Abstract
Objective:
Breast cancer is the most common female cancer worldwide and is the second most commonly diagnosed cancer in Indian women. This study evaluates the differences between pre- and post-menopausal breast cancer women regarding risk factors, nature of disease presentation, tumor characteristics, and management.
Methods:
This is a prospective observational study, conducted in the Oncology Department of St. Ann's Cancer Hospital, for a period of 6 months from January to August 2012. Data on basic demography, clinical and pathological tumor profile, and treatment details were collected prospectively for each patient based on patient interviews and medical records.
Findings:
Among 100 female patients taken up for the study, 48 were premenopausal and 52 had reached menopause. The mean age of presentation for breast carcinoma was a decade earlier in these patients compared with western patients. The risk factors for both pre-and post-menopausal breast cancer were found similar other than late menopause in postmenopausal patients. Having dense breast tissue was a predominant risk factor among all women. Late presentation was the common phenomenon in almost all patients. The treatment given was not based on any standard guidelines due to inadequate public health policies.
Conclusion:
Late stage at presentation of breast cancer is the main problem and possesses a challenge to the health care community. In order to reduce the burden of breast cancer, a multi-sectorial approach and evidence-based strategies aiming at early detection and effective management of the disease are required.
Keywords: Breast cancer, diagnosis, epidemiology, Indian women, postmenopausal, premenopausal
INTRODUCTION
Breast cancer is the most common female cancer worldwide. Global burden of breast cancer will increase to over 2 million new cases/year by 2030.[1] The incidence of breast cancer is rising in India (22.9%) and is now the second most commonly diagnosed cancer in women after cervical cancer.[1] The age-standardized mortality rate for breast cancer in India was found to be 11.1/100,000 where globally it was 12.5/100,000 according to International Agency for Research on Cancer report in 2008.[1]
Although many risk factors may increase the chance of having breast cancer, it is not yet known just how some of these risk factors cause cells to become cancerous[2,3]. Risk factors can be divided into un-modifiable risk factors and risk factors related to lifestyle choices. Un-modifiable risk factors are gender, age, genetic factors, family history, personal history of breast cancer, dense breast tissue, menstrual periods, breast radiation early in life, and treatment with diethylstilbestrol. Risk factors related to lifestyle are not having pregnancy history or pregnancy at late ages, recent use of birth control pills, using hormone therapy after menopause, not breast-feeding, alcohol, being overweight or obese, lack of exercise, and induced abortion[3,4,5,6,7,8,9].
Menopause does not cause cancer, but the risk of developing cancer increases as a woman ages. A woman who experiences menopause after age 55 has an increased risk of ovarian, breast, and uterine cancers. The risk is greater if a woman also began menstruating before age 12. A longer exposure to estrogen increases a woman's risk of breast cancers. Therefore, women who have been through natural menopause are more likely to develop cancer around as twice as high because of hormonal factors[3].
Hence, this study can provide a guide in improving an individual's knowledge about breast cancer and helps to understand more about breast cancer. The results of this study emphasize the most prevailing risk factors in this population, the appropriateness of the diagnostic tests used and treatment given. This can bring into light the present scenario of the disease and also the specific areas in which special care and precautions are needed to suppress the rising incidence of breast cancer.
The main aim of this study is to evaluate the difference between pre- and post-menopausal breast cancer women regarding risk factors, nature of disease presentation, tumor characteristics and management. The study also aims to find out the prevalence of breast cancer, to identify the clinical presentation, risk factors diagnostic methods, and the different types of treatment patterns used. It also assesses the impact of treatment given and women's knowledge about breast cancer.
METHODS
This is a prospective cross-sectional study conducted in the Oncology Department of St. Ann's Cancer and General Hospital, Warangal, for a period of 8 months (January 2012-August 2012). A total of 100 female patients who were histologically or cytologically confirmed with breast cancer were included in the study.
Patients were divided into two groups based on their menopausal status as premenopausal and postmenopausal. Patients who had no menstrual flow since 12 months were considered as postmenopausal and the rest were considered as premenopausal. Patients who had natural menopause were only included in the study population. Patients who have undergone hysterectomy or who were having any other ovarian problems and whose menopausal status was not specified and who had a personal history of cancer other than breast cancer were also excluded from the study.
