Abstract
Cardiac hypertrophy is controlled by a highly connected signaling network with many effectors of cardiac myocyte size. Quantification of the contribution of individual pathways to specific changes in shape and transcript abundance is needed to better understand hypertrophy signaling and to improve heart failure therapies. We stimulated cardiac myocytes with 15 hypertrophic agonists and quantitatively characterized differential regulation of 5 shape features using high-throughput microscopy and transcript levels of 12 genes using qPCR. Transcripts measured were associated with phenotypes including fibrosis, cell death, contractility, proliferation, angiogenesis, inflammation, and the fetal cardiac gene program. While hypertrophy pathways are highly connected, the agonist screen revealed distinct hypertrophy phenotypic signatures for the 15 receptor agonists. We then used k-means clustering of inputs and outputs to identify a network map linking input modules to output modules. Five modules were identified within inputs and outputs with many maladaptive outputs grouping together in one module: Bax, C/EBPβ, Serca2a, TNFα, and CTGF. Subsequently, we identified mechanisms underlying two correlations revealed in the agonist screen: correlation between regulators of fibrosis and cell death signaling (CTGF and Bax mRNA) caused by AngII; and myocyte proliferation (CITED4 mRNA) and elongation caused by Nrg1. Follow-up experiments revealed positive regulation of Bax mRNA level by CTGF and an incoherent feedforward loop linking Nrg1, CITED4 and elongation. With this agonist screen, we identified the most influential inputs in the cardiac hypertrophy signaling network for a variety of features related to pathological and protective hypertrophy signaling and shared regulation among cardiac myocyte phenotypes.
Keywords: cardiac hypertrophy, phenotypic screen, cardiac myocytes, signal transduction, CTGF, CITED4
1. Introduction
Cardiac hypertrophy develops with increased biochemical and mechanical stresses on the heart [1] and is a major predictor of heart failure and sudden cardiac death [2–4]. Specific features of cardiac hypertrophic remodeling depend on the type of cardiac stress [5], [6]. Physiological stresses such as exercise lead to hypertrophy without cardiac dysfunction, but pathological stresses such as high blood pressure and myocardial infarction lead to hypertrophy with increased fibrosis, cell death, and cardiac dysfunction [7]. Moreover, myocytes grow distinctly in response to different mechanical stimuli. Pressure overload induces concentric hypertrophy, characterized by thickening of the heart wall, and volume overload of the heart induces eccentric hypertrophy, characterized by myocyte elongation and dilation of the heart wall [8]. Since eccentric hypertrophy is a greater risk to patients than concentric hypertrophy [9], increased knowledge of the unique signaling pathways controlling myocyte shape will be important in improving therapies for heart failure. Previous work suggests that myocyte size is regulated by common signaling pathways and myocyte shape is regulated by distinct signaling pathways [10], [11]. But little is known about the specific signaling pathways that induce distinct characteristics of hypertrophy [12], [13].
Many signaling pathways and genes manage the hypertrophic response [14]. While many of the components of this network have been identified, the distinct contributions to different features of hypertrophy such as shape, fibrosis, and cell death between pathways is not well understood [15]. Furthermore, the pathways governing hypertrophy are highly connected with much cross-talk between pathways [16]. It is unclear how all of the parts of such an interconnected network function together as a coordinated system that can induce distinct, context-dependent hypertrophy features. Commonly measured markers of hypertrophy such as cell size and fetal gene expression are not markedly differentially regulated between receptor pathways in the signaling network [16].
Here, we test the hypothesis that features such as myocyte shape, fibrosis, cell death, and inflammation may better differentiate hypertrophic signaling pathways. We quantified differential regulation of 5 shape features using high-throughput myocyte imaging and transcript levels of 12 genes induced by 15 predominant hypertrophic agonists. These genes have previously been associated with phenotypes such as fibrosis, cell death, contractility, proliferation, angiogenesis, inflammation, and the fetal cardiac gene program, providing a phenotypic signature for each agonist. We clustered pathway inputs and outputs to identify a network map linking input modules to output modules. Among these, we found strong correlations between Bax and CTGF mRNA abundance in response to AngII and between myocyte elongation and CITED4 mRNA abundance in response to Nrg1. Follow-up experiments validated these correlations, revealing regulation of pro-apoptotic Bax by fibrosis marker CTGF and negative regulation of myocyte elongation by CITED4 gene expression.
2. Methods
2.1 Cell culture and microscopy
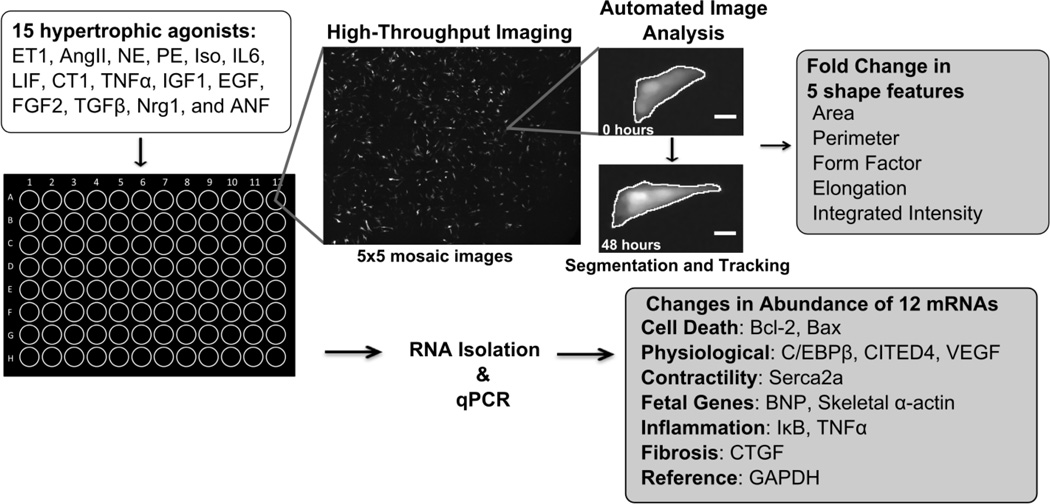
Cardiac myocytes were harvested from 1 to 2 day old Sprague Dawley rats using the Neomyts isolation kit (Cellutron, Baltimore, MD). Myocytes were cultured in plating media (Dulbecco’s modified Eagle media, 17% M199, 10% horse serum, 5% fetal bovine serum, 100 U/mL penicillin, and 50 mg/mL: streptomycin) at a density of 100,000 cells per well of a 96-well plate coated with SureCoat (a combination of collagen and laminin, Cellutron). All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the University of Virginia Institutional Animal Care and Use Committee. Two days after isolation, myocytes were transfected with GFP under a cardiac myocyte specific troponin T promoter[17] using Lipofectamine 2000 (Invitrogen, Carlsbad, California; transfection efficiency: 10–15%). Two days after transfection, myocytes were imaged using automated image acquisition scripts, which collect a 5 × 5 grid of images in each well of interest in the 96-well plate (Fig. 1)[18]. Images were collected using an Olympus IX81 inverted microscope with 10X UPlanSApo 0.40 NA objective, Orca-AG CCD camera (Hamamatsu, Bridgewater, NJ), automated stage (Prior Scientific, Rockland, MA), and IPLab software (Scanalytics, Fairfax, VA).
