Abstract
Transformation by tyrosine kinase oncogenes in myeloid malignancies, including BCR-ABL in chronic myeloid leukemia, FLT3ITD in acute myeloid leukemia (AML) or JAK2V617F in myeloproliferative neoplasms (MPN), is associated with increased growth and cytoskeletal abnormalities. Using targeted approaches against components of the superoxide-producing NADPH-oxidases, including NOX2, NOX4 and the common p22phox subunit of NOX1-4, myeloid cells were found to display reduced cell growth and spontaneous migration. Consistent with a role of NOX as regulators of membrane proximal signaling events in non-phagocytic cells, NOX2 and NOX4 were not involved in the excess production of intracellular reactive oxygen species and did not significantly increase oxygen consumption. All NOX family members are controlled in part through levels of the rate-limiting substrate NADPH, which was found to be significantly elevated in tyrosine kinase oncogene transformed cells. Also, reduced phosphorylation of the actin filament crosslinking protein MARCKS in response to suppression of p22phox hints at a novel effector of NOX signaling. MARCKS was also found to be required for increased migration. Overall, these data suggest a model whereby NOX links metabolic NADPH production to cellular events that directly contribute to transformation.
Keywords: Myeloid neoplasia, tyrosine kinase oncogene, signal transduction, NADPH oxidase, migration
Introduction
Constitutive activation of tyrosine kinase oncogenes (TKOs) due to mutations is frequently associated with myeloid leukemias and myeloproliferative disorders. For example, the BCR-ABL oncogene is associated with enhanced proliferation and viability of myeloid cells in chronic myelogenous leukemia (CML),1 the JAK2V617F point mutation is an activating mutation in patients with myeloproliferative disorders including polycythemia vera, essential thrombocythemia, idiopathic myelofibrosis, and myelodysplastic syndromes2 and activating mutations of the FMS-like receptor tyrosine kinase 3 (FLT3), such as internal tandem duplications (ITD), are frequently linked to acute myeloid leukemia (AML).3 Cells transformed by these and other TKOs are associated with elevated levels of intracellular reactive oxygen species (ROS), defined here specifically as superoxide radicals, hydrogen peroxide and hydroxyl radicals.4-6 This effect of TKOs on ROS is not limited to these specific oncogenes and can also be found in other cancers, such as through oncogenic forms of the receptor tyrosine kinase MET in lung cancer7 or in normal cells in response to chronic stimulation with growth factors.8 Even though it is appreciated that elevated ROS are found in many different types of cancers, their exact contribution to disease initiation, maintenance or progression is not well understood. In proliferating cells, increased ROS are required for growth and viability.9
In metabolically active cells, mitochondria are a major source of ROS, mostly as a byproduct of the electron transport chain10, but cellular processes, such as enzymatic reactions involving cytoplasmic NADPH reductase and others, may also nonspecifically contribute to the pool of ROS. The role of NADPH oxidases (NOX) in the excess production of ROS and their function in myeloid leukemia cells is not well understood. In contrast to mitochondria, the primary function of NOX is the production of superoxide radicals from molecular oxygen.11 Vast amounts of ROS can be produced in phagocytic cells for respiratory burst function but only small amounts may be required for the modulation of signaling mechanisms. NOX are multi-subunit transmembrane enzymes and their expression varies in different cell types.12 The family of human NOX proteins consists of five members (NOX1 to 5) and two related dual oxidases (DUOX1 and 2). NOX1, 2 and 3 show a high degree of homology and require p40phox, p47phox and p67phox subunits and activation by Rac. In addition, p22phox subunit is important for the stability and functioning of NOX1 to 4.11,13,14 On the other hand, NOX5 (absent in mice) does not require p22phox.11
Our current study was designed to determine the role of NOX in various cellular processes including ROS generation, cell growth and migration in hematologic malignancies transformed by TKOs. Using patient derived myeloid cell lines dependent on activated tyrosine kinases for transformation, including KU812 (BCR-ABL), HEL (JAK2V617F) and Molm13 (FLT3ITD), the function of NOX proteins was determined. shRNA knockdown of p22phox, NOX2 and NOX4 demonstrated that these proteins are crucial regulators of cell growth and migration but may not be involved in the excess production of intracellular ROS levels. NOX proteins can be regulated at different levels and we found that tyrosine kinase oncoproteins were associated with increased amounts of the rate-limiting substrate NADPH. NOX signaling may be mediated through MARCKS (Myristoylated alanine-rich C-kinase substrate) phosphorylation at its regulatory site. Since NOX2 and NOX4 do not appear to be required for the excess production of ROS, the biological and biochemical effects are expected to be through proximal effectors that depend on a localized increase in intracellular ROS.
Materials and Methods
Cells
The murine BaF3 cells expressing BCR-ABL, JAK2V617F or FLT3ITD were maintained as described.15 For ROS measurements cells were starved in medium without interleukin 3 (IL3). Human cell lines KU812 (Ph+; CML) and Molm13 (FLT3ITD+; AML) were grown in RPMI 1640 (Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS; Lonza, Walkersville, MD). HEL cells (JAK2V617F+; erythroleukemia) were grown in medium containing sodium pyruvate (1mM; Invitrogen, Carlsbad, CA). In some experiments, cells were treated with metabolic analogs, including 2-deoxyglucose (dose response or 4.5mg/mL, Sigma, St. Louis, MO) and 3-bromopyruvate (100μM, Sigma), the flavoprotein inhibitor diphenyleneiodonium (DPI, 5μM, Sigma) or kinase inhibitors, including imatinib (1μM; Novartis, Basel, Switzerland), Jak inhibitor (1μM; Calbiochem, Gibbstown, NJ), and midostaurin (50nM; Novartis). 293T cells were grown in Dulbecco's Modified Eagle Medium (DMEM; Mediatech) supplemented with 10% FBS.
