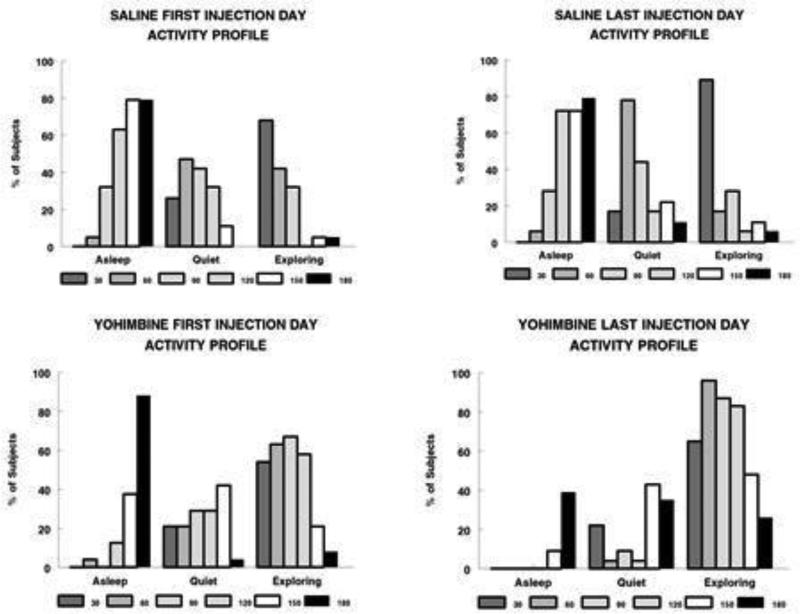
Figure 1 summarizes the key observations of behavioral effects of repeated injections of YOH (n=24) or SAL (n=19). Activities that included horizontal or vertical (rearing) movements, digging in shavings, sniffing, grooming, and eating or handling food pellets were collapsed into the category “Exploring”. There were almost no recorded instances of drinking from water bottles. Behavioral profiles from the first and final (sixth) injections are shown in Figure 1, and specific significant differences are summarized in Table 1. Two main observations are the differences between SAL and YOH which were sustained across all six injections, and the lack of behavioral habituation to YOH between first and last injections. Observations were made at 30 min intervals for 3 hr post-injection. Whereas SAL-injected rats were initially active immediately post-injection, rats reverted to quiet movements and sleep by the end of the session, consistent with the circadian timing of their natural sleep cycle. In contrast, the YOH rats showed delays in returning to sleep and sustained ‘exploring’ activity following the first injection. As a result of the multiple injection sessions, during the final injection session, SAL rats shifted more quickly from ‘exploring’ to ‘quiet’ to asleep, compared with the first SAL injection. More YOH rats were ‘exploring’, for a more extended time during the session, as well as less ‘asleep’ time at the sixth YOH injection compared to the first. This suggests that not only did habituation to the repeated YOH paradigm not occur, but that perhaps sensitization to the treatment was occurring, at least at the behavioral level. Finally, there were some unique observations in the YOH-treated rats: flushing of the snout and paws; ‘lying on belly’ behavior within the first 60 min post-injection; and piloerection. These were very infrequent but only observed in the YOH rats.
Figure 1.
Acute (3hr) effect of YOH (n=24) or SAL (n=19) injections on behavior patterns. Data are shown for the first and last (#6) injection day. Data are expressed as the percent of subjects receiving either YOH or SAL that manifest main behaviors of ‘Asleep’, ‘Quiet’, or ‘Exploring’ behavior at 30 minute intervals following injections (see “Methods” for further details). Each rat was scored ‘0’ or ‘1’ for each behavior at each time point, across all injection sessions.
TABLE 1.