Figure 1. 15 ligand hypertrophic agonist screen in neonatal rat cardiac myocytes, measuring changes in 5 shape features and abundance of 12 mRNAs.
Myocytes were treated for 48 hours with 1 of 15 hypertrophic agonists. Fold change in 5 shape features was calculated using our automated image acquisition and analysis platform. We measured fold change in abundance of 12 mRNAs related to cell death, physiological hypertrophy, contractility, fetal genes, inflammation, and fibrosis using qPCR.
2.2 Quantifying changes in shape
After initial images were collected, myocytes were rinsed and cultured in serum-free media containing one of three concentrations of a hypertrophic agonist (dilution factor = 10, intermediate concentration listed): 1 µM Atrial Natriuretic Factor (ANF), 1 µM Angiotensin II (AngII), 1 nM Cardiotrophin-1 (CT1), 10 nM Epidermal Growth Factor (EGF), 100 nM Endothelin-1 (ET1 while maintaining cell health in the serum free control condition), 20 ng/mL Fibroblast growth factor-2 (FGF2), 10 nM Insulin Growth Factor-1 (IGF1), 10 ng/mL Interleukin-6 (IL6), Isoproterenol (ISO), 1 nM Leukemia Inhibitory Factor (LIF), 1 µM Norepinephrine (NE), 10 ng/mL Neuregulin-1 (Nrg1), 1 µM Phenylephrine (PE), 1 ng/mL Transforming Growth Factorbeta (TGFβ), or 10 ng/mL Tumor Necrosis Factor-alpha (TNFα). A comprehensive list of agonist concentrations is shown in Supplementary Table S1. After 48 hours, myocytes were imaged again. 48 hour stimulation allowed for robust changes in cell size and shape to be measured while maintaining cell health in serum free conditions.
Changes in myocyte area, perimeter, form factor, elongation, and integrated fluorescence intensity were calculated using automated custom Matlab image analysis algorithms. Form factor is a measure of circularity and is calculated as 4π*area/perimeter2. A circle therefore has a form factor value of 1 and all other shapes have form factors less than 1. Elongation is a ratio of the major axis length to the minor axis length of the myocyte. All shape measurements were recorded from two wells from three independent myocyte isolations.
2.3 Quantifying changes in transcript abundance
48 hours after stimulation with the hypertrophic agonists, total RNA was purified from myocytes given the intermediate concentration of the agonist using the RNeasy Mini kit (Qiagen, Valencia, CA). Complementary DNA was synthesized from 85.5 ng of total RNA using the iScript cDNA synthesis kit (Bio-Rad). mRNA levels of twelve genes (Bcl-2, Bax, C/EBPβ, CITED4, VEGF, Serca2a, BNP, Skeletal α-actin, IκB, TNFα, CTGF, and GAPDH) were measured using qPCR (BioRad CFX Connect) using iTaq Universal SYBR Green Supermix (Bio-Rad), 2 ng of cDNA, and 400 nM of each primer set. GAPDH served as internal control. Gene-specific primers were designed on PrimerQuest (Integrated DNA Technologies, Inc.) A list of primers used is shown in Supplementary Table S2. Data were analyzed using the comparative CT method with efficiency correction[19]. Measurements were collected from three independent myocyte isolations.
2.4 siRNA knockdown
Two days after isolation, 10 nM silencer select siRNA (Ambion) was transfected into cells using Lipofectamine RNAiMAX, as described by the manufacturer’s protocol. Two siRNA sequences were tested for each target. Cells were transfected again the next day with 10 nM siRNA in fresh medium. 24 hours following the second transfection, a given hypertrophic agonist was applied (0.1 µM Ang II, 10 ng/mL Nrg1, or 1 nM LIF). 48 hours after addition of the agonist, RNA was purified from the myocytes as described above. For CITED4 knockdown, Lipofectamine 2000 was used with the first transfection so that the cTnt-GFP plasmid could be introduced simultaneously for subsequent imaging.
2.5 CITED4 adenoviral overexpression
To express CITED4 in neonatal rat ventricular myocytes the full-length cDNA for CITED4 with either an N or C-terminal Flag tag was cloned into the pENTR vector (Life Technologies) and subsequently subcloned into an adenoviral destination vector. Transfection into HEK 293 cells resulted in significant virus production within one week. After purification the viral titer was determined by immunocytochemistry to viral coat proteins (Adeno-X, Clontech). Neonatal rat cardiac myocytes cultured on laminin-coated coverslips were treated with adenoviral constructs expressing CITED4 or lacZ for 12 hours. Myocytes were then cultured in serum free media containing the thymidine analogue EdU (10µM) for an additional 24 hours before imaging. A multiplicity of infection (MOI) of 30 was used for overexpression experiments. After fixation cultures were stained with antibodies against sacromeric actin (α-actinin) to identify cardiac myocytes and antibodies for Ki67 or EdU detection for determination of proliferation. Myocytes were imaged with a Zeiss 510 laser scanning confocal microscope with a 40X objective. 60 high power frames (HPFs) were acquired and analyzed from 3 independent experiments.