Semi-quantitative real-time PCR
NOX and DUOX gene expression was measured by semi-quantitative real-time PCR using specific primers (Invitrogen) (Supplementary Table 1) and the products confirmed by DNA sequencing (not shown). Total RNA was extracted (RNeasy kit, Qiagen, Valencia, CA) to synthesize cDNA (Taqman Reverse Transcription Reagents, Applied Biosystems, Foster City, CA) for semi-quantitative real-time PCR (Power SYBR green PCR master mix) using a 7500 Real-Time PCR System (both Applied Biosystems).
Targeted knockdown using lentiviral approaches
Knockdown of NOX2, NOX4 or p22phox was done using an shRNA method. Three different constructs (RNAi Screening Facility, Dana-Farber Cancer Institute) were used (A-C) out of a pool of up to five. Lentiviruses containing shRNA were generated by co-transfecting 293T cells with viral packaging vectors, pMD2.GVSV-G and pCMVΔ8.91 (RNAi Screening Facility) and shRNAs using the TransIT (Mirus, Madison, WI) reagent. Cells were infected in the presence of polybrene (5μg/mL; Millipore, Temecula, CA) and selected in medium containing puromycin (3μg/mL; Sigma). The efficiency of knockdown was confirmed by semi-quantitative real-time PCR and immunoblotting.
Immunoblotting
Immunoblotting was performed as described previously.15 The antibodies used were as follows: Polyclonal rabbit antibodies against NOX2 (Millipore), NOX4 (recognizes the 32 kDa isoform 4; Novus Biologicals, Littleton, CO), p22phox (Santa Cruz Biotechnology), pMARCKS (phospho-Ser152/156; Cell Signaling, Danvers, MA), pERK1/2 (phospho-Thr202/Tyr204; Cell Signaling) and pGSK3β (phospho-Ser9; Cell Signaling); monoclonal rabbit antibodies against β1 integrin (EP1041Y; Abcam, Cambridge, MA) and pAkt (C31E5E, phospho-Thr308; Cell Signaling); mouse monoclonal antibodies against β-actin (AC-15; Sigma).
Isolation of membrane fractions
Knockdown of NOX2 was confirmed by immunoblotting of the proteins in the enriched membrane fractions. Briefly, cells were incubated in hypotonic lysis buffer containing Tris (10mM, pH 8.0; Invitrogen), EDTA (1mM; Sigma), Na3VO4 (1mM; Sigma), and protease inhibitor cocktail (Complete; Roche Diagnostics) for 10 min and homogenized. Nuclei were separated by centrifuging at 1500g for 5 min. Supernatant was separated and ultracentrifuged at 100,000g for 1h at 4°C. The proteins in the membrane pellet were used for immunoblotting.
Measurement of intracellular and extracellular ROS
The relative levels of intracellular ROS were measured using the redox-sensitive dye 2′,7′-dichlorofluorescein diacetate (DCF-DA; Calbiochem, La Jolla, CA)8 or by MitoSOX Red (Invitrogen) staining. Cells (1×106) were washed with PBS (Mediatech), incubated with DCF-DA (20μM) for 5 min at 37°C or with MitoSOX Red (5μM) for 10 min at 37°C, subsequently washed twice in PBS and analyzed on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). In control experiments, extracellular superoxide production was measured using a chemiluminescence assay (Diogenes Cellular Luminescence Enhancement System, National Diagnostics, Atlanta, GA). Cells (10×106) were washed twice with PBS before treatment with 50 μM phorbol 12-myristate 13-acetate (PMA, Sigma) at 37°C for 10 min. Diogenes reagent was added and superoxide measured (Monolight 3010 luminometer, Pharmingen, San Diego, CA) continuously for 100s to verify a linear increase in luminescence.
Oxygen consumption
A Clark-type oxygen electrode connected to a Mitocell MT200A respirometer and a Model 782 oxygen meter (Strathkelvin Instruments, North Lanarkshire, Scotland) was used to measure oxygen consumption. The amount of oxygen consumed was continuously recorded for 30 min at 37°C in cells pretreated with tyrosine kinase inhibitors or in cells with p22phox knockdown. The rate of oxygen consumption was compared to starved, untreated or control shRNA transfected cells.
Migration assay
Migration of HEL and Molm13 cells with p22phox knockdown was measured using Transwell inserts (8μm) (Corning Incorporated, Corning, NY). Matrigel invasion chambers (8μm) (BD Biosciences) were used to measure migration of KU812 cells with p22phox knockdown. The relative number of migrated cells in the bottom chamber was counted after 6-8 h and compared to control cells.
Measurement of NADPH levels
Cellular NADPH levels (106 cells) were measured using an NADPH assay kit (Abcam) according to the manufacturer's instructions without modifications and relative levels were quantified on a SpectraMax 190 microplate reader (Molecular Dynamics, Sunnyvale, CA).
Targeted knockdown using siRNA
Knockdown of MARCKS was done using specific siRNA and compared to scrambled siRNA (Santa Cruz Biotechnology Inc., Santa Cruz, CA). HEL cells were transfected with siRNAs in 100 μL Nucleofector solution V (Amaxa, Gaithersburg, MD) according to the manufacturer's instructions using the Nucleofector device. Expression of MARCKS was analyzed by real-time PCR 24h after transfection.
