Abstract
Dendritic cells are potent antigen presenting cells that have been shown to have significant antitumor effects in vitro and in vivo. However, the therapeutic efficacy of dendritic cells as an immunotherapeutic treatment has been limited by both immunologic tolerance and active immunosuppression in the tumor microenvironment. To address this problem, we examined the ability of concurrent systemic chemotherapy and local, fractionated radiation to augment intratumoral dendritic cell injections in a mouse model of squamous cell carcinoma. Intratumoral injections of dendritic cells alone did not have a significant antitumor effect in mice with squamous cell carcinoma flank tumors, but the addition of chemoradiation resulted in significant tumor regression. Concurrent chemoradiation alone resulted in slower tumor growth, but no complete tumor regressions. The combination of chemoradiation and intratumoral dendritic cell injections resulted in improved survival and complete tumor regression in 30% of mice. Mice with complete tumor regression were partially resistant to the repeat challenge with relevant tumor sixty days after treatment. These findings were partially dependent on the presence of CD4+ T cells, CD8+ T cells, and NK cells. Chemoradiation may augment intratumoral dendritic cell injections through increased intratumoral apoptosis as well as decreased intratumoral regulatory T cells. This work suggests a possible role for the use of intratumoral dendritic cell therapy with more traditional chemoradiation strategies.
INTRODUCTION
Squamous cell carcinoma accounts for over 97% of malignancies in the upper aerodigestive tract (UADT) and, historically, surgery followed by adjuvant radiotherapy has been the mainstay treatment for most tumors in this region. Over the last 20 years, however, chemotherapy and radiation have been increasing used as the primary treatment modalities for squamous cell carcinoma of the head and neck (SCCHN).1, 2 Despite the potential for end-organ preservation in many head and neck subsites with chemoradiation strategies as opposed to up-front surgery, most studies have not demonstrated a significant survival advantage with chemoradiation protocols. Many patients continue to succumb to loco-regional and distant recurrences despite the initial response of disease to chemoradiation.
The modulation of the immune system in patients with SCCHN is an attractive concept given the fact that these patients have profound immune defects that are associated with increased recurrence.3 These immune defects include escape from immune recognition by the tumor downregulating tumor HLA expression 4 and the direct inhibition of immune defenses by the tumor secretion of VEGF 5, PGE2 6, 7, TGF-β 8 , and IL-10 8. Patients also have reduced peripheral blood levels of CD4+ and CD8+ T cells 9, defective IFN-γ secretion 10 and impaired maturation of 11 plasmacytoid dendritic cells, and high levels of inhibitory Treg cells.12
One approach for modulating the immune system has been the use of dendritic cell vaccines. Dendritic cells are potent antigen-presenting cells with the capability of presenting tumor-associated antigens that stimulate primary and secondary T and B cell responses in vitro and in vivo.13 Dendritic cells have been used as antitumor vaccines in both preclinical and clinical studies with encouraging results.14–16 Various strategies have been used to “load” dendritic cells with tumor antigens including the use of tumor RNA17, defined tumor peptides18, and whole tumor cell lysates.19, 20 However, loading dendritic cells for large scale clinical trials or routine clinical use could prove difficult and prohibitive. An alternative strategy is to perform intratumoral injections of unpulsed dendritic cells to allow for in-vivo-in situ priming in the tumor inflammatory milieu.21, 22 Monitoring of relevant antigen-specific immune responses with this approach can be accomplished without affecting therapeutic efficacy by pulsing dendritic cells with keyhole limpet hemocyanin (KLH) prior to intratumoral administration (manuscript in preparation). This strategy has the potential to overcome both the need for in vitro loading of dendritic cells and the difficulties arising from trafficking of dendritic cells to immunologically relevant sites. Importantly, studies from our laboratory have shown that the trafficking of intratumoral-administered DCs traffick more efficiently to tumor-draining lymph nodes from radiated tumors than from untreated tumors.23 Head and neck cancers are ideal for this treatment because they are routinely accessible to direct visualization owing to their location in the UADT, thus allowing directed tumor injections.
Despite encouraging preclinical and clinical data in dendritic cell vaccines and other immunotherapy strategies, the overall therapeutic efficacy of these treatment approaches has been limited for the primary treatment of solid tumor malignancies.24 Another strategy has been the use of chemotherapy or radiation in an effort to augment the antitumor effects of various immunotherapy treatment approaches. A number of chemotherapeutic agents have been shown to have immunopotentiating effects that enhance tumor vaccines in both preclinical and clinical models.22, 25, 26 In addition, fractionated radiation has been shown to augment the therapeutic efficacy of intratumoral dendritic cell vaccination in a mouse model of melanoma and sarcoma.21 The mechanisms by which chemotherapy and radiation augment tumor immunotherapy are unclear and the process is most likely multifactorial. Several mechanisms have been suggested including the uncovering of relevant tumor antigens through necrosis or apoptosis of the tumor, altering the tumor milieu to favor antitumor immune responses, depleting immune suppressive cells, as well as lymphopenia-induced homeostatic expansion of T cells.