3. Results
3.1 Differential effects on myocyte size and shape
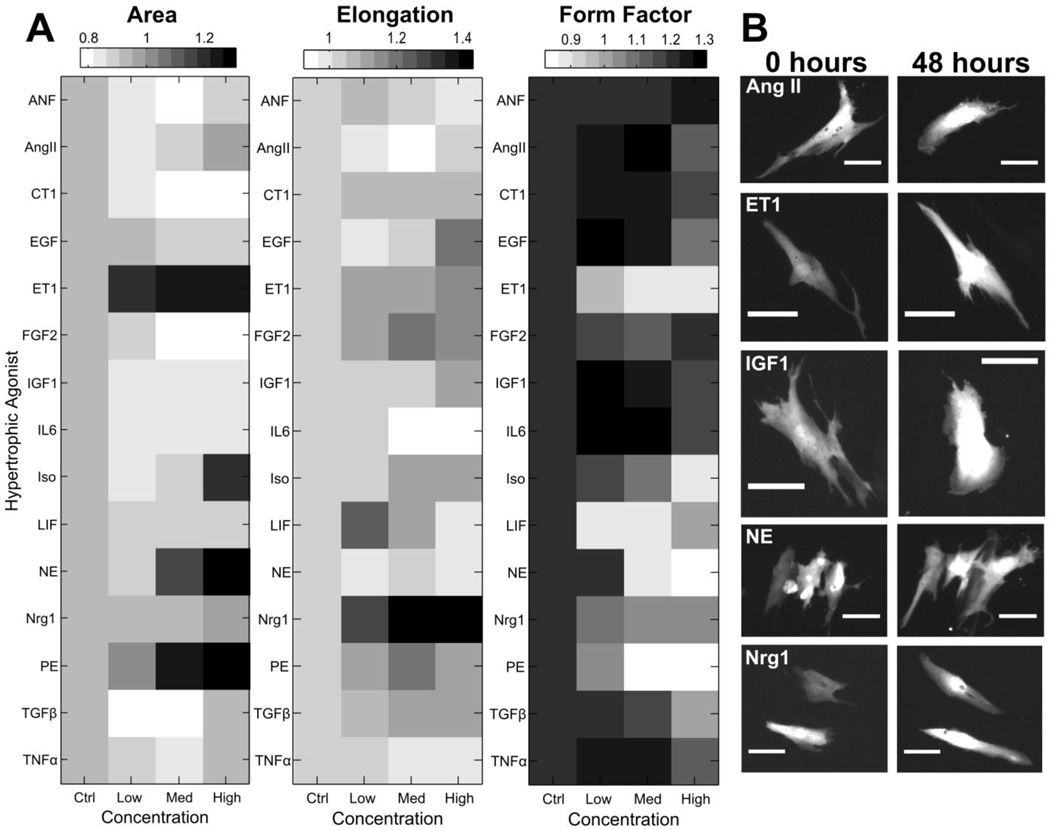
Our automated microscopy and image analysis platform[18] enables quantitative comparison of changes in area, elongation, form factor, perimeter, and integrated fluorescence intensity between signaling pathways (Fig. 1). Fold changes in each of these shape features was calculated after 48 hour stimulation with three different concentrations of 15 hypertrophic agonists (Fig. 2A, Supplementary Fig. S1–S2). These data reveal distinct regulation of shape among the 15 hypertrophic agonists. The α-adrenergic agonists PE, NE, and ET1 caused the largest increases in cell area. While many of the agonists caused minimal changes to cell size, other shape changes were affected more dramatically. This is most evident with Nrg1 stimulation, which did not significantly affect cell area but caused the greatest increases in myocyte elongation. The α-adrenergic agonists ET1, NE, and PE decreased form factor due to increases in the number of cell protrusions. Nrg1 stimulation most dramatically increased elongation with LIF and FGF also causing high elongation responses at specific concentrations. Interestingly, many of the agonist dose responses revealed biphasic regulation of myocyte elongation, as can be seen with LIF, FGF, and PE. Representative myocyte images of the response to a subset of the hypertrophic agonists studied are shown in Figure 2B and illustrate the diversity of shape responses observed among the agonists.
Figure 2. Hypertrophic agonist dose responses reveal diverse monophasic and biphasic effects on myocyte shape.
A) Heat maps of median fold changes in area, elongation, and form factor induced by three different concentrations of hypertrophic agonists, with a dilution factor of 10. B) Representative images of cardiac myocytes treated with a subset of the hypertrophic agonists tested, revealing unique changes in shape among the hypertrophy pathways (Scale bar: 50 µm).
3.2 Single cell data reveal differential regulation of shape features
Previous studies have hypothesized that due to the substantial cell-cell variability, single cell data from even control (untreated) conditions may contain valuable information about relationships between cellular phenotypes[20], [21]. To test this hypothesis, correlations between each of the five shape features were calculated using either the median response to the intermediate concentration of each hypertrophic agonist (Figure 3A) or single-myocyte data from control conditions (N=724 myocytes) (Figure 3B).
Figure 3. Intrinsic variability in single-cell data from untreated conditions is sufficient to capture distinct modes of myocyte shape regulation.
Pearson correlation coefficients were calculated using either A) median fold change in shape feature data from myocytes stimulated with the intermediate concentration of each of the 15 agonists or B) single-cell data from the control myocytes (N=724 myocytes) of fold change in shape features after 48 hours.
Single cell data from untreated cells allowed identification of several interesting correlations in cell shape metrics. Area and elongation have a small correlation coefficient (0.07), indicating that these features depict distinct features of cell shape and may be regulated by different pathways. As expected, area and perimeter are highly correlated (0.82) since these features both primarily describe cell size, however, some variation remains due to differences in shape. For example, LIF significantly increased perimeter without increasing cell area. Fluorescence intensity provides a reporter of troponin T promoter activity that, while highly correlated with cell size, is independent of cell area [18]. This correlation therefore indicates that larger myocytes typically have increased troponin T promoter activity. Form factor is moderately anti-correlated with both perimeter and elongation. Form factor is a measure of cell circularity, and therefore decreases with both elongation and increased numbers of cell protrusions. Form factor and area have a small correlation coefficient (−0.26), indicating that these features capture distinct features and may be regulated by different pathways. Thus single-cell data from even a single treatment condition can harness cell-cell variability to reveal new relationships between myocyte phenotypes.
Correlations derived using the median responses to receptor agonists were qualitatively similar, but higher in magnitude than the correlations derived using single-cell control data. Median-derived correlations coefficients are larger since the agonists that highly affected cell size in this data set also typically had larger effects on many of the other shape metrics. For example, the α-adrenergic agonists had the largest effects on cell area, perimeter, intensity, and form factor.
3.3 Differential effects on myocyte transcript abundance
For each of the 15 hypertrophic agonists, we measured fold change in mRNA abundance of 12 genes related to cardiac myocyte hypertrophy. Genes were selected to capture a diverse set of maladaptive and adaptive features of hypertrophy for each agonist. We measured mRNA abundance of genes previously related to cell death (Bax, which promotes apoptosis and necrosis[22], and anti-apoptotic Bcl2 [23], [24]), physiological hypertrophy (C/EBPβ, which is down-regulated with exercise [25], CITED4, which is upregulated with exercise and associated with myocyte proliferation [25], and pro-angiogenic VEGF [26]), contractility (Serca2a [27]), fetal genes (BNP and skeletal α-actin [28]), inflammation (IκB [29] and TNFα [30], [31]), and fibrosis (connective tissue growth factor, CTGF [32]).