Statistical analysis
For statistical comparison between groups, the Student's t-test was used. p values of less than 0.05 were considered significant. Error bars represent SEM (standard error of the mean) of at least three independent experiments.
Results
NOX proteins are expressed in cell lines transformed by oncogenic tyrosine kinases
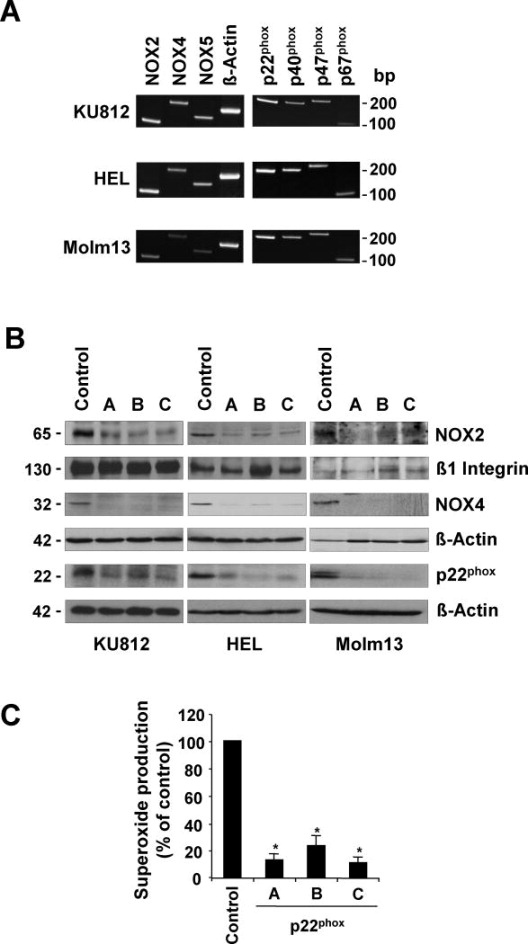
Hematopoietic cells expressing TKOs associated with these diseases, including BCR-ABL, JAK2V617F and FLT3ITD, have been found to display elevated levels of intracellular ROS.4-6 Recently, NOX have been implicated in various cancers, however, their role in hematologic malignancies is not well understood. Using patient-derived KU812 (BCR-ABL), HEL (JAK2V617F) and Molm13 (FLT3ITD) cells, we determined the expression of the various NOX components. Semi-quantitative real-time PCR detected expression of NOX2, NOX4, and NOX5 as well as p22phox, p40phox, p47phox, and p67phox in these cells (Figure 1A). The results also indicated that NOX4 and NOX5 were expressed with a Ct value at least 3-fold higher compared to NOX2 (not shown). We did not observe expression of NOX1, NOX3, DUOX1 and DUOX2 in these cells. Interestingly, murine BaF3 cells expressing BCR-ABL, JAK2V617F and FLT3ITD only expressed NOX1, NOX2 and NOX4 (data not shown). The gene for NOX5 is absent in the murine genome. NOX proteins are dependent on reduced NADPH, which is oxidized for the production of superoxide radicals and this process can be inhibited by diphenyleneiodonium (DPI). In initial experiments, DPI (5μM) was found to strongly reduce ROS levels in KU812 (69.6±0.4%), HEL (77.1±0.5%) and Molm13 (72.2±0.9%) cells (Suppl. Fig. 1). Nevertheless, this small molecule drug was originally identified as an inhibitor of mitochondrial respiration, may have additional effects on carbon metabolism and is now considered to be a flavoprotein inhibitor.16 We therefore sought to determine the role of NOX proteins in ROS production and transformation by using a specific genetic approach with lentiviral-based shRNA knockdown. The expression of NOX2 and NOX4 was targeted since it is common to both murine and human cells. In addition, p22phox which is required for stability and functioning of NOX1 to 4 was stably knocked down, therefore also controlling for functional redundancy between the NOX genes.11,13,14 The efficiency of knockdown using three different shRNA constructs was confirmed by real-time PCR (data not shown) and most importantly, the efficient reduction in protein levels was confirmed by immunoblotting (Figure 1B). Since KU812 and HEL cells are not respiratory burst competent,17 we confirmed that NOX proteins were functionally silenced in Molm13 cells with p22phox knockdown. Superoxide production in response to the respiratory burst activator PMA was found to be reduced by 76.9% to 89.1%, using three different lentiviral constructs targeting p22phox in these cells (Figure 1C).
Figure 1. Expression and targeting of NOX proteins in cells transformed by tyrosine kinase oncogenes.
A-B. Patient derived KU812, HEL and Molm13 cells were used to determine the expression of NOX proteins and their functional components. A. Gene expression of NOX2, NOX4, NOX5, p22phox, p40phox, p47phox, p67phox and actin was determined by RT-PCR. B. Cellular expression of NOX2, NOX4, and p22phox proteins was determined in response to targeted knockdown with specific or control shRNA, as indicated. C. Superoxide production in response to PMA (50 μM) treatment was measured using a chemiluminescence method in Molm13 cells containing control shRNA or p22phox-targeting shRNA (n=3, *p<0.05).