Data on clinical presentation, evident findings of cancer, diagnostic tests and diagnosis and tumor characteristics were collected from medical records available at the central registry of the hospital. Information about patient's personal habits, risk factors, well-being was obtained by interviewing either the patient or the patient's caretakers.
The two study groups are later compared and evaluated regarding the risk factors associated, nature of disease presentation, diagnostic pattern, tumor characteristics and treatment modalities.
Statistical analysis was performed using SPSS statistical software (SPSS, Chicago, IL, USA), version 17. Multivariate logistic regression analysis was used to calculate odds ratio and 95% confidence interval (CI) for risk factors associated with breast cancer. In order to measure the odds ratio, we have taken premenopausal as controls because they have relatively reduced risk compared with postmenopausal. P < 0.001 was considered to be statistically significant.
RESULTS
Among 100 female patients taken up for the study, 48 were premenopausal and 52 had reached menopause. In 52 postmenopausal patients, 42% attained menopause at age <45 years and 58% attained menopause at age >45 years.
In this study, the age at diagnosis ranged between 24 and 80 years with a mean age of 47.7 years. More than half of the patients (54%) were diagnosed between the age of 40 and 60 years. About 26% were aged younger than 40 years and 20% were aged older than 60 years at presentation.
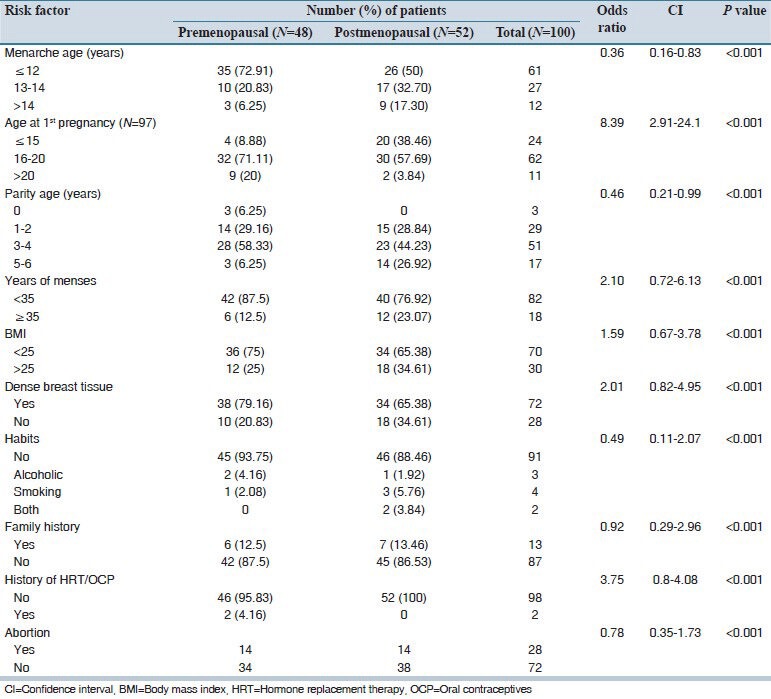
Nearly 68% patients were illiterates and 18% had primary education and the rest of 14% had secondary education. Most of the patients were housewives and few were daily wage labors. A summary of risk factors distribution among pre - and post-menopausal women was shown in Table 1.
Table 1.
Distribution of risk factors among pre- and post-menopausal patients
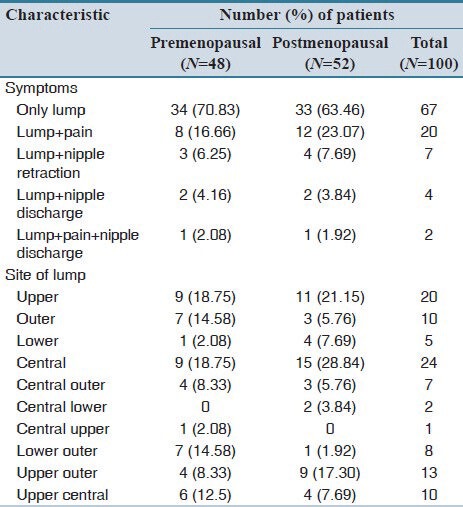
All patients presented with a lump in the breast. Pain, nipple retraction and nipple discharge were present in some patients along with a lump in the breast.