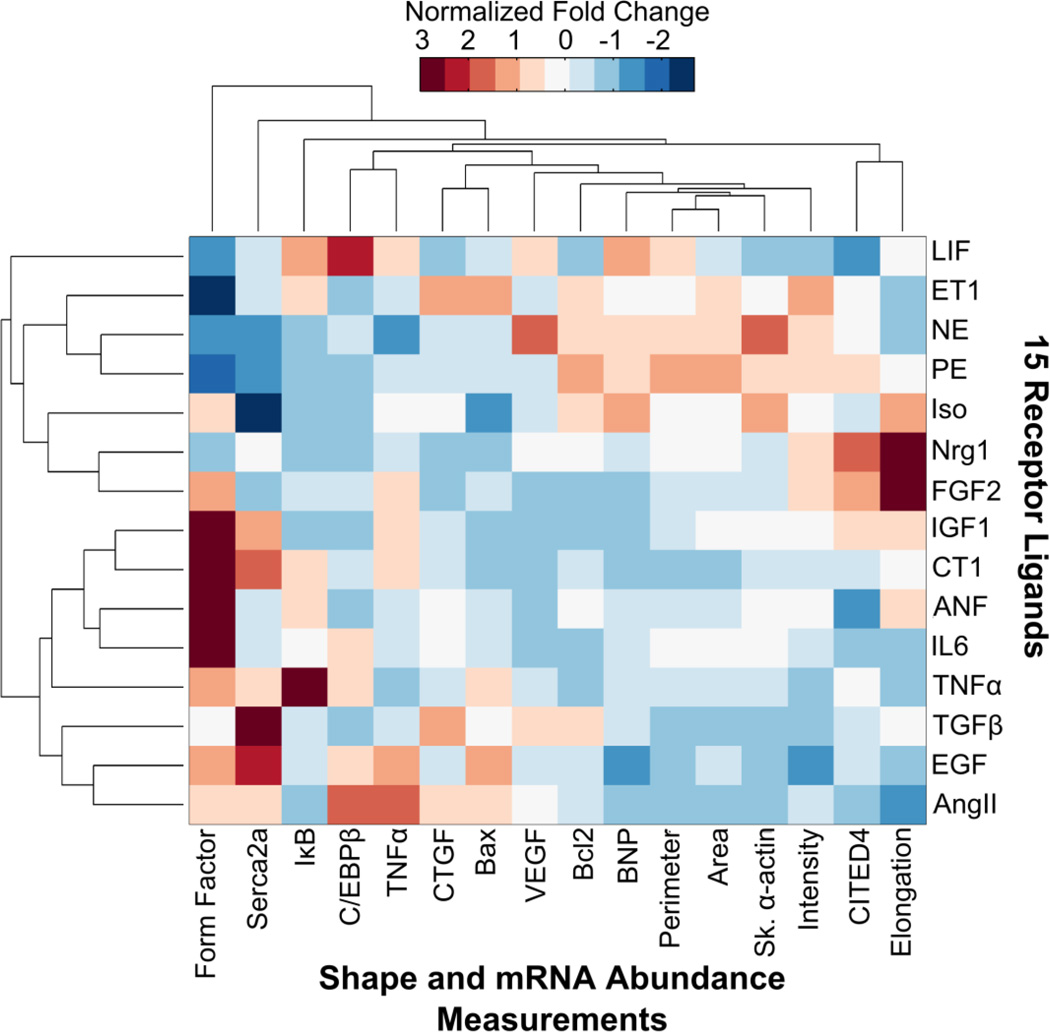
We performed hierarchical clustering of the hypertrophic agonist screen data of fold change in mRNA abundance and shape compared to control of myocytes treated with the intermediate concentration of each agonist (Fig. 4). Each output (column), was normalized before clustering to give a mean of 0 and a standard deviation of 1. This allowed us to compare outputs that had different effect sizes. Bar graphs showing data before normalization with standard error and p-values for each output is provided in the supplement (Supplementary Fig. S3–S18).
Figure 4. Agonist screen identifies distinct hypertrophy phenotypic signatures.
Hierarchical clustering of the ligand screen data of fold change in output compared to control. Each output (columns) was normalized before clustering to give a mean of 0 and a standard deviation of 1. Red indicates an agonist generates relatively high levels of a given output compared to the other agonists tested and blue indicates relatively low levels. While each agonist significantly affected at least one of the hypertrophic phenotypic outputs, agonists had unique signatures of hypertrophy.
The combined mRNA abundance and shape data reveal that the hypertrophic agonists generate distinct phenotypic signatures of hypertrophy (Fig. 4). CITED4 and elongation were notably upregulated by Nrg1 and FGF2, CTGF was upregulated by ET1, Ang II, and TGFβ, Bax was upregulated by ET1, Ang II, and EGF, and C/EBPβ was upregulated with Ang II and LIF. Remarkably, while many of the agonists had minimal effects on cell area, the effects on other hypertrophy phenotypic outputs were much more prominent. For example, while LIF did not significantly increase myocyte area, LIF led to significant increases in BNP, C/EBPβ, and IκB. Moreover, nearly every agonist significantly upregulated VEGF mRNA abundance, which is important for myocyte growth without cardiac dysfunction [33] (Supplementary Fig. S13). Hierarchical clustering revealed new relationships among the outputs measured, identifying correlations between CITED4 and Elongation, TNFα and C/EBPβ, and CTGF and Bax. Notable correlations among inputs were identified between NE and PE, Nrg1 and FGF2, and EGF and Ang II.
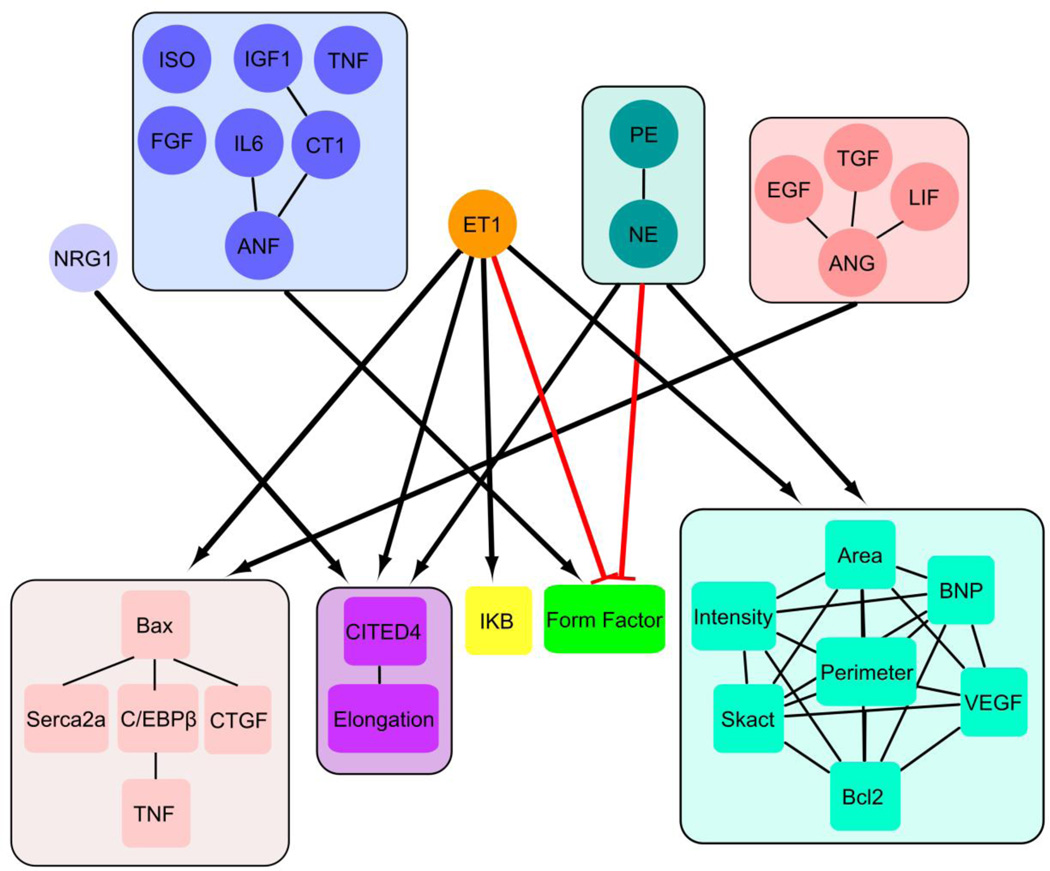
3.4 Clustering reveals modular input-output relationships
Through k-means clustering of the data in Fig. 4, we identified highly correlated input and output modules and a network map linking input modules to output modules (Fig. 5). We identified five modules within the inputs and five modules within the outputs. Lines between species within a module indicate P<0.01 for a given correlation. Interestingly, many maladaptive outputs grouped together in one of the modules: Bax, C/EBPβ, Serca2a, TNFα, and CTGF. More beneficial outputs, Bcl2 and VEGF, grouped together with fetal genes, BNP and skeletal α-actin, and myocyte area, perimeter, and fluorescent intensity. Nrg1 and ET1 were each in a module by themselves, indicating especially unique regulation of hypertrophic outputs. CITED4 and Elongation were highly correlated and in a distinct module from the other outputs measured.