Statistical Analysis of Behavioral Responses to SAL or YOH Injections
| A. The pattern of the specific behavioral response (Asleep, Quiet, or Exploring) across the 180-min observation period differs between SAL and YOH (p<0.05 for comparisons listed below) on each Injection Day. / |
| Injection 1—SAL vs. YOH for Asleep, Quiet, or Exploring (6 timepoints) |
| Injection 2-- “ |
| Injection 3-- “ |
| Injection 4-- “ |
| Injection 5-- “ |
| Injection 6-- “ |
| B. The individual response (Asleep, Quiet, or Exploring) pattern across the 180 minute observation period shifted as a function of Injection Day, for either YOH or SAL (P<0.05 for comparisons listed below). |
| YOH-Asleep |
| YOH-Quiet |
| YOH-Exploring |
| SAL-Asleep |
| SAL-Quiet |
| SAL-Exploring |
| C. The pattern of change of behavioral response (Asleep, Quiet, or Exploring) across the six injection days differs between YOH and SAL (p<0.05 for comparisons listed below). |
| SAL vs. YOH—Asleep (6 timepoints x 6 injections) |
| SAL vs. YOH—Quiet “ |
| SAL vs. YOH—Explore |
Effect of YOH injections on food intake and body weight
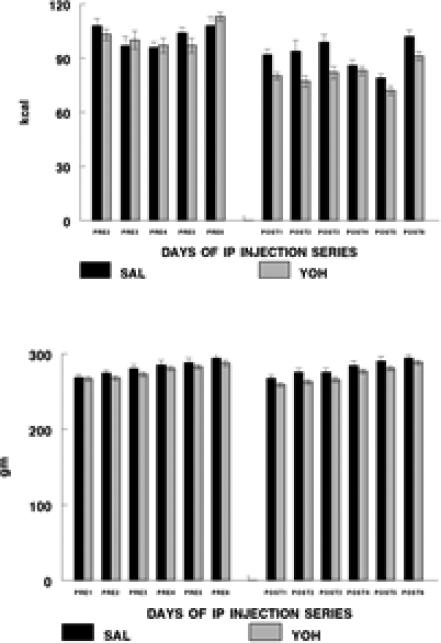
Food intake and body weight in response to the YOH and SAL injections are shown in Figure 2. Whereas food intake during the 24-hr prior to each injection did not differ between YOH and SAL conditions, food intake during post-injection days was decreased for both YOH and SAL rats, with an additional significant overall effect of YOH vs. SAL on post-injection days. However, there was no difference in cumulative food intake across the entire injection period for SAL vs. YOH conditions. Both YOH and SAL rats gained weight across the time of the injection paradigm. However, body weight of YOH rats was consistently decreased across the time of the injection paradigm compared with SAL rats (both pre-injection and post-injection days). The effect of YOH on body weight was not sustained in the post-YOH week (data not shown). Thus, YOH had acute but not sustained effects on food intake and body weight.
Figure 2.
Food intake (kcal; Figure 2 upper) and body weight (gm; Figure 2 lower) of rats receiving SAL (n=14-15) or YOH (n=19) injections. The left group represents measurements from the 24hr prior to an injection (‘pre-injection’); data were not collected during the 24 hr prior to the first injection. The right group represents measurements from the injection days (24 hr ‘post injection’).
Effect of YOH injections on HPA axis activity
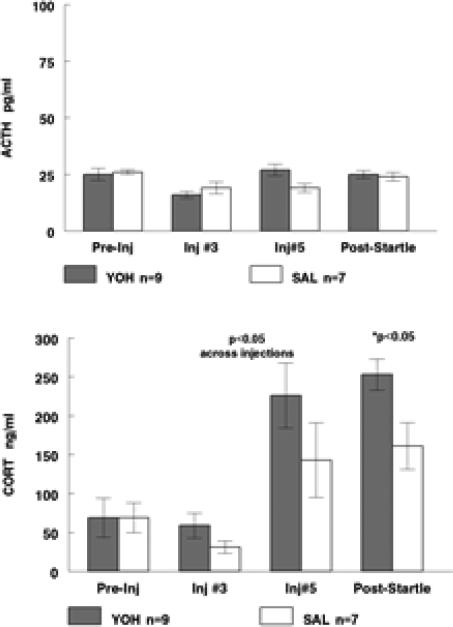
In the cohort of rats tested for startle response, ACTH and CORT were measured and data are shown in Figure 3. Baseline (“Pre-Inj”) ACTH and CORT were matched between YOH and SAL groups. CORT levels were significantly elevated between baseline and Inj 5 days, for both YOH and SAL. Across injections, CORT was significantly elevated (p=0.03) in the YOH vs. SAL condition (individual injection Days 3 or 5 did not achieve statistical significance). Across injections, ACTH levels did not differ between YOH and SAL, although there was a trend for ACTH levels to be elevated in the YOH rats on Inj 5 day. CORT levels were significantly elevated (p=.02) in the YOH vs. SAL rats when measured after startle testing, whereas there was no effect of prior YOH on ACTH levels. These findings suggest that altered responsivity of the adrenal gland to ACTH or other stimuli, rather than hypothalamic-pituitary activation, was responsible for the elevation of CORT during and after the YOH injection regimen.