Overall 54% patients presented with a history of 2-6 months duration, 23% presented with history for more than 12 months duration and 15% presented with history of 7-12 months duration of symptoms.
About 51 patients had a lump in the right breast and 49 patients had a lump in the left breast. In the majority of the patients, the lump was present in the central quadrant and upper quadrant [Table 2].
Table 2.
Distribution of clinical presentation and site of lump between studied patients
Biopsy was the conformation test in all patients; core needle biopsy was done in 81 patients and fine-needle aspiration cytology (FNAC) was done only in 19 patients. Abdominal ultrasound, chest X-ray, complete blood count, and renal function tests were also done to all patients regularly as a part of diagnosis and also for metastatic workout.
Infiltrating duct cell carcinoma was the most prominent histopathological type accounting about 85 of total cases in which 39 were premenopausal and 46 were postmenopausal women. Totally, eight patients including five premenopausal and three postmenopausal women had lobular carcinoma. Six patients had medullary carcinoma in which four were premenopausal and two were postmenopausal women. Fibroadenoma was found in only one postmenopausal patient.
Overall 75 patients were diagnosed with stage 3 disease in which 39 were premenopausal and 36 were postmenopausal women. Totally, 16 patients were diagnosed with stage 2 consisting equal number of pre-and post-menopausal women. Six postmenopausal women were diagnosed with stage 4 disease in which 3 had spine metastasis, one with brain metastasis and 2 with nodal reoccurrence. Majority of postmenopausal patients were progesterone positive, whereas the majority of premenopausal patients were estrogen positive.
Overall 92 patients were given adjuvant treatment, five patients were given neoadjuvant treatment and three were given palliative treatment as they had advanced disease. A total of 95 patients underwent surgery and modified radical mastectomy (MRM) was the only type of surgery done to all these patients.
Majority of the patients (79%) received Adriamycin, cyclophosphamide and paclitaxel (AC → T) based chemotherapy and 18% patients received cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) based chemotherapy. Totally, 96 patients were given radiotherapy. Adjuvant radiotherapy was given to 93 patients at a dose of 5000 cGy for 25 fractions and palliative treatment was given to three patients at a dose of 3000 cGy for 10 fractions.
After surgery, swelling and pain in the limbs were the most common adverse effects experienced by the majority of patients. During chemotherapy, most of the patients experienced vomiting, nausea, loss of appetite and alopecia. During radiotherapy, dehydration and fatigue were the mostly reported adverse effects.
DISCUSSION
Some previous studies stated that breast cancer is a disease of older women and its incidence increases with age, and it is rare below the age of 20 years.[10,11,12] Majority of patients in this study were between the third and fifth decade of their life similar to studies reported from India and other Asian countries.[11] A majority of premenopausal patients were in third and fourth decade of their life and the majority of postmenopausal were in fifth and sixth decade of their life. Even young women who had family and professional commitments hold major proportions in the study population. More than 50% of women included in the study were diagnosed before the age of 50 years, in contrast to the western settings where only 23% of women younger than 50 years presented with breast cancer.[8]
Majority of the patients were from a rural background, which was contradictory to the previous reports from India as well as United States, which show a higher incidence in urban population compared to the rural population.[10]
The incidence of breast cancer in this study was found slightly more in postmenopausal women than in premenopausal women. In postmenopausal women majority of them attained menopause after the age of 45 years.