Figure 5. Clustering reveals modular input-output relationships in hypertrophy signaling.
Diagram of the 5 input modules and 5 output modules generated by k-means clustering of the hypertrophic agonist screen data. Links between input modules and output modules are marked if the median normalized fold change in output between each input-output pair in the modules was >|0.70|with red lines indicating negative effects. Lines marked between components within a module indicate P<.01 from a given correlation. Clustering identified new relationships and modules among inputs and outputs, including correlations between CTGF and Bax and between CITED4 and myocyte elongation.
We also calculated the median normalized fold change in output between each inputoutput pair within an input module and an output module. Bold lines connecting input modules and output modules were diagrammed (Fig. 5) if the median normalized fold change in output between each input-output pair in the module was >|0.7|, with red lines indicating negative effects. The Ang II and ET1 modules had a high effect on the module with Bax and C/EBPβ. ET1 had high effects on every output module, and was the only module to markedly affect IκB. The α-adrenergic receptors had high effects on the module with cell size, fetal genes, and VEGF. Nrg1 upregulated the CITED4 and elongation module and the ANF module was the only group that increased form factor. This network map reveals shared regulation among maladaptive features of hypertrophy, which could enhance the development of pathological hypertrophy.
3.5 Pro-fibrotic CTGF upregulates Bax mRNA abundance
Given the maladaptive effects of myocyte death and fibrosis on cardiac hypertrophy, we wanted to further investigate our measured correlation between pro-cell death Bax and profibrotic CTGF. While Bax/CTGF correlation could conceivably arise from a shared upstream pathway without causal relationships between Bax and CTGF, we hypothesized that CTGF mechanistically regulates Bax mRNA abundance in cardiac myocytes. To test this hypothesis, we performed a series of follow-up experiments. First, we confirmed the presence of CTGF in myocytes with immunofluorescence imaging (Supplementary Fig. S19–20).
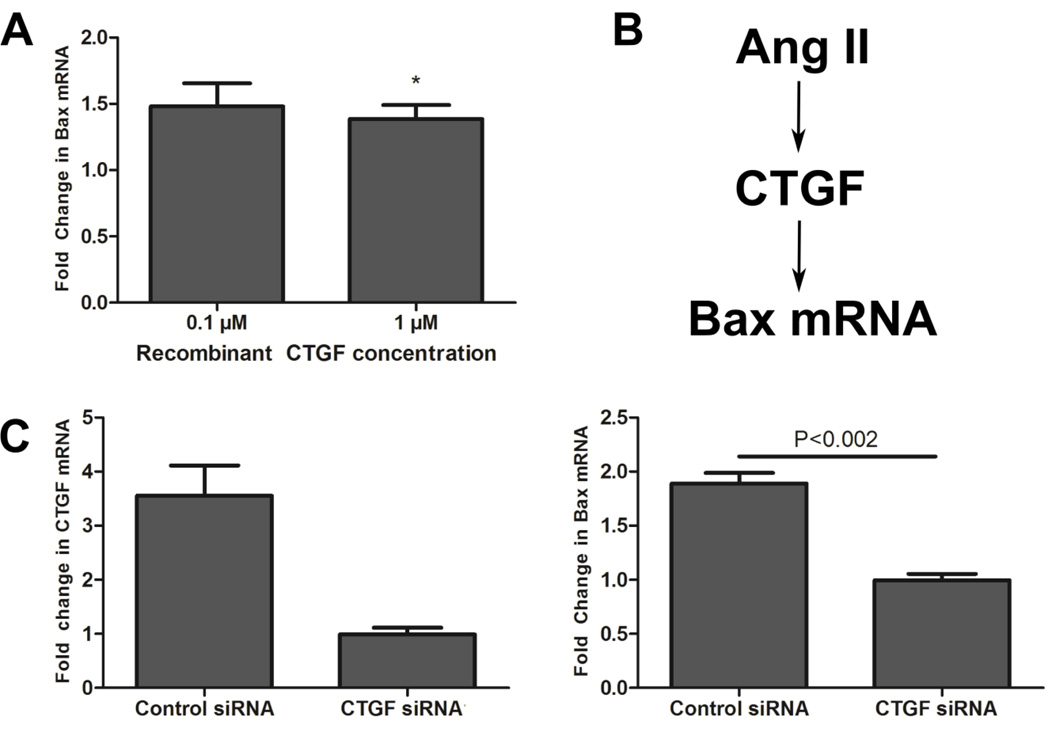
We next tested whether recombinant CTGF was sufficient to increase Bax mRNA abundance. Five days after isolation, we applied 0.1 or 1 µM recombinant human CTGF protein (Life Technologies) to the cardiac myocytes. After 48 hours we measured fold change in Bax mRNA using qPCR as described earlier. As hypothesized, application of recombinant CTGF to myocytes did lead to increased Bax mRNA abundance (Fig. 6A).
Figure 6. CTGF increases Bax mRNA abundance.
A) Mean +/− SE fold change in Bax mRNA after 0.1 µM or 1 µM recombinant CTGF was applied to myocytes for 48 hours. Bax mRNA abundance was quantified with qPCR. 1 µM CTGF significantly increased the Bax mRNA level in cardiac myocytes (P<.05, Student’s t-test). B) Schematic of hypothesized regulation of Bax mRNA by CTGF. Since CTGF induced increases in Bax mRNA in myocytes we hypothesized that regulation of Bax expression by CTGF may have led to the correlation between these features in our ligand screen data. C) Mean +/− SE fold change in CTGF (left) and Bax (right) after CTGF mRNA was knocked down by siRNA transfection and myocytes were stimulated with 0.1 µM Ang II for 48 hours. Knockdown of CTGF led to significantly decreased Bax mRNA compared to control siRNA (P<0.002, Student’s t-test).
To further study the CTGF/Bax interplay under conditions mimicking pathological stress, we studied the effect of CTGF knockdown with siRNA on Bax mRNA abundance in Ang IItreated myocytes. Since recombinant CTGF increased Bax mRNA abundance, we hypothesized that knockdown of CTGF would lead to decreased Bax mRNA abundance. Ang II was chosen as an agonist because it induced large increases in abundance of the output module containing Bax and CTGF without greatly affecting many other outputs. As hypothesized, knockdown of CTGF decreased Ang II-dependent increases in Bax mRNA (Fig. 6C). Data for both CTGF siRNA constructs is shown in Supplementary Fig. S21. Together, these results indicate a signaling pathway from CTGF to Bax mRNA regulation in cardiac myocytes and reveal a role for CTGF in Ang II-induced increases in Bax mRNA abundance. While CTGF plays a significant role in Ang II-induced Bax regulation, Bax has other known regulators [34–37]. Thus in addition to promoting fibrosis, agonists causing increased CTGF may also enhance cell death signaling, potentially exacerbating maladaptive hypertrophic remodeling of the heart.