Figure 3.
ACTH (pg/ml; Figure 3 upper) and CORT (ng/ml; Figure 3 lower) at baseline (collected before the start of the injection series), on two separate injection days (Inj#3 and Inj#5), and after startle response testing (four days after the conclusion of the injection series). There was an independent effect of YOH injection to increase CORT (across Inj#3 and Inj#5).
Effect of prior YOH injections on behavioral tests
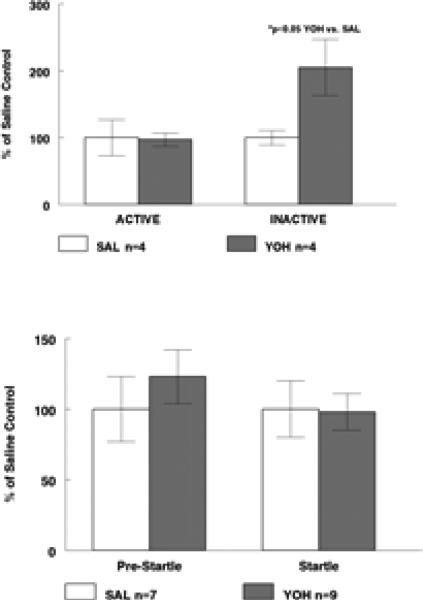
Observations from both behavioral paradigms suggested that prior YOH made the rats more non-specifically active. Thus, in the sucrose self-administration paradigm begun three days post-injections (Figure 4, upper), there was no difference in lever presses on the “Active” lever that yielded a sucrose reward, between SAL and YOH rats (459±122 vs. 447±48 cumulative presses). However, there was a significant increase in presses on the “Inactive” lever (which yielded no reward nor any other stimulus) in prior-YOH rats vs. SAL rats (78±16 vs. 38±4 cumulative presses). In a separate cohort, startle response and pre-startle movements were measured four days after the recurrent injection paradigm was finished, and data are shown in Figure 4 (lower). Informal observation prior to the startle test suggested that the YOH rats were more active than the SAL rats. This was borne out by the pre-startle movement score of 38±10 vs. 18±5 immediately prior to the first startle; however across all ten pre-startle periods in the test session, this was not statistically significant (38±6 vs. 31±7). There was no difference in response to the startle stimulus between YOH and SAL rats averaged across the ten startle events (535±72 vs. 548±111).
Figure 4.
Effect of prior history of recurrent YOH on two behavioral paradigms, fixed-rati sucrose self-administration (Figure 4 upper) and startle response (Figure 4 lower). Prior YOH resulted in a significant increase in presses on the “Inactive” Lever (no rewards) with no effect on “Active” Lever presses. Prior YOH had no significant effect on Pre-Startle movements or on Startle response, tested across ten startle episodes in the session. Data are shown as normalized to respective SAL-control values; see “Results” for data.