Inconsistent results were published regarding age at menarche and breast cancer risk.[13] Some studies reported that younger age at menarche increased breast cancer risk only in premenopausal women, while some reported increased risk only for postmenopausal women.[11,12,13] In some studies done previously, age at menarche was found to be associated with both pre-and post-menopausal breast cancer while in another studies, it had no association with either pre- or post-menopausal breast cancer.[11,12,13] In this study, early onset of menarche was found to be associated with both pre- and post-menopausal patients as the majority of the patients in either groups attained puberty at an age of <12 years. The median age of menarche worldwide is 14 years with a range from 11 to 18 years. Some studies done on Indian women showed that the risk of both premenopausal and postmenopausal breast cancer decreased with delay in the onset of menarche.[10]
Late menopause increases the risk of breast cancer. Postmenopausal women have a lower risk of breast cancer than premenopausal women of the same age and childbearing pattern. Risk increases by almost 3% for each year older at menopause (natural or surgery induced), thus women who has attained menopause at 55 years rather than 45 years, has approximately 30% higher risk.[10,14] In this study, majority of the women reached menopause after the age of 45 years.
Risk of developing breast cancer increased in both pre-and post-menopausal patients who had early onset of menarche and late menopause possibly due to the increase in the duration of hormonal exposure.
Early age at first full-term pregnancy (FP) is inversely related to breast cancer risk[15]. This association perhaps reflects either a pregnancy induced maturation of mammary cells, and thus making them less susceptible to carcinogenic transformation or a long-lasting hormonal change or both. In this study, it was found that the majority of the women were at an age younger than 30 during their first pregnancy. However, information regarding the age at last pregnancy was lacking. Late age at last FP also has been found to be associated with a higher risk of breast cancer, but not in all studies.[12] More studies must be done to investigate the association between age at any FP and breast cancer risk.
High parity has generally been associated with low breast cancer risk in previous epidemiological studies.[15,16] Null parity was associated with an overall increased risk of breast cancer.[10] Contradictory to the previous studies and available literature it was found that many women in this study presented with breast cancer despite of high parity.
Practicing breast feeding was believed to minimize the risk of breast cancer in both pre-and post-menopausal patients. The longer the duration of breastfeeding by women, the greater protection and the risk is relatively reduced by 4% for every 12 months of breastfeeding.[13] But in this study, it was found that breast feeding was not protective against breast cancer in both pre- and post-menopausal women, which was conflicting with the majority of the western studies.[12] One of the largest studies done in India to examine the relationship between breastfeeding and breast cancer risk has shown mixed results; it reported that increased duration of breastfeeding was associated with a significantly decreased risk of premenopausal breast cancer, but no effect was seen in women with postmenopausal breast cancer.[10]
A study done in Mumbai showed a nonsignificant protective effect for breast feeding.[10] But due to small sample size of this study, results were probably underpowered to detect significant differences.
One study done in China reported that induced abortion was associated with an increased risk for breast cancer and with a dose-response relationship.[17] In this study, also similar finding was observed, but more studies should be done to prove it significantly. The FP first causes mammary cell proliferation and differentiation and thus presumably reduces the susceptibility to carcinogenesis. Researchers supposed that an early interruption of a pregnancy may lead to enhanced proliferation of breast tissue with subsequent carcinogenic change.[16,17]
Most of the patients, both pre-and post-menopausal patients had dense breast tissue. Dense breast tissue means there is more gland tissue and less fatty tissue that is associated with epithelial proliferation and stromal fibrosis.[18,19] The relation between these histological features and risk of breast cancer may be explained by the known actions of growth factors that play an important role in breast development and carcinogenesis. Breast cancers originate in epithelial cells, so greater areas of fibroglandular tissue may reflect a greater number of cells that are at risk of carcinogenesis and/or an increased rate of epithelial proliferation.[18,19] Unfortunately, dense breast tissue can also make it harder for doctors to spot problems on mammograms.[20,21]
The data from Indian studies overall suggests that the role of known risk factors on the development of breast cancer within the Indian population is unclear and a large multi-center study would be of benefit to try and understand the shifting trends in breast cancer incidence in this population.
The nature of disease presentation and tumor characteristics were found independent of the menopausal status and were related to the stage of the disease. Lump in the breast was the chief presenting complaint of all the women in this study as reported in various studies.[11,12,14,22] No patient presented with isolated complaint of pain or nipple discharge or nipple retraction. During patient interview, it was found that almost all women found a lump in their breast by themselves, but due to lack of knowledge about breast cancer they were not able to detect their disease. The problem of late presentation is mainly due to rural background, poverty and lack of awareness. Hence by educating the masses on self-breast examination and screening techniques, they can detect their disease themselves which could also help in early diagnosis of the disease.