3.6 CITED4 negatively regulates cardiac myocyte elongation
Clustering analysis had revealed prominent correlation between CITED4 mRNA abundance and myocyte elongation. Since this correlation has interesting implications for co regulation of shape and gene expression, we decided to investigate this relationship further. CITED4 is upregulated with exercise and associated with myocyte proliferation [25], making it a potential therapeutic target for cardiac regeneration. Moreover, eccentric hypertrophy due to myocyte elongation is associated with severe cardiac dysfunction [9], making signaling regulating myocyte elongation an especially important area for further investigation. While elongation/CITED4 correlation could arise from a shared upstream pathway without causal relationships between CITED4 and elongation, we hypothesized that CITED4 mechanistically regulated elongation in cardiac myocytes.
To test this hypothesis, we knocked down CITED4 with siRNA (Fig. 7A) and measured the effects on Nrg1 and LIF-induced elongation of cardiac myocytes. Nrg1 and LIF were chosen since they both induce myocyte elongation, but had different effects on CITED4 mRNA abundance (strong upregulation vs. no effect respectively, see Supplementary Fig. S7). CITED4 knockdown did not affect elongation in control myocytes and significantly increased Nrg1-induced elongation (Fig. 7B–C). Data for both CITED4 siRNA constructs is shown in Supplementary Fig. S22. These data are both consistent with Nrg1-induced elongation and upregulation of CITED4 as measured in the agonist screen (Fig. 4) and indicate that CITED4 negative regulates myocyte elongation, forming an incoherent feedforward loop [38]. Moreover, while LIF did not specifically upregulate CITED4 expression, CITED4 knockdown below basal levels also increased elongation in LIF treated myocytes.
Figure 7. CITED4 knockdown enhances Nrg1 and LIF-induced myocyte elongation via an incoherent feed-forward loop.
A) Mean +/− SE of CITED4 mRNA abundance. CITED4 mRNA was knocked down by siRNA transfection. CITED4 mRNA levels were quantified using qPCR. Myocytes were treated with control, 10 ng/mL Nrg1, or 1 nM LIF for 48 hours after transfection with control or CITED4 siRNA. B) Median and interquartile range of fold change in myocyte elongation for control, Nrg1, and LIF treated myocytes. Nrg1 and LIF-treated myocytes significantly increased elongation after CITED4 knockdown (two-tailed Mann Whitney U test, N~700 myocytes), indicating that CITED4 negatively regulates elongation. C) Representative images of control myocytes and Nrg1-treated myocytes with control siRNA or CITED4 siRNA (Scale bar: 100 µm). CITED4 knockdown enhanced Nrg1-induced myocyte elongation. D) Minimal mathematical model of CITED4 regulation of myocyte elongation, fit to CITED4 mRNA and elongation data in control siRNA conditions. E) Incorporating measured levels of CITED4 mRNA knockdown, the model accurately predicted increased Nrg1- and LIF-dependent myocyte elongation in CITED4 siRNA conditions (an independent experimental validation).
To test whether the proposed incoherent feed-forward loop can quantitatively explain the measured relationships between CITED4 mRNA abundance and myocyte elongation, we developed a minimal mathematical model using ordinary differential equations (Fig. 7D). Equations and a detailed schematic of the model are provided in the Supplement (Supplementary Fig. S23). In the model, Nrg1 stimulates myocyte elongation and CITED4 expression, LIF stimulates myocyte elongation, and CITED4 expression negatively regulates myocyte elongation. These processes are modeled using saturating, Michaelis-Menten form kinetics, with CITED4 negative regulation of elongation only occuring if the myocytes have increased elongation compared to control (fold change in elongation > 1). CITED4 mRNA and elongation are also influenced by constant basal production and linear degradation terms.
We fit the model parameters to CITED4 and elongation data from control siRNA experiments using nonlinear least-squares fitting (Fig. 7D) and then independently validated the predicted the change in myocyte elongation given the experimentally determined amount of CITED4 knockdown (Fig. 7E). Note that the model predictions of elongation after CITED4 knockdown closely matched the elongation data, even though the model was not fit to this condition (Fig. 7E). We next explored whether alternative simpler models may also be able to predict the data. We calculated the sum of squared errors of prediction (SSE) to quantitatively compare the appropriateness of selecting one model over another, where a smaller SSE indicates a higher predictive ability. We found that negative regulation of elongation by CITED4 (Supplementary Fig. S24) and a minimum elongation threshold for negative regulation by CITED4 (Supplementary Fig. S25) were necessary for our model predictions of elongation to validate against our independent experimental observations with CITED4 siRNA. Indeed, model variants lacking CITED4 regulation of elongation (SSE = 0.055) or the elongation threshold (SSE = 0.029) performed worse in experimental validation tests than the default model (SSE = 0.016). Our experimental and modeling results imply that CITED4 mRNA abundance and myocyte elongation were correlated in our hypertrophic agonist screen due to coincident regulation by Nrg1. Moreover, Nrg1, CITED4, and myocyte elongation form an incoherent feedforward loop where CITED4 negatively regulates elongation.
3.7 CITED4 overexpression increases cardiac myocyte size and myocyte proliferation
Our minimal model of CITED4 and elongation required incorporating an elongation threshold above which CITED4 negatively regulated elongation (Supplementary Fig. S25). This was initially validated in our CITED4 siRNA experiments where CITED4 knockdown further enhanced elongation in Nrg1 and LIF treated myocytes, but not with control myocytes (Figure 7A–C). As a corollary, our model predicts that overexpression of CITED4 does not affect elongation of unstimulated cardiac myocytes (Figure 8). To test this hypothesis, we treated neonatal rat cardiac myocytes with adenoviral constructs expressing CITED4, or lacZ as a control. As predicted by the model, increased expression of CITED4 did not significantly affect myocyte elongation (Figure 8A–B). However, overexpression of CITED4 did increase the average cell area, fraction of myocytes expressing proliferation markers Ki67 and EdU, and myocyte number (Figure 8C–F), demonstrating activity of the construct. Therefore, CITED4 overexpression induces myocyte proliferation, as previously reported [25], and myocyte hypertrophy consistent with the phenotype of physiologic hypertrophy.
Figure 8. Overexpression of CITED4 induces myocyte proliferation and hypertrophy without affecting elongation.
Neonatal rat ventricular myocytes were treated with either CITED4-expressing adenovirus or lacZ control. Experiments were performed 24 hours after infection. Data was collected from 3 independent experiments with ~500 myocytes per condition, reported as mean +/− SE. A) Representative images of lacZ and CITED4-expressing myocytes labeled for theproliferation marker Ki67 (red), α-actinin (green), and DAPI (blue). B) Model prediction and independent experimental validation of change in myocyte elongation after CITED4 overexpression. CITED4 overexpression without an elongation stimulus such as Nrg1 or LIF did not affect myocyte elongation, as predicted by our model. C) Myocyte area, D) percentage of myocytes positive for proliferation markers Ki67 and E) EdU, F) and quantification of cardiac myocyte cell number. HPF indicates high-power field. CITED4 overexpression induced increases in myocyte area and myocyte proliferation without affecting elongation.