Effect of prior YOH injections on CNS activation patterns
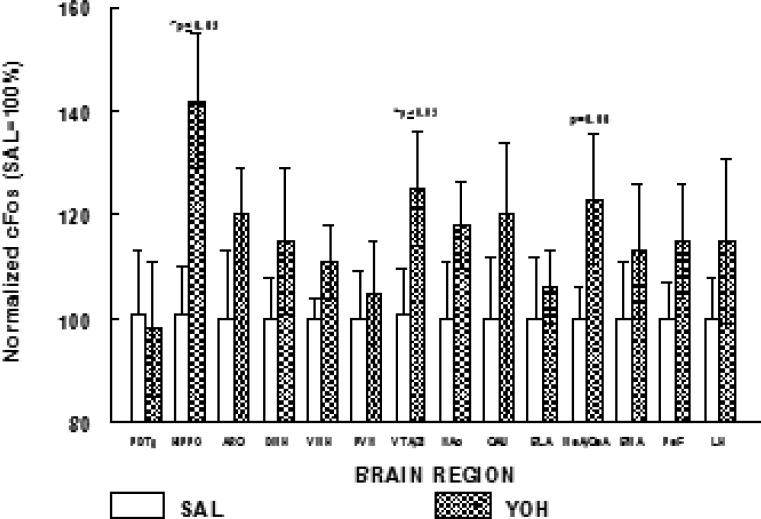
In two cohorts of rats, quantitative cFos mapping throughout the CNS was carried out to test whether activation was increased in the CNS of rats that had a prior history of YOH vs. SAL (brains obtained 3-4 days after the finish of the injection series). Stereotaxic coordinates and raw data for the SAL control group are shown in Table 2. For ease of comparison, in Figure 5 SAL control cFos counts for each brain region are normalized to 100%, and cFos counts for the respective region from YOH rats are expressed relative to the SAL control. We did not observe YOH-enhanced activation within the brainstem, as exemplified on the graph by the pontine dorsal tegmentum (PDTg). From midbrain forward, an overall pattern across CNS regions, of increased cFos expression, was observed in YOH vs. SAL condition (p<0.05). Specific comparisons showed increased cFos in the YOH hippocampus (HIPPO, which included quantitation of the dentate gyrus and CA2 regions) and YOH ventral tegmental area/zona inserta (VTA/ZI, regions enriched in dopaminergic neuronal cell bodies) (p<0.05 for YOH vs. SAL for each area). cFos expression in the medial/central nuclei of the amygdala (MeA/CeA) showed a trend to be increased (p=0.06) in the YOH vs. SAL rats.
TABLE 2.
Stereotaxic Coordinates for cfos Quantitation and Control Data
| DV | Lateral (+/-mm from midline) | A—P (mm from bregma) | cFos Counts | |
|---|---|---|---|---|
| PDTG | −6.8-- −7.8 | 0.0—0.6 | −9.2-- −10.04 | 55±8 |
| ARC | 9.6 | 0.0 – 0.6 | −2.3-- −3.6 | 82±11 |
| PVN | 8.0 | 0.0 –0.6 | −1.8-- −2.12 | 104±10 |
| DMH | 8.2 --8.8 | 0.0 – 0.6 | -2.56-- −3.14 | 94±10 |
| VMH | 9.4 – 10.2 | 0.0 – 0.6 | −2.12-- −3.14 | 66±8 |
| LH | 8 – 8.1 | 1.2 – 2.6 | −1.8-- −3.6 | 141±14 |
| LH peF | 8.0 – 9.0 | 1.1 – 1.4 | −1.8-- −3.6 | 164±11 |
| NAc shell/ | 6.4 - 7.6 | 0.6 – 1.0 | +1.7-- +0.75 | 125±15 |
| NAc core | 6.4 -7.6 | 1.1 – 2.0 | +1.7-- +0.75 | |
| VTA/ | 7.8 – 8.6 | 0.8 – 1.4 | −4.8-- −5.3 | 165±19 |
| −7.4-- −7.8 | 0.6—2.8 | −2.3-- −3.8 | ||
| BLA | −7.8-- −8.2 | 4.2—5.0 | −1.6-- −2.2 | 81±10 |
| MeA/CeA | −7.6-- −9.4 | 2.6—4.4 | −2.3-- −3.8 | 247±16 |
| BMA | −8.8-- −9.2 | 3.4—4.2 | −2.3-- −3.8 | 133±15 |
| CAU | −3.4-- −6.6 | 1.4—5.2 | −3.8-- −1.6; 0.7-1.7 | 154±19 |
Figure 5.