In light of these observations, we undertook experiments to determine whether concurrent chemoradiation could augment the therapeutic efficacy of intratumoral DC treatments in a mouse model of squamous cell carcinoma. In this study, we demonstrated that mice with palpable, squamous cell carcinoma flank tumors treated with chemoradiation and intratumoral dendritic cell injections had significantly slower tumor growth than control animals and approximately 30% of treated animals had a complete and durable response. Complete response mice were resistant to subsequent tumor challenge and treated mice demonstrated elevated interferon-γ (IFN- γ) secretion to relevant tumor. This antitumor effect was dependent on CD4+ and CD8+ T cells as well as NK cells. The mechanisms of action for chemoradiation improving intratumoral dendritic cell injections may involve both the depletion of immunosuppressive Treg cells in tumor as well as improving antigen presentation with increased tumor apoptosis.
MATERIALS AND METHODS
Mice
Female C3H/HeNCr MTV- mice were purchased from the National Cancer Institute (Frederick, MD) and housed in specific pathogen-free conditions at the Animal Maintenance Facility of the University of Michigan Medical Center. The mice used for experiments were 8 weeks of age or older. Experiments were conducted under an experimental protocol that was reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan (UCUCA # 9270).
Tumor
SCCVII is a poorly immunogenic squamous cell cancer line that spontaneously arises in C3H mice 27. The tumor cell line was maintained in vitro in complete media (CM). Complete media consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 0.1 mM nonessential amino acids, 1mM sodium pyruvate, 2 mM fresh L-glutamine, 100 µg/mL streptomycin, 100 units/mL penicillin, 50 µg/mL gentamicin, 0.5 µg/mL Fungizone (all from Life Technologies, Inc. Gaithersburg, MD), and 0.05 mM 2-mercaptoethanol (Sigma, St. Louis, MO).
Chemotherapy and Ionizing Radiation
Mice were placed in plastic restraining devices to ensure immobilization during radiation as well as to allow radiation to be delivered directly to the tumor. Local tumor irradiation was delivered in five consecutive daily fractions (Days 7–11) using the PANTAK Therapax DXT 300 Model X-Ray Unit (Cast Haven, CT). The total radiation dose administered was 15 Gy (3 Gy × 5 days). Chemotherapy was given by intraperitoneal injections on Days 7 and 8 during fractionated radiation. Cisplatin (SICOR Pharmaceuticals, Inc. Irvine, CA) was administered at a concentration of 4 mg/kg and 5-fluorouracil (American Pharmaceutical Partner, Inc. Schaumburg, IL) was administered at a concentration of 50 mg/kg.
Generation of Bone Marrow-Derived Dendritic Cells
Erythrocyte-depleted bone marrow cells from flushed marrow cavities of femurs and tibias of naïve syngeneic mice were cultured in CM supplemented with 10 ng/mL granulocyte macrophage colony stimulating factor (GM-CSF) and 10 ng/mL IL-4 (Pepro Tech, Inc. Rocky Hill, NJ) at 1 × 106 cells/mL. On day 5, dendritic cells were harvested by gentle pipetting and enriched by 14.5% metrizamide (Sigma, St. Louis, MO)-CM gradient. The low density interface was collected by gentle pipette aspiration. The dendritic cells were washed twice with PBS and resuspended at 1 × 106 cells/0.1 mL HBSS for i.t. injection. The resulting dendritic cell population was > 80% positive for co-expression of MHC II and CD86.
Treatment Protocol
C3H mice were inoculated s.c. in the mid-right flank with 2.5 × 105 SCCVII tumor cells on day 0. Radiation was performed days 7–11 while concurrent chemotherapy was given on days 7 and 8. Unpulsed DCs (106 in 50ul PBS) were administered i.t. on days 7, 14, and 21. Treatment was designed to start when tumors became palpable. Control groups of mice received either no treatment (i.t. PBS), i.t. DCs alone, or chemoradiation alone. Tumors were measured in a blinded fashion two to three times per week in the largest perpendicular diameter using vernier calipers, and the size was measured as tumor area (mm2). Data are reported as the average tumor ± SE of five or more mice/group. Survival was followed and recorded as the percentage of surviving animals over time (in days) after tumor inoculation.