4. Discussion
Quantitative understanding of the distinct contributions of signaling pathways to specific changes in shape and mRNA expression is needed to better understand and ultimately control the molecular circuits governing cardiac hypertrophy. Here, we performed a screen of 15 hypertrophic agonists in neonatal rat ventricular myocytes and quantified differential regulation of 5 shape features and transcript abundance of 12 genes. While cultured myocytes do not fully reproduce the complexity of the heart, they are essential for scalable high-throughput studies, and neonatal myocytes are the best characterized cellular system for studying myocyte hypertrophy [39]. We plated myocytes on SureCoat for optimal cell health, but consideration of other substrates would be interesting in future work, particularly in studies of mechanotransduction pathways such as integrin signaling.
Previous work has established a role for these 15 agonists in hypertrophy [14], [16], but the contributions of these pathways to distinct hypertrophic features and relative dominance among the agonists to specific phenotypic outputs was less characterized. We previously compared changes in shape and sarcomere organization between four of the hypertrophic agonists, PE, Iso, IGF1, and TNFα [11]. We extended this work to measure transcript abundance in addition to shape with a more diverse panel of agonists. This allowed us to better characterize differential regulation of maladaptive and adaptive hypertrophy features and identify transcripts regulating Bax mRNA and myocyte elongation.
Previous work has demonstrated systems analysis of a single ligand screen in other cell types [40] and high-content screening of cardiac myocyte size in fixed cells after perturbation with microRNA libraries [41]. Here, we used live cell imaging of cardiac myocytes to measure fold-changes in 5 shape features. Live-cell imaging using plasmid-based transfection allows for isolation of RNA from the same myocytes we used to measure shape changes, conspicuous cell boundaries suitable for automated segmentation from monolayers of myocytes, and measurement of fold-changes in shape of individual myocytes, which has less dispersion than raw data without tracking [18].
While many commonly measured markers of hypertrophy such as cell size and fetal gene expression are not substantially differentially regulated between hypertrophy pathways [16], measuring outputs related to shape, fibrosis, cell death, inflammation, and proliferation revealed distinct phenotypic signatures among the hypertrophy pathways. Correlation coefficients among the shape features further reveal differential regulation of cell area, elongation, and form factor. Distinct regulation of elongation is of substantial interest due to the in vivo significance of concentric and eccentric hypertrophy [5]. We used our data of differential regulation of outputs to identify modules within inputs and outputs. These modules revealed shared regulation of many maladaptive features such as cell death, fibrosis, and inflammation. Shared regulation of maladaptive hypertrophy features could provide insight into the observation of distinct in vivo presentations of pathological and physiological hypertrophy [7]. Additionally, protective outputs VEGF and Bcl2 were in a module with measures of cell size and hypertrophy (fetal gene program). VEGF is important for adaptive growth of the heart [26], [42], and Bcl2 would further protect the heart during growth [43], [44]. While we examined cell size, shape and mRNA at 48 hours, additional causal relationships may be revealed by examining earlier timepoints.
Hierarchical clustering of the ligand screen data revealed correlation between mRNA abundance of pro-fibrotic CTGF and pro-cell death Bax. Correlation between these two features is interesting given association with cardiac myocyte death and dilated cardiomyopathy and heart failure [45], [46] and association of fibrosis with diastolic dysfunction and arrhythmias [47], [48]. Previous work has shown that CTGF stimulates proliferation in fibroblasts and endothelial cells [49] but apoptosis in human breast cancer cells[50] and human aortic smooth muscle cells [51], [52]. CTGF protein levels are increased in both the infarct region and viable myocardium after myocardial infarction in rats indicating involvement by CTGF in early and late stage cardiac remodeling [53]. Our follow-up experiments showed that increased CTGF was sufficient to increase Bax mRNA abundance and that knocking down CTGF decreased Ang II-dependent increases in Bax mRNA. Pathological hypertrophy is associated with increased fibrosis and cell death and shared regulation between CTGF and Bax could accelerate development of cardiac dysfunction. Therapies for hypertrophy that decrease CTGF could therefore provide some protection from cell death in addition to protection from fibrosis.
We identified an incoherent feed forward loop for Nrg-1-induced elongation where Nrg-1 increased myocyte elongation and CITED4 mRNA abundance, while CITED4 mRNA negatively regulates myocyte elongation. Further study of the dynamics of changes in myocyte elongation and CITED4 mRNA abundance will be needed to fully explore the biological significance of this type 1 incoherent feed forward loop. Previous studies of this signaling motif in other systems have shown that incoherent feed forward loops can induce pulses in signaling and accelerate signaling response time [38], [54]. Previous work has also shown Nrg1 and LIF-induced myocyte elongation [55]. Nrg1 also induced the largest increases in CITED4 mRNA, which has been previously shown to induce myocyte proliferation [25]. Therapeutic importance of Nrg1 signaling has been shown in previous mouse studies. Knockout of Erb-b4, a member of the Nrg1 signaling pathway, led to dilated cardiomyopathy and premature death in mice [56]. Moreover, administration of Nrg1 to rats with cardiac dysfunction reduced hypertrophy and increased fractional shortening and ejection fraction [57].
While Nrg1 induced myocyte elongation at 48 hours, coincident increases in CITED4 mRNA worked to attenuate elongation. Since eccentric hypertrophy has been linked to higher risk for systolic dysfunction and heart failure[9], [58], there has been great interest in learning about specific signaling pathways that regulate myocyte elongation [59], [60]. Increased CITED4 may therefore be beneficial to the heart by preventing high levels of myocyte elongation in addition to its previously studied role in exercise induced myocyte proliferation. However, it will be important in future studies to determine CITED4’s role in regulation of adaptive phases of eccentric hypertrophy versus maladaptive phases that lead to heart failure [15], [61]. Moreover, we showed that overexpression of CITED4 increased myocyte area and proliferation. While CITED4 and area were not as highly correlated as CITED4 and elongation, we did observe high CITED4 mRNA levels with ET1, PE, and NE, the agonists causing the highest increases in cell area.
Conversely, LIF increased myocyte elongation without increased CITED4 mRNA to buffer elongation, making it a potentially more deleterious inducer of elongation. CITED4 knockdown below basal levels still enhanced LIF-induced elongation, indicating basal CITED4 expression in the myocytes that protects against elongation signaling. Previous work has demonstrated the importance of ERK5 signaling in LIF-induced elongation [62]. LIF also significantly increased abundance of C/EBPβ mRNA, a gene down-regulated in exercise-induced hypertrophy [25]. Moreover, while skeletal α-actin and BNP were similarly regulated, LIF selectively increased BNP and not skeletal α-actin. High BNP levels have been associated with increased cardiovascular morbidity and mortality [63], [64]. These findings indicate LIF signaling may be especially detrimental in pathological hypertrophy.
5. Conclusions
In summary, by measuring 5 shape features and 12 mRNAs we quantified distinct phenotypic signatures for 15 major hypertrophic agonists. This result is significant given the high level of cross talk in the hypertrophy signaling network and lack of differential regulation between pathways in commonly measured hypertrophy readouts such as fetal gene expression [16]. Follow-up experiments identified positive regulation of Bax mRNA abundance by CTGF, negative regulation of myocyte elongation by CITED4, and increased cell size with CITED4 overexpression.