The incidence of breast cancer was more in the upper and central quadrants of either side probably because of larger volume of breast tissue is present in that quadrants.[6]
For the diagnosis of breast carcinoma, core needle biopsy was done in the majority of the patients and a positive predictive outcome was obtained in most of them. FNAC was done in those patients who were in stage 1 or 2 whose tumor size is comparatively less. Apart from these tests abdominal ultrasound, chest X-ray, complete blood count, renal function tests and cardiac tests were done periodically to assess the patient condition and also as a part of metastatic workup.
As reported in most of the previous studies, infiltrating duct cell carcinoma was the prominent histopathological type.[11,12,20,21] Other types include lobular, medullary and fibro-adenoma. There was not a single case of carcinoma in situ reported. Assessment of hormonal status and lymph nodes was useful in determining treatment and also as a tool for prognostication. Most of the patients were found either estrogen positive or progesterone positive and all patients were lymph positive.
Majority of the patients (75%) were diagnosed at stage 3 collaborating with the epidemiological data.[1] Stage 4 disease was presented only by postmenopausal women probably due to advanced age. Patients with stage 1 disease were half to the number of patients with stage 4 disease. This reflects that the population lacked awareness of the disease.
Treatment of breast cancer should be multi-dimensional and multi-disciplinary in nature and must be given based on the stage of the disease. Optimized treatment can be enhanced when diagnosis is made early.
Majority of the patients irrespective of their stage of disease received adjuvant treatment in which surgery was complemented by either chemotherapy or radiotherapy or both. Usually combination of both chemotherapy and radiotherapy was given after surgery. Adjuvant treatment was found very fruitful in both early and advanced breast cancer. In early breast cancer, it reduces the risk of local recurrence and in advanced breast cancer it delays locoregional recurrence, reduces growth of systemic metastasis and prolongs the life of the patient.
Advanced breast cancer poses enormous management problems because of extensive lesions, which often result in difficult operative and post-operative problems such as flap necrosis, wound infection and early locoregional recurrence of breast cancer. The treatment of these patients posed a greater problem than anticipated. Only palliative radiotherapy was offered to patients in an advanced stage.
The only surgical procedure used was MRM. Patient ignorance, absence of proper treatment units and poor follow-up are the main reasons for a low rate of breast conserving surgery.
Chemotherapy plays a major role in the treatment of breast cancer. Intensive use of chemotherapy is indicated from 4 to 12 cycles in invasive breast cancer. In our unit, AC → T was given for 8 cycles and CMF was given for 6 cycles. CMF was given to elderly patients and those who had cardiac complications.
Hormonal therapy was not given to the patients after completion of treatment which is very essential. It was found that hormonal therapy was not followed by patients mainly due to financial problems.
Patients recovered well after the treatment despite of the adverse effects, but it had a lot of impact on their well-being. Patients were physically, emotionally and also psychosocially very weak mainly due to the lack of proper financial assistance, or poor support from family and poverty. Lack of proper knowledge about the disease made them make false assumptions and interpretations, which developed a fear among them.
Finally, the mean age of presentation for breast carcinoma is a decade earlier in these patients compared to western patients. The risk factors for both pre-and post-menopausal patients were found similar other than late menopause in postmenopausal patients. The main reasons for late stage presentation are lack of awareness, poverty and absence of screening modalities. The treatment given was not based on any standard guidelines due to inadequate public health policies. Owing to lack of awareness, lack of funding, lack of infrastructure, and lack of public health schemes, breast cancer screening, and early detection are not yet available even though there is increasing rate of incidence.
The prerequisites for early detection of breast cancer are cost-effective screening modalities along with propagation of self-breast examination and clinical breast examination. Women should be informed about the benefits and harms of screening and research should be oriented toward assessing individual's risk and incorporating it to optimize the effectiveness of screening.
This study has certain limitations. First, the prevalence of breast cancer cases was studied rather than the incidence. Second, the sample size was small which resulted in wider CIs. Third, information about genetic risk factors was absent. Despite of limitations, this study can be useful in understanding the epidemiology of breast cancer in this region.