Effect of prior YOH injection (n=12) on CNS cFos expression patterns (brain activation). Data are normalized to SAL controls (n=12) for each brain region (PDTg, dorsal pontine tegmental area; HIPPO, hippocampus; ARC, arcuate; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; PVN, paraventricular nucleus; VTA/ZI, ventral tegmental area/zona inserta; NAc, nucleus accumbens (core and shell); CAU, caudate nucleus; BLA, basolateral nucleus of the amygdala; MeACeA, medial and central nuclei of the amygdala; BMA, basomedial nucleus of the amygdala; PeF, perifornical region; LH, lateral hypothalamus).
DISCUSSION
The main finding of this study is that a recurrent (semi-chronic) moderate stressor (YOH) experience results in prolonged CNS activation and enhanced responsivity of the adrenal cortex. Importantly, our behavioral assessment during the YOH regimen demonstrated that the rats did not accommodate to the recurrent experience but if anything seemed to become behaviorally sensitized. This was manifest by an increase in the extent and duration of “Exploring” behavior observed in the quantitative comparison between first and last sessions (YOH within-subjects). The treatment regimen had acute effects on food intake and body weight only during the two week period when injections were given. Somewhat surprisingly, even SAL injections resulted in decreased food intake, however this effect was further enhanced in the YOH group. In addition, measurement of CORT levels after Injection#5 demonstrated elevations of CORT in both SAL and YOH rats relative to their pre-injection baseline levels. These effects in the SAL rats appeared to be disconnected from the behavioral responses observed during the 3-h post-injection period: That is, by the last injection the SAL rats more quickly returned to an expected quiescent state (SAL within-subjects). ACTH levels were not elevated in the YOH vs. SAL rats, nor were they elevated relative to respective baselines when measured at Inj#3 or Inj#5. This would imply that medial hypothalamic corticotrophin-releasing factor (CRF or CRH) was likewise not elevated. Since CRF is a known suppressor of food intake and catabolic factor, a further implication of the unchanged ACTH levels is that this pathway is not responsible for the decreased food intake and body weight we observed throughout the 2wk injection paradigm. Finally, our goal was to design a model that would have prolonged sequelae after the repeated activation of stress/anxiety pathways, and our post-hoc data suggest that we were somewhat successful in achieving this.
We hypothesized that performance in both post-hoc behavioral paradigms would be increased as a result of prior YOH experience. That is, we hypothesized that the startle response would be enhanced, and that sucrose self-administration would likewise be enhanced. In fact what we observed were suggestions of enhanced non-specific activity in the post-YOH rats. Rather than increasing lever-pressing for sucrose in the self-administration paradigm, lever-pressing on a bar which yielded no reward (“Inactive”), but is utilized as a index of non-specific activity, was increased. Further we had an observer report, as well as a statistical trend for ‘pre-startle’ movements captured within seconds before the actual startle event, to be increased, but this trend did not reach statistical significance. There was no increase in the response to the startle per se, which would have been a finding consistent with increased activity in CNS anxiety pathways. Both increased sucrose self-administration and increased startle responding would have been predicted in response to a single acute injection of YOH immediately prior to a behavioral session. Although this startle paradigm (26) has been shown to be modulated by in vivo treatments, it is possible that a more stringent sucrose self-administration paradigm might be necessary to discern an increased drive for sucrose responding (i.e., the ‘fixed ratio’ paradigm might already reflect maximum possible responding). It is also possible that other behavioral components of sucrose self-administration, i.e., acquisition, and post-extinction reinstatement, may be the key behavioral phases that would be affected by the prior YOH regimen.