Supplementary Material
Highlights.
Screen of 15 receptor agonists measuring differential regulation of hypertrophy
Measured fold changes in 5 shape features and transcript levels 12 genes
Clustering revealed network map linking input modules to output modules
Follow-up experiments revealed positive regulation of Bax expression by CTGF
CITED4 knockdown enhanced Nrg1 and LIF-induced myocyte elongation
Acknowledgments
We thank Renata Polanowska-Grabow and Bryan Piras for technical assistance. This work is supported by the National Science Foundation (pre-doctoral fellowship to K.R., CAREER grant #1252854) and the National Institutes of Health Grant (#HL094476 and #HL05242).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison R, Savage D, Kannel W, Castelli W. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 4.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 5.Berlo JHV, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maillet M, Van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 10.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass GT, Ryall KA, Katikapalli A, Taylor BE, Dang ST, Acton ST, Saucerman JJ. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2011;52:923–930. doi: 10.1016/j.yjmcc.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molkentin JD. The transcription factor C/EBPbeta serves as a master regulator of physiologic cardiac hypertrophy. Circ Res. 2011;108:277–278. doi: 10.1161/RES.0b013e31820ff484. [DOI] [PubMed] [Google Scholar]
- 13.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–8314. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 15.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryall KA, Holland DO, Delaney KA, Kraeutler MJ, Parker AJ, Saucerman JJ. Network reconstruction and systems analysis of cardiac myocyte hypertrophy signaling. J Biol Chem. 2012;287:42259–42268. doi: 10.1074/jbc.M112.382937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad KM, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2010;18:43–52. doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryall KA, Saucerman JJ. Automated imaging reveals concentration dependent delay in reversibility of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2012;53:282–290. doi: 10.1016/j.yjmcc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Sachs K, Perez O, Pe’er D, Lauffenburger Da, Nolan GP. Causal protein-signaling networks derived from multiparameter single-cell data. Science. 2005;308:523–529. doi: 10.1126/science.1105809. [DOI] [PubMed] [Google Scholar]
- 21.Izu LT, Chen-Izu Y. Une cellule type? J Mol Cell Cardiol. 2012;52:921–922. doi: 10.1016/j.yjmcc.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 24.Nadal-Ginard B. Myocyte Death, Growth, and Regeneration in Cardiac Hypertrophy and Failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 25.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara K, Saito Y, Takano M, Arai Y, Yasuno S, Nakagawa Y, et al. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. EMBO J. 2003;22:6310–6321. doi: 10.1093/emboj/cdg601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, et al. Requirement of nuclear factor-kappaB in angiotensin II-and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–2325. doi: 10.1161/01.CIR.0000164237.58200.5A. [DOI] [PubMed] [Google Scholar]
- 30.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 31.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 32.Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2004;37:603–606. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baines CP, Molkentin JD. Adenine nucleotide translocase-1 induces cardiomyocyte death through upregulation of the pro-apoptotic protein Bax. J Mol Cell Cardio. 2009;46:969–977. doi: 10.1016/j.yjmcc.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho Kinase up-regulate bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 36.Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, et al. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–616. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405:407–415. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook SA, Clerk A, Sugden PH. Are transgenic mice the “alkahest” to understanding myocardial hypertrophy and failure? J Mol Cell Cardiol. 2009;46:118–129. doi: 10.1016/j.yjmcc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- 41.Jentzsch C, Leierseder S, Loyer X, Flohrschütz I, Sassi Y, Hartmann D, et al. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol. 2012;52:13–20. doi: 10.1016/j.yjmcc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Jaba IM, Zhuang ZW, Li N, Jiang Y, Martin KA, Sinusas AJ, et al. NO triggers RGS4 degradation to coordinate angiogenesis and cardiomyocyte growth. J Clin Invest. 2013;123:1718–1731. doi: 10.1172/JCI65112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–793. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- 44.Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 Protein, an Inhibitor of Apoptosis, and Bax, an Accelerator of Apoptosis, in Ventricular Myocytes of Human Hearts With Myocardial Infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 45.Dorn GW. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 47.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 48.Assayag P, Carré F, Chevalier B, Delcayre C, Mansier P, Swynghedauw B. Compensated cardiac hypertrophy: arrhythmogenicity and the new myocardial phenotype. I. Fibrosis. Cardiovasc Res. 1997;34:439–444. doi: 10.1016/s0008-6363(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 49.Rupérez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 50.Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective Tissue Growth Factor Induces Apoptosis in Human Breast Cancer Cell Line MCF-7. J Biol Chem. 1999;274:37461–37466. doi: 10.1074/jbc.274.52.37461. [DOI] [PubMed] [Google Scholar]
- 51.Hishikawa K, Nakaki T, Fujii T. Connective tissue growth factor induces apoptosis via caspase 3 in cultured human aortic smooth muscle cells. Eur J Pharmacol. 2000;392:19–22. doi: 10.1016/s0014-2999(00)00115-1. [DOI] [PubMed] [Google Scholar]
- 52.Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Fujii T, Luscher TF. Overexpression of Connective Tissue Growth Factor Gene Induces Apoptosis in Human Aortic Smooth Muscle Cells. Circulation. 1999;100:2108–2112. doi: 10.1161/01.cir.100.20.2108. [DOI] [PubMed] [Google Scholar]
- 53.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 54.Mangan S, Itzkovitz S, Zaslaver A, Alon U. The incoherent feed-forward loop accelerates the response-time of the gal system of Escherichia coli. J Mol Cell Cardiol. 2006;356:1073–1081. doi: 10.1016/j.jmb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src / FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.García-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 57.Sawyer DB, Caggiano A. Neuregulin-1β for the treatment of systolic heart failure. J Mol Cell Cardiol. 2011;51:501–505. doi: 10.1016/j.yjmcc.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–122. doi: 10.1016/j.amjcard.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sciaretta S, Sadoshima J. New insights into the molecular phenotype of eccentric hypertrophy. J Mol Cell Cardiol. 2010;49:153–156. doi: 10.1016/j.yjmcc.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakaoka Y, Shioyama W, Kunimoto S, Arita Y, Higuchi K, Yamamoto K, et al. SHP2 mediates gp130-dependent cardiomyocyte hypertrophy via negative regulation of skeletal alpha-actin gene. J Mol Cell Cardiol. 2010;49:157–164. doi: 10.1016/j.yjmcc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Langenickel T, Pagel I, Höhnel K, Dietz R, Carvajal JA, Delpiano AM, et al. Differential regulation of cardiac ANP and BNP mRNA in different stages of experimental heart failure. Am J Physiol Heart Circ Physiol. 2000;278:H1500–H1506. doi: 10.1152/ajpheart.2000.278.5.H1500. [DOI] [PubMed] [Google Scholar]
- 64.Gardner D. Natriuretic peptides: markers or modulators of cardiac hypertrophy? Trends Endocrinol Metab. 2003;14:411–416. doi: 10.1016/s1043-2760(03)00113-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.