Tyrosine kinase oncogenes increase oxygen consumption
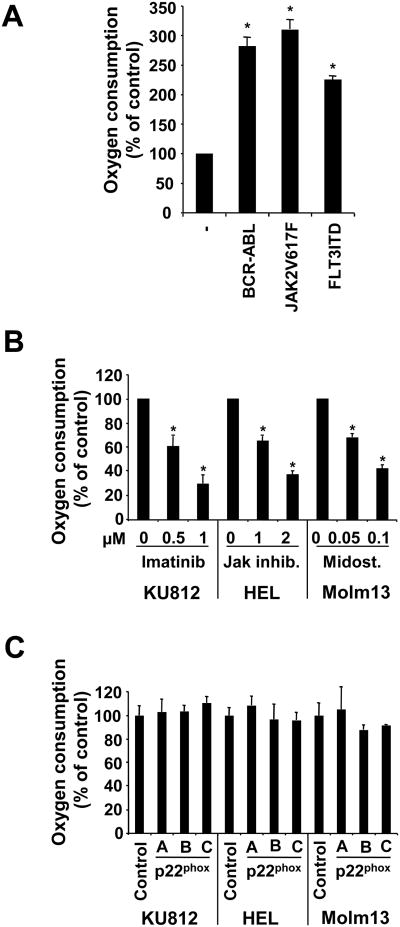
Univalent reductions of molecular oxygen by stepwise electron transfer is required for the production of various ROS, generating sequential intermediates starting with superoxide radicals (O2•-), to hydrogen peroxide (H2O2) and to hydroxyl radicals (•OH). Thus, changes in oxygen consumption are an indirect measurement of potential changes in ROS. In BaF3 cells transformed by TKOs, the oxygen consumption was significantly increased (BCR-ABL: 182.1±14.8%; JAK2V617F: 210±15.8%; FLT3ITD: 125.7±6.8% increase) (Figure 2A). Consequently, in KU812, HEL and Molm13 cells, oxygen consumption was reduced in a dose dependent manner in response to their respective tyrosine kinase inhibitors, including imatinib (1μM; 70.6±6.5%), Jak inhibitor (2μM; 62.5±2.9%) and midostaurin (100nM; 57.3±2.5%) (Figure 2B). Oxygen consumption was also measured in KU812, HEL and Molm13 cells with p22phox knockdown. We did not observe significant difference in oxygen consumption between control cells and cells with targeted knockdown, suggesting that NOX do not consume a significant amount of molecular oxygen (Figure 2C). We had previously demonstrated that mitochondria are a significant source of intracellular ROS in cells transformed by BCR-ABL, using electron transport chain inhibitors to block mitochondrial ROS production18 and similar data were obtained in cell line models used here (not shown).
Figure 2. Oxygen consumption in cells transformed by tyrosine kinase oncogenes.
A Clarke-type electrode and temperature-controlled chamber were used to measure cellular oxygen consumption. A. BaF3 cells expressing either BCR-ABL, JAK2V617F or FLT3ITD were compared to the parental cell line. B-C. KU812, HEL and Molm13 cells were treated with tyrosine kinase inhibitors (B) or cells with p22phox knockdown were used (C). (n=3, *p<0.05).
Mitochondrial ROS are increased in tyrosine kinase oncogene-transformed cells
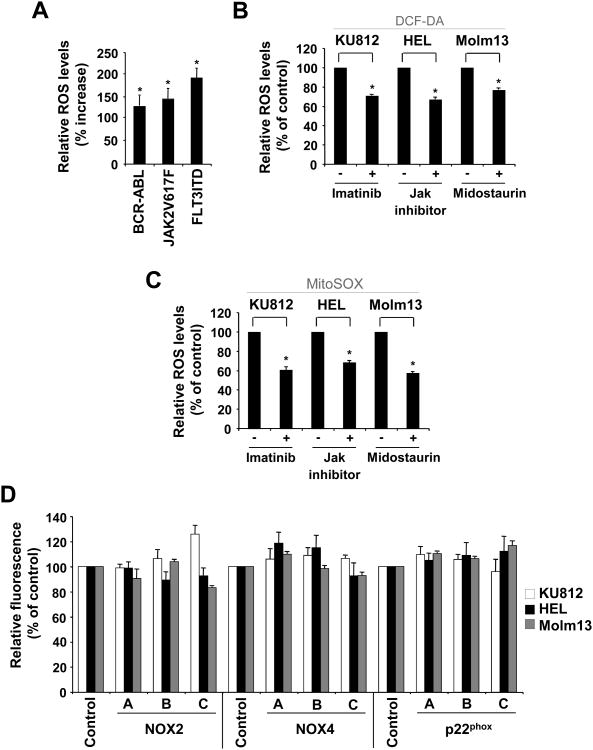
Next, we measured intracellular levels of ROS directly using redox-sensitive fluorochromes. In murine BaF3 cells, intracellular levels of ROS are elevated in the presence of BCR-ABL (129.4±21.9%), JAK2V617F (145.7±20.7%) and FLT3ITD (193.1±20.6%), when compared to the parental cell line (Figure 3A). Also, in KU812, HEL and Molm13 cells, the presence of their respective kinase inhibitor led to a reduction in ROS levels, including imatinib (1μM; 29.1±2.6%), Jak inhibitor (1μM; 33.2±1.9%) and midostaurin (50nM; 23.0±1.1%) (Figure 3B). These data confirm previous findings suggesting that TKOs are sufficient and required for the increase in ROS4,19. Our previous data using the hexokinase inhibitor 2-deoxyglucose suggested that elevated ROS in BCR-ABL transformed cells are dependent, at least in part, on cellular glucose (carbon) metabolism18 and similar results were found in TKO transformed BaF3 cells as well as KU812, HEL and Molm13 cells (Suppl. Fig. 2). In order to further confirm the role of mitochondria in ROS production, we also used the mitochondrial redox sensitive fluorochrome MitoSOX Red. Consistent with the above data, KU812, HEL and Molm13 cells, displayed reduced MitoSOX Red fluorescence in response to tyrosine kinase inhibitors, including imatinib (1μM; 38.9±2.5%), Jak inhibitor (1μM; 31.4±1.8%) and midostaurin (50nM; 41.9±1.1%), respectively (Figure 3C). In additional control experiments, we confirmed that elevated ROS indeed require an active electron transport chain using CML, AML and polycythemia vera patient specimens (not shown). Next, using DCF-DA staining, the amount of intracellular ROS was measured in control and NOX-targeted shRNA containing cells. Consistent with a lack in change of oxygen consumption in p22phox knockdown cells, there was no significant reduction in ROS levels in cells with NOX2, NOX4 and p22phox knockdown compared to control cells (Figure 3D). In order to evaluate clonal effects as a result of stable integration and selection for cells with targeted knockdown, transient knockdown with the p22phox construct was also performed. Also in these experiments, we did not observe a significant reduction in intracellular ROS (not shown). The data imply that NOX proteins do not significantly participate in the excess production of intracellular ROS in TKO transformed cells.