Quantitation of IFN-γ
Spleen or tumor-draining lymph node (TDLN) of 24-day treated mice were harvested and a single-cell suspension created by mincing the tissue between two frosted glass microscope slides and passing through a 40um or 70um filter. Red cells were lysed using red cell lysing buffer (150 mM NH4Cl, 0.1mM EDTA, 10mM NaHCO3). Splenocytes or TDLN were cultured with or without radiated SCCVII tumor cells at a concentration of 1:5 overnight in 24 well plates. Supernatants were harvested and assayed for IFN-γ by enzyme-linked immunosorbent (ELISA) assays (BD Biosciences Pharmingen, San Diego, CA).
In vivo T-cell and NK cell depletion
Depleting ascites were prepared from hybridomas producing rat IgG2b mAb against murine CD4 (GK1.5, L3T4), CD8 (2.43, Lyt2.2), and NK cells (PK.136, NK1.1) (all from ATCC). To deplete CD4/CD8 T cells or NK cells in vivo, mice were given 0.2 ml of ascites fluid diluted to 1ml i.p. with PBS 1 week prior to treatment and this was repeated weekly to maintain depletion. Rat immunoglobulin (RIg; Sigma Chemical Co.) was inoculated in a separate group of animals as a control for the rat antimouse depleting antibodies. These depletion studies were validated by flow cytometry of splenocytes using PE-conjugated anti-CD4, anti-CD8, and anti-NK cell mAbs (all from BD Biosciences Pharmingen, San Diego, CA) that were noncompeting with the above antibodies used for in vivo depletion. Greater than 98% of the relevant cell subsets were depleted.
Detection of Intracellular IFN-γ and IL-2
Spleens were harvested from day 29 mice after various treatments. Single-cell suspensions were generated and red cells lysed with ACK. Splenocytes were cultured for 24 hours in CM alone or co-cultured 5:1 (spleen: SCCVII) with SCCVII tumor cells. After 24 hours, purified CD3 (2µg/ml) (BD Biosciences Pharmingen, San Diego, CA), CD28 (2µg/ml) (BD Biosciences Pharmingen, San Diego, CA), and Golgiplus (1 µg/ml) (BD Biosciences Pharmingen, San Diego, CA) were added for 4 hours at 37° C. The cells were washed 3 times with 1 × PBS then surface-stained with CD3-PE, CD4-PE-cy7, and CD8-Per-cp-cy5.5 antibodies (BD Biosciences Pharmingen, San Diego, CA). The cells were stored overnight in Fixation/ Permeabilization buffer (EBiosciences, San Diego, CA) at 4 ° C. The cells were then stained in Permeabilization Buffer (EBiosciences, San Diego, CA) with antibodies to IL-2-FITC and IFN-γ-FITC (BD Biosciences Pharmingen, San Diego, CA) for 30 minutes at 4 ° C in the dark. Analysis was performed with flow cytometry.
In Vitro Detection of Tumor CD95 and MHC II
SCCVII tumor were cultured in 12 well plates at a concentration ranging from 5 ×105 to 1.5 × 106 cells / well. For the chemoradiation experiment, cells were cultured for 24 hours in complete media alone, cisplatin (5µg/ml)/ 5-fluorouracil (400ng/mL), CM after treatment with a single 3Gy dose of radiation, or treated with a combination of radiation and chemotherapy with the previously described doses. Tumors were harvested and stained for MHC-II and CD95 and analyzed by flow cytometry. For the dendritic cell cytokine experiment, SCCVII tumor cells were cultured for 24 hours in media alone, IL-10 (25ng/ml), IL-6 (100ng/ml), IL1a (10ng/ml), IL-12 (12.5ng/ml), and TNF-α (100ng/ml). Dendritic cells were generated as previously described and then stimulated with LPS (100ng/ml) for 24 hours and co-cultured with SCCVII in a ratio 1:1. Wells were washed multiple times to eliminate DC prior to staining with MHC-II and CD95 and flow cytometry of SCCVII tumor cells.
Detection of Apoptotic Cells and Quantitation of Treg cells
Apoptotic cells were measured using an Apoptag® Plus Peroxidase In Situ Apoptosis Kit (Millipore, Billerica, MA) as described by the manufacturer for paraffin-embedded tissue. Apoptotic cells were counted on 10 randomly selected high-powered fields from 24-day tumors of treated mice.
For the measurement of Treg cells, single-cell suspensions of spleen, blood, tumor draining lymph node (TDLN) and tumor from mice 24 days after tumor inoculation were generated and lymphocytes were isolated using a Ficoll gradient. Lymphocytes were stained on the surface with CD4-PerCp (BD Biosciences Pharmingen, San Diego, CA) and intracellular staining was performed with FoxP3-PE (eBioscience, San Diego, CA). Results are reported as the percentage of FoxP3+ cells that are CD4+.