We quantitated cFos expression throughout the CNS at a timing consistent with the post hoc behavioral paradigms. This measurement would reflect the ‘post hoc baseline’ for CNS activation of rats treated with prior SAL or YOH. We did not observe increased activation of any region of the medial hypothalamus in the YOH (vs SAL) rats: cFos expression was comparable in the ARC, PVN, DMH, and VMH. We did observe increased activation in specific limbic regions, for example the HIPPO (which reflected quantitation of the dentate gyrus and CA2 regions). The HIPPO is a key regulator of, and responder to, stressful stimuli so this observation is consistent with a prolonged effect of stress on this region. We further observed increased activation of the medial and central amygdalar nuclei, regions that would be activated in response to stress/anxiety experiences [16,20,22]; and the VTA/ZI midbrain region which contains a strong investment of dopaminergic neuronal cell bodies. Activation of these two areas would be consistent with both motivation-related and anxiety-related behavioral outcomes; we speculate that the degree of enhanced activation we observed may not have been substantial enough to impact outcomes in the behavioral paradigms tested here. Key CNS sites that are implicated in the motivational reinstatement process include the central nucleus of the amygdala (CeA) [27]; the VTA; and the ventral hippocampus [28,29]. CRF/CRF1 receptors in the amygdala have been implicated in food- or sucrose-reinstatement by cues or by acute YOH administration [28,29]; and alpha2-adrenergic receptors are implicated in food pellet self- administration [30]. As mentioned above, a self-administration paradigm that included evaluation of reinstatement may have shown an effect of the prior YOH.
Research from numerous laboratories over the past decade has demonstrated substantial functional connectivity between the medial hypothalamus (particularly the ventromedial region (VMH) and paraventricular nucleus (PVN)) which plays a key role in energy homeostasis and stress responses; and circuitries that mediate motivated behavior and limbic function (emotional memory), including multiple sub-regions of the amygdala, the ventral tegmental area (VTA) and nucleus accumbens (NAc), and frontal or prefrontal cortical areas. These CNS functional connections may account for the food intake and body weight effects that we observed. We measured cFos post hoc and observed no significant elevations of cFos in the medial hypothalamus of YOH rats, but the food intake and body weight effects had abated after the injection paradigm was completed (cFos measurements acutely following YOH and SAL injections would be necessary to confirm involvement of the medial hypothalamus).
Results from several human studies suggest that HPA axis function may be altered in patients with PTSD, a syndrome of extreme anxiety. However, increases, decreases, or no change of pituitary (ACTH)/adrenal (glucocorticoids) hormones have been reported. Recent research suggests that, in one population of PTSD patients, neither adrenal nor pituitary sensitivities are altered, and the authors suggest that defective HPA function may be specific and individual, based upon a combination of experienced trauma, genetic factors, and individual history [31,32]. Yehuda [33] has posited that both variations in patient testing conditions, as well as the challenge tests themselves, may not allow for mechanistic determinations of HPA problem(s), although enhanced responsivity to glucocorticoids is one possible mechanism. In their rat PTSD model [26], Rasmussen and colleagues have identified subpopulations of low and high responders to startle testing, and only the high startle responders exhibited elevated corticosterone levels following the PTSD paradigm. This supports the concept that multiple factors may impact on HPA axis function in association with traumatic experience. Our model demonstrated enhanced CORT in response to the startle test, where both prior-SAL and prior-YOH rats showed elevated CORT compared with pre-injection (within-subjects) baselines, and a further elevation in the YOH compared with SAL rats.
In conclusion, we tested a model of prior recurrent activation of CNS pathways of stress/anxiety on subsequent neuroendocrine, behavioral, and CNS activation patterns. The recurrent YOH paradigm was clearly efficacious during its administration, and modest but significant post hoc activation within the CNS suggests a prolonged effect of the treatment, with possible impact on neuroendocrine functioning. The lack of substantial effect on the specific behaviors we tested, for which stress and anxiety pathway activation should have had an effect, suggest that the model, although encouraging, is perhaps not quite robust enough. Higher or more prolonged YOH dosing may have greater post hoc behavioral efficacy, which could be tested in future studies. It is becoming clear that environmental factors such as diet and availability of ‘rewarding’ foods, as well as events or stimuli that are stressful, combine and interact in a complex fashion, as suggested by recent research from Christiansen, Herman, and colleagues [34,35]. Future studies are warranted to delineate the nature of, and strength of, such interactions.
Highlights.
■ Recurrent yohimbine administration increases exploratory behaviors in the rat.
■ Prior recurrent yohimbine increases cFos in limbic regions of the rat CNS.
■ Prior recurrent yohimbine in the rat increases corticosterone response to startle.