Figure 3. ROS in cells transformed by tyrosine kinase oncogenes.
Intracellular ROS levels were measured by DCF-DA (A, B, D) or MitoSox (C) staining in tyrosine kinase transformed cells or in response to targeted approaches. A. BaF3 cells expressing either BCR-ABL, JAK2V617F or FLT3ITD were compared to the parental cell line (left panel). B-D. KU812 (BCR-ABL), HEL (JAK2V617F) and Molm13 (FLT3ITD) cells were compared to kinase inhibitor treated cells (18h), as indicated (B-C) or control cells were compared to cells with NOX2, NOX4 and p22phox knockdown (D). (n=3, *p<0.05).
NOX proteins regulate growth and migration
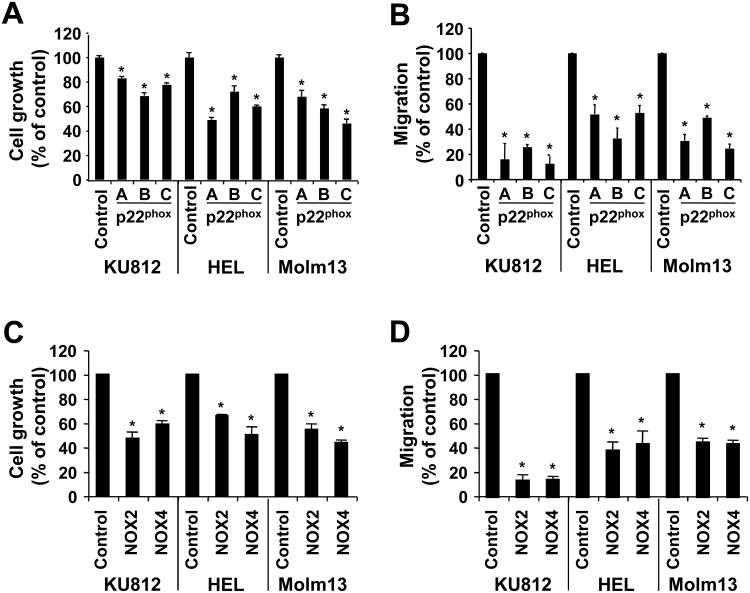
Finally, the effect of the knockdown on cell growth and migration using cells with p22phox knockdown was tested. We found significant reduction in cell growth (72 h culture) in KU812 (17.1-31.6%, p<0.05), HEL (27.9-50.9%, p<0.05) and Molm13 (32.0-54.0%, p<0.05) cells compared to control shRNA-transfected cells (Figure 4A). Significant changes in viability were not detected under these experimental conditions (not shown). Similar to cell growth, a drastic reduction in migration compared to control cells was observed in KU812 (74.3-87.6%, p<0.05), HEL (47.1-67.6%, p<0.05) and Molm13 (51.0-75.6%, p<0.05) cells (Figure 4B). These data did not indicate whether growth and migration were regulated through NOX2 or NOX4. Using the same approach, we first measured potential changes in cell growth in single population with NOX2 and NOX4 knockdown. Consistent with p22phox knockdown, NOX2- and NOX4-targeted approaches in the cell lines tested led to a significant reduction of growth by 34.2-52.8% and 41.2-56.5% (p<0.05), respectively (Figure 4C). We also found that in these cells both NOX2 (55.3-86.1% reduction, p<0.05) and NOX4 (56.5-85.6% reduction, p<0.05) were required for optimal migration (Figure 4D). We did not detect a significant change in cell growth during the migration assays. Interestingly, BaF3 cells that are transformed by the TKOs tested are fully dependent on the transforming tyrosine kinase activity for growth and spontaneous migration, in contrast to parental BaF3 cells, which do not proliferate or migrate under identical experimental conditions (not shown). These data suggest that NOX proteins play an important role in growth and migration of hematopoietic cells transformed by TKOs.
Figure 4. p22phox, NOX2 and NOX4 are required for optimal growth and migration.
KU812, HEL and Molm13 cells were used to determine the biological consequences of p22phox, NOX2 and NOX4 knockdown. Growth (A, C) and relative migration (B, D) were determined in KU812, HEL and Molm13 cells with p22phox knockdown (A-B) and NOX2 or NOX4 knockdown (C-D) as well as in control cells (n=3, *p<0.05).