In order to reduce the burden of breast cancer, a multi-sectorial approach and evidence based strategies aiming at early detection and effective management of the disease should be implemented. Hence, public health programs that ensure access to appropriate, affordable diagnostic tests and treatment must be introduced.
AUTHORS’ CONTRIBUTION
All authors contributed the idea of research, design of study, data analysis and manuscript preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Atlanta: American Cancer Society; 2013. American Cancer Society. Breast Cancer Facts and Figures 2013. [Google Scholar]
- 3.Cooper K. Springhouse: Springhouse Corp; 1998. Pathophysiology Made Incredibly Easy. [Google Scholar]
- 4.Fauci A, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. 17th ed. New York: The McGraw-Hill Companies; 2008. Harrison's Principle's of Internal Medicine; pp. 516–22. [Google Scholar]
- 5.Gallucci BB. Selected concepts of cancer as a disease: From the Greeks to 1900. Oncol Nurs Forum. 1985;12:67–71. [PubMed] [Google Scholar]
- 6.Kumar V, Abbas AK, Fausto N, Mitchell R. 8th ed. Philadelphia: Elsevier Saunders; 2007. Robbins Basic Pathology; pp. 173–224. [Google Scholar]
- 7.Atlanta: American Cancer Society; 2009. American Cancer Society. Recommendations for the Early Detection of Cancer. [Google Scholar]
- 8.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th ed. New York: Springer; 2010. AJCC Cancer Staging Manual; pp. 347–69. [Google Scholar]
- 9.Henderson IC. Risk factors for breast cancer development. Cancer. 1993;71(6 Suppl):2127–40. doi: 10.1002/1097-0142(19930315)71:6+<2127::aid-cncr2820711602>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu DS, Sandhu S, Karwasra RK, Marwah S. Profile of breast cancer patients at a tertiary care hospital in North India. Indian J Cancer. 2010;47:16–22. doi: 10.4103/0019-509X.58853. [DOI] [PubMed] [Google Scholar]
- 11.Pathy NB, Yip CH, Taib NA, Hartman M, Saxena N, Iau P, et al. Breast cancer in a multi-ethnic Asian setting: Results from the Singapore-Malaysia hospital-based breast cancer registry. Breast. 2011;20(Suppl 2):S75–80. doi: 10.1016/j.breast.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Butt Z, Haider SF, Arif S, Khan MR, Ashfaq U, Shahbaz U, et al. Breast cancer risk factors: A comparison between pre-menopausal and post-menopausal women. J Pak Med Assoc. 2012;62:120–4. [PubMed] [Google Scholar]
- 13.Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 15.Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER, et al. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol. 2000;151:715–22. doi: 10.1093/oxfordjournals.aje.a010266. [DOI] [PubMed] [Google Scholar]
- 16.Jiang AR, Gao CM, Ding JH, Li SP, Liu YT, Cao HX, et al. Abortions and breast cancer risk in premenopausal and postmenopausal women in Jiangsu Province of China. Asian Pac J Cancer Prev. 2012;13:33–5. doi: 10.7314/apjcp.2012.13.1.033. [DOI] [PubMed] [Google Scholar]
- 17.Adesunkanmi AR, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15:399–409. doi: 10.1016/j.breast.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 19.Butt S, Borgquist S, Anagnostaki L, Landberg G, Manjer J. Parity and age at first childbirth in relation to the risk of different breast cancer subgroups. Int J Cancer. 2009;15(125):1926–34. doi: 10.1002/ijc.24494. [DOI] [PubMed] [Google Scholar]
- 20.Laura W. Victoria: The Cancer Council NSW; 2011. Understanding Breast Cancer-A Guide for People with Cancer, their Families and Friends; p. 76. [Google Scholar]
- 21.Vinod R, Ganesan P. New Delhi: INDOX Cancer Research Network; 2011. Risk Factors for Breast Cancer in India: An INDOX Case-Control Study. [Google Scholar]
- 22.Geneva: World Health Organization; 2007. WHO's Fight against Cancer Strategies that Prevent, Cure and Care. Guide for Effective Programs. [Google Scholar]