ACKNOWLEDGMENTS
Dianne Figlewicz Lattemann is a Senior Research Career Scientist, Biomedical Laboratory Research Program, Department of Veterans Affairs Puget Sound Health Care System, Seattle, Washington; and the research in this paper has been supported by NIH grant DK40963. Susan Hill was supported by University of Washington Training Grant NIH T32DK007120, and by the University of Washington Diabetes Endocrinology Research Center Grant NIH P30DK017047. Alfred J. Sipols was supported by the University of Latvia grant Y5-AZ17-ZF-N-003. The authors thank the laboratory of Dr. Dennis Rasmussen (VAPSHCS) for assistance with the startle response measurements. We thank Ms. Amalie Alver and Ms. Lydia Wang for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Joes KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci. 2011;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dallman MF, Pecoraro NC, laFleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor DB, Jones F, Conner M, et al. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008;27(1 Suppl):S20–31. doi: 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- 4.Conner FM, Fletcher W. Stress and snacking: A diary study of daily hassles and between meal snacking. Psychology and Health. 1999;14:51–63. [Google Scholar]
- 5.Pagoto SL, Schneider KL, Bodenlos JS, Appelhans BM, Whited MC, Ma Y, Lemon SC. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity. 2012;20:200–5. doi: 10.1038/oby.2011.318. [DOI] [PubMed] [Google Scholar]
- 6.Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adapatation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med. 2010;235:1150–62. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- 7.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, LeMoal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci. 1996;93:8716–20. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deroche V, Piazza PV, Maccari S, LeMoal M, Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res. 1992;584:309–13. doi: 10.1016/0006-8993(92)90911-r. [DOI] [PubMed] [Google Scholar]
- 9.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alchohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–74. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 10.Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Charney DS, Heninger GR, Redmond DE. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- 17.Figlewicz DP. The CNS physiology of food reward: current insights and future directions. Neurobiology of Food and Fluid Intake. In: Stricker E, Woods SC, editors. Handbook of Behavioral Neurobiology. 2nd Edition. Plenum Publishers; New York: 2004. pp. 43–60. [Google Scholar]
- 18.Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 22.Debiec J, LeDoux J. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory. Treatment implications for PTSD. Ann NYAS. 2006;1071:521–4. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- 23.Figlewicz DP, Bennett-Jay JL, Kittleson S, Sipols AJ, Zavosh A. Sucrose self-administration and CNS activation in the rat. American Journal of Physiology. 2011;300:R876–84. doi: 10.1152/ajpregu.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figlewicz DP, Jay JL, Acheson MA, Magrisso IJ, West CH, Zavosh A, Benoit SC, Davis JF. Moderate high fat diet increases sucrose self-administration in young rats. Appetite. 2013;61:19–29. doi: 10.1016/j.appet.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. Atlas of the rat brain in stereotaxic coordinates. 5th Edition. Elsevier Academic Press; San Diego CA: 2005. [Google Scholar]
- 26.Rasmussen DD, Crites NJ, Burke BL. Acoustic startle amplitude predicts vulnerability to develop post-traumatic stress hyper-responsivity and associated plasma corticosterone changes in rats. Psychoneuroendocrinology. 2008;33:282–91. doi: 10.1016/j.psyneuen.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–6. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RJ, Aston-Jones G. alpha2adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–9. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, Peskind ER, Raskind MA. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol Psychiatry. 2001;50:238–45. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 32.Radant AD, Dobie DK, Peskind ER, Murburg MM, Petrie EC, Kanter ED, Raskind MA, Wilkinson CW. Adrenocortical responsiveness to infusions of physiological doses of ACTH is not altered in posttraumatic stress disorder. Frontiers Behav Neurosci. 2009;3:1–8. doi: 10.3389/neuro.08.040.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann NYAS. 2006;1071:137–66. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen AM, DeKloet AD, Ulrich-Lai YM, Herman JP. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav. 2011;103:111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen AM, Herman JP, Ulrich-Lai YM. Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry. Stress. 2011;14:205–215. doi: 10.3109/10253890.2010.531331. [DOI] [PMC free article] [PubMed] [Google Scholar]