Tyrosine kinase oncogenes increase NADPH levels but not NOX expression
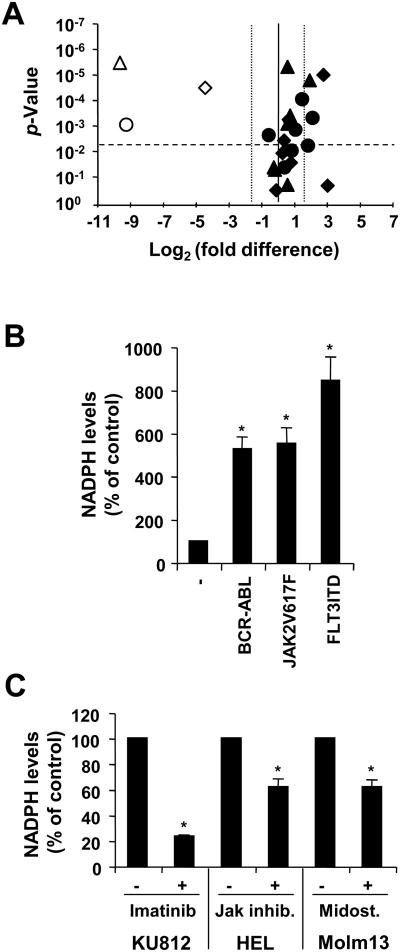
The functional regulation of NOX proteins in cells has been well characterized and might depend in part on the cellular context and the NOX isoforms expressed. NOX2 and NOX4 activities can be both regulated through changes in expression and through the availability of their main substrate NADPH. The regulation of NOX2 is more complex than NOX4 and may be influenced by additional factors and signaling molecules, which are not measured here. Initially, we looked for changes in the expression of NOX components in KU812, HEL and Molm13 in response to tyrosine kinase inhibition (Figure 5A). We did not observe a significant reduction in NOX components, suggesting that the associated tyrosine kinase oncoproteins are not likely to regulate NOX through elevated levels. Moreover, some NOX components were even found to be increased, suggesting that the oncogenic tyrosine kinase activities lead to a reduction in the levels of some components, depending on the cellular background. Next, cellular NADPH levels were measured, which are in particular critical for the regulation of the constitutively active NOX420 but are also rate-limiting for NOX2.21,22 The cellular NADPH levels were significantly increased in BaF3 cells transformed by TKOs, (BCR-ABL: 427.9±54.2%; JAK2V617F: 453.1±70.9%; FLT3ITD: 743.5±108.7% increase) (Figure 5B). Similarly, in KU812, HEL and Molm13 cells, NADPH levels were reduced in response to imatinib (1μM; 70.6±0.8%), Jak inhibitor (1μM; 62.5±6.1%) and midostaurin (50nM; 57.3±5.5%), respectively (Figure 5C). Thus, changes in NADPH levels may constitute an important mechanism for the regulation of NOX proteins by TKOs. Additional experiments would be required to determine whether the observed changes in NADPH levels are sufficient to effectively alter NOX activities and cause changes in biological phenotypes.
Figure 5. Tyrosine kinase oncogenes increase NADPH levels but not NOX expression.
A. Changes in gene expression of NOX and their components in response to imatinib (1μM; ●) in KU812 cells, Jak inhibitor (1μM; ◆) in HEL cells and midostaurin (50nM; ▲)) in Molm13 cells were determined by real-time PCR relative to their respective p-values (volcano plot). Open symbols indicate changes in expression of Pim1. The horizontal dashed line indicates the p=0.005 significance level and the vertical dotted lines indicate 3-fold changes. B-C. NADPH levels were measured in whole cell extracts. BaF3 cells expressing BCR-ABL, JAK2V617F and FLT3ITD were compared to parental BaF3 cells (B). Untreated KU812, HEL and Molm13 cells were compared to cells treated with imatinib (1μM), Jak inhibitor (1μM) and midostaurin (50nM), respectively (C).
p22phox is required for phosphorylation of MARCKS, a regulator of migration
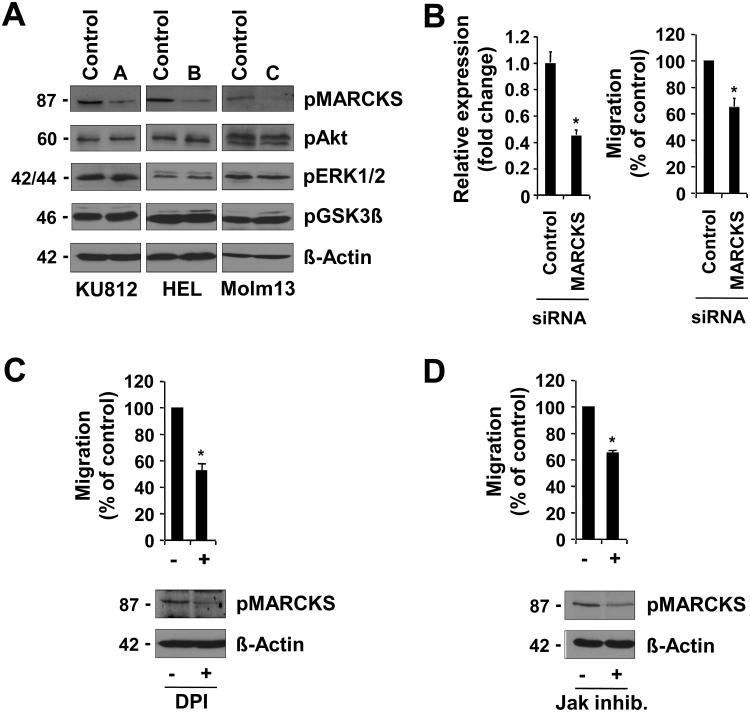
In an attempt to identify signaling mechanisms regulated through NOX, we looked at proteins known to be involved in the regulation of cytoskeletal reorganization in cells with p22phox knockdown. We found that phosphorylation of the actin cross-linking protein MARCKS at the regulatory Ser152/156 was specifically reduced in KU812, HEL and Molm13 with p22phox knockdown (Figure 6A, top panel). As a negative control, we did not detect changed phosphorylation at the activation sites of AKT (Thr308), ERK1/2 (Thr202/Tyr204) or GSK3β (Ser9) (Figure 6A, bottom panels). To test whether MARCKS would be required for migration in transformed cells, we knocked down expression of this protein with siRNA pools targeting MARCKS and compared them to non-targeting siRNA. HEL cells were used due to their high degree of transfectability. Initially, knockdown of MARCKS was confirmed by real-time PCR and led to a 55.0±3.0% (n=3; p<0.05) reduction in expression levels compared to control transfected cells (Figure 6B, left panel). Consistent with the above data, knockdown of MARCKS also led to a 35.2 ±5.7% (n=3; p<0.05) reduction in migration of HEL cells (Figure 6B, right panel). Similar data were obtained in KU812 cells with MARCKS knockdown (not shown). Molm13 cells, however, were found to be difficult to transfect. In order to confirm the regulation of MARCKS phosphorylation, we treated HEL cells with the flavoprotein inhibitor DPI, which is expected to block NOX activity (Figure 6C), as well as an inhibitor of the oncogenic JAK2 kinase (Figure 6D). As expected, treatment with both inhibitors significantly reduced migration (DPI, 47.7% reduction; Jak inhibitor, 35.3% reduction) as well as MARCKS phosphorylation. Overall, these data suggest an important role for NOX in MARCKS phosphorylation and a requirement for this protein in cell migration. Future experiments would be necessary to define a link between MARCKS phosphorylation and disease development.
Figure 6. Functional MARCKS expression is required for migration.
A. KU812, HEL and Molm13 cells with p22phox knockdown were used to determine phosphorylation of MARCKS, Akt, ERK1/2, or GSK3β and compared to control shRNA-expressing cells. B. HEL cells were transfected with either control siRNA or MARCKS-specific siRNA. Relative expression (left panel) and migration (right panel) were determined 24h after transfection. C-D. HEL cells were left untreated or treated for 6h with DPI (5μM) (C) or for 18h with Jak inhibitor (1μM) (D) and relative migration (top) or MARCKS phosphorylation (bottom) were determined. (n=3, *p<0.05).
Discussion
ROS are important signaling intermediates that participate in the regulation of different biological activities, including cell signaling, growth, angiogenesis and differentiation, but ROS can also be involved in processes that are detrimental to normal growth, such as apoptosis and DNA damage.9 In phagocytic cells the role of superoxide-producing NOX proteins has been well established as crucial effectors of host defense mechanisms. Mutations that lead to loss of NOX function result in the impaired ability of phagocytic cells to defend against pathogens.23 The expression of NOX in non-phagocytic cells would imply additional functions and their role in myeloid neoplasms or other malignancies is just beginning to emerge. Our experiments in cells with targeted knockdown of NOX2, NOX4 and p22phox indicate that these proteins are not significantly involved in the excess production of ROS. Consistent with our data, Naughton et al. did not observe changes in ROS in response to targeting NOX2 using murine BaF3 cells, but found NOX4 to be required for BCR-ABL induced ROS and growth.24 However, NOX4 knockdown data also demonstrated that ROS were still reduced when no changes in NOX4 protein levels were observed at a later time point. In the absence of additional off target effects, this would imply that subliminal changes in NOX4 are sufficient to effect oxidative stress. Our data using three patient derived cell lines and p22phox as well as NOX4 knockdown, do not support a role of NOX4 in the regulation of the excess production of ROS in human models. It has been hypothesized that small amounts of ROS produced by NOX proteins may regulate membrane proximal signaling events through redox-sensitive proteins in a variety of cells.25 Woo et al. have also recently demonstrated that inactivation of detoxifying peroxiredoxins in proximity to the membrane may restrict elevated ROS to the site of production26, thus conveying specificity to this process without significantly increasing intracellular ROS.
We observed significant growth reduction in KU812, HEL and Molm13 cells with p22phox, NOX2 and NOX4 knockdown. NOX activity has previously been shown to be involved in transformation, such as by overexpression of NOX1 in Ras transformed NIH3T3 fibroblasts, causing increased cell growth and tumor formation in athymic mice.27 In pancreatic adenocarcinoma, NOX4 showed a prosurvival role and knockdown resulted in apoptosis, likely through AKT and apoptosis signal-regulating kinase 1 dependent mechanisms.28 Similarly, inhibition of NOX4 was also shown to suppress cell growth in melanoma cells associated with partial G2-M cell cycle arrest.29 In addition to growth, our data also suggested that NOX proteins are required for migration. Interestingly, we also found in data not shown that inducible expression of active Rac (a component of the functional NOX2 complex) can regulate migration independent of excess production of ROS. Also, NOX can show specific subcellular localization, such as membrane ruffles, lipid rafts, and lamellipodia, leading to localized production of ROS that is likely to be required for cell-matrix adhesions, cytoskeletal reorganization and cell migration.25
The primary targets of NOX-produced superoxide are not well characterized and may include Rho family GTPases, protein tyrosine phosphatases or other proteins with redox-sensitive cysteines.9 Our data demonstrate that knockdown of p22phox leads to reduced phosphorylation of MARCKS in transformed cells. Also, MARCKS phosphorylation required the active JAK2V617F kinase in HEL cells, suggesting a link between the transforming kinase activity and NOX function. MARCKS was originally described as myristoylated protein kinase C substrate. Phosphorylation of MARCKS is thought to disrupt its interaction with acidic phospholipids, resulting in translocation to the actin cytoskeleton, where it regulates actin function.30 Cells containing oncogenic tyrosine kinases are already associated with spontaneous migration but the regulatory mechanisms are not well understood.31 Whereas MARCKS is shown here to be a crucial regulator of this biological effect, the potential role of MARCKS kinases, including protein kinase C isoforms, and the redox mechanisms involved are not known. Preliminary data suggest that knockdown of p22phox does not affect global activation of PKC isoforms (not shown) and it is therefore likely that p22phox specifically targets individual kinases or phosphatases to regulate the phosphorylation of MARCKS. Our data do not exclude a potential role of MARCKS in cell growth but suggest that there may be different molecules involved that mediate a growth advantage through NOX signaling pathways.
Growth and migration in cells transformed by TKOs depends on their active kinase activity but it is not known how this is linked to NOX. Our data suggest that in the leukemic cell lines tested that all NOX2 and NOX4 components are readily expressed and that this expression does not depend on the oncogenic tyrosine kinase activity. NOX2 and NOX4 can be regulated at different levels. For example, NOX2 requires active RAC, which has already been associated with transformation by BCR-ABL.32 In contrast, NOX4 is constitutively active and may be regulated in part through changes in its expression levels. Our data hint at a potential regulation of NOX activities at the substrate level. NOX activity depends on the presence of molecular oxygen and NADPH, which is the rate-limiting substrate for the generation for superoxide, in particular for NOX4.20 We show that the relative levels of NADPH in cells transformed by TKOs are elevated. Consequently, treatment with tyrosine kinase inhibitors limits the availability of the major NOX substrate NADPH. There are multiple sources of NADPH in the cell, but a major mechanism includes the catabolic pentose phosphate pathway. Further characterization of changes in this and related catabolic pathways in myeloid malignancies will provide a better understanding of the regulation of NOX.
Our previous data suggested that glucose metabolism and mitochondrial ROS are important for cell growth and survival in BCR-ABL transformed cells.4,18 Here we expand this finding and suggest that the superoxide-producing NOX are important regulators of migration in myeloid cells transformed by TKOs. It is not known whether this phenotype is specific for these diseases and it would be interesting to see whether cells transformed by other mechanisms or different types of cancer display a similar dependency on NOX for increased migration. NOX effectors are expected to be specifically regulated through NOX-induced ROS for the regulation of processes such as invasion and metastasis. Therefore, NOX and downstream effectors would make ideal drug targets and blocking NOX mediated biological functions could have beneficial effects in leukemia therapy.
Supplementary Material
Acknowledgments
Financial support This work is supported in part by National Institutes of Health grants (5R01CA134660-03, M.S.), (5R01CA129501-02 and 5R01CA125541-03, R.S.), and (5P01CA66996-12, J.D.G.), a Leukemia and Lymphoma Society SCOR grant (J.D.G.) and by grants from the American Cancer Society, the U.S. Department of Defense and the Adams Barr Program in Innovative Cancer Research (M.S.).
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Walz C, Sattler M. Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloid leukemia (CML) Crit Rev Oncol Hematol. 2006;57:145–164. doi: 10.1016/j.critrevonc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerji L, Sattler M. Targeting mutated tyrosine kinases in the therapy of myeloid leukaemias. Expert Opin Ther Targets. 2004;8:221–239. doi: 10.1517/14728222.8.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 5.Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P, et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111:3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 7.Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, Shapiro GI, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8:620–627. [PubMed] [Google Scholar]
- 8.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, et al. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–2935. [PubMed] [Google Scholar]
- 9.Rodrigues MS, Reddy MM, Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal. 2008;10:1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 10.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 11.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 12.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes MS, Reddy MM, Gonneville JR, Deroo SC, Podar K, Griffin JD, et al. BCR-ABL promotes the frequency of mutagenic single strand annealing DNA repair. Blood. 2009;114:1813–1819. doi: 10.1182/blood-2008-07-172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland PC, Clark MG, Bloxham DP, Lardy HA. Mechanism of action of the hypoglycemic agent diphenyleneiodonium. J Biol Chem. 1973;248:6050–6056. [PubMed] [Google Scholar]
- 17.Yoshie O, Majima T, Saito H. Membrane oxidative metabolism of human eosinophilic cell line EoL-1 in response to phorbol diester and formyl peptide: synergistic augmentation by interferon-gamma and tumor necrosis factor. J Leukoc Biol. 1989;45:10–20. doi: 10.1002/jlb.45.1.10. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 19.Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, et al. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- 20.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisimoto Y, Motalebi S, Han CH, Lambeth JD. The p67phox activation domain regulates electron flow from NADPH to flavin in flavocytochrome b558. J Biol Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 22.Nisimoto Y, Ogawa H, Miyano K, Tamura M. Activation of the flavoprotein domain of gp91phox upon interaction with N-terminal p67phox (1-210) and the Rac complex. Biochemistry. 2004;43:9567–9575. doi: 10.1021/bi0400249. [DOI] [PubMed] [Google Scholar]
- 23.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Naughton R, Quiney C, Turner SD, Cotter TG. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- 25.Ushio-Fukai M. Compartmentalization of Redox Signaling through NADPH Oxidase-derived ROS. Antioxid Redox Signal. 2008;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H(2)O(2) Accumulation for Cell Signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 29.Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 30.Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattler M, Quinnan LR, Pride YB, Gramlich JL, Chu SC, Even GC, et al. 2-methoxyestradiol alters cell motility, migration, and adhesion. Blood. 2003;102:289–296. doi: 10.1182/blood-2002-03-0729. [DOI] [PubMed] [Google Scholar]
- 32.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.