Statistics
For comparison of treatment groups, 1-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test was performed. All statistical analysis was performed with GraphPad Prism software (San Diego, CA). Statistical significance was defined as p < 0.05, unless otherwise stated.
RESULTS
Intratumoral dendritic cell injection augments the antitumor effects of chemoradiation
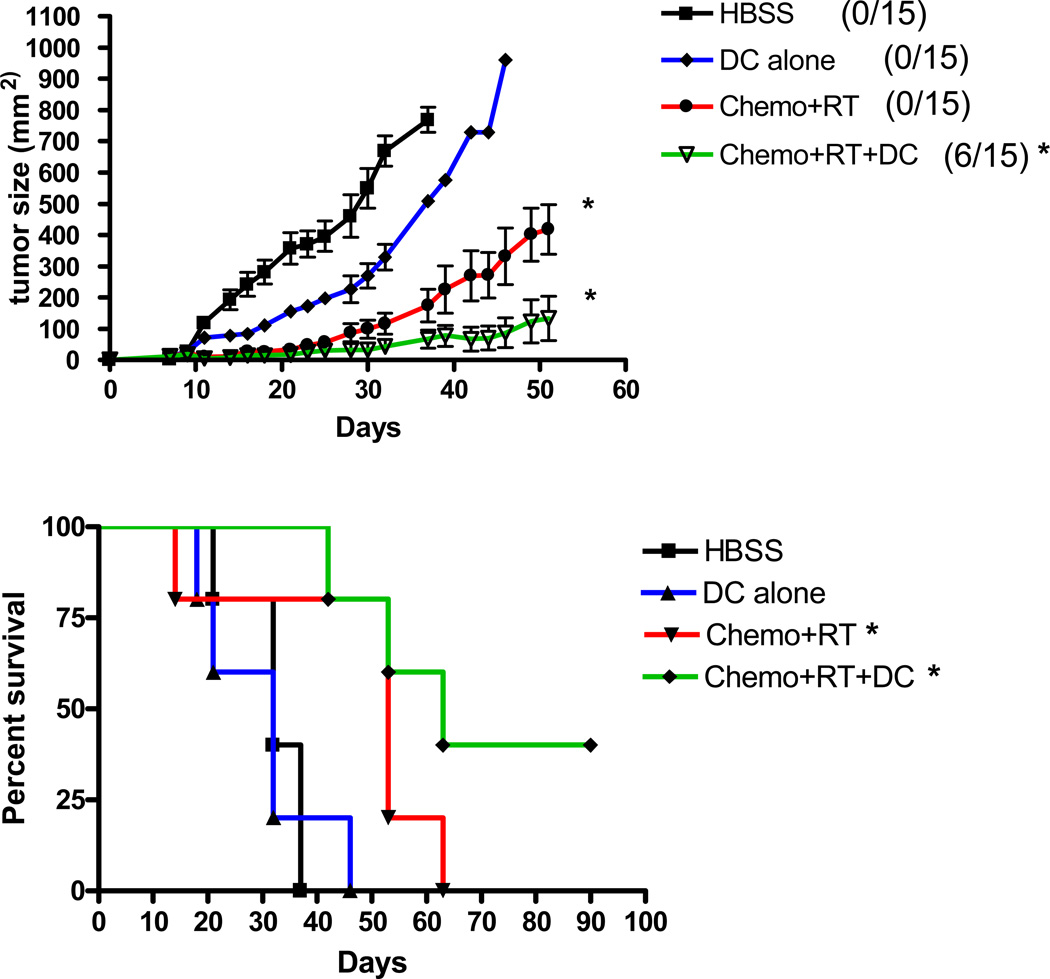
Experiments were performed to determine the effect of adjuvant intratumoral dendritic cell injections in a therapeutic model of chemoradiation. C3H/HeN mice were injected s.c. in the rear flank with 2.5 × 105 SCCVII squamous carcinoma cells and were selected for inclusion in the study when tumors were palpable at day 7 after injection. Without treatment, mice succumbed to local tumor progression between 3 and 5 weeks after tumor inoculation (Figure 1). Therapy with local fractionated radiation to the tumor was performed at various doses for 5 daily fractions (3, 5, 7, and 9 Gy/day) to determine a dose that would be similar to what would be delivered clinically and still allow sufficient tumor growth to test the vaccination strategy. In addition, cisplatin (2, 4, 6, and 8 mg/kg body weight) and 5-fluorouracil (50 and 100 mg/kg body weight) at various combined doses were tested. Cytoreduction was dose dependent and a dose of 4mg/kg cisplatin and 50 mg/kg 5-fluorouracil on 2 consecutive days resulted in a 50% reduction in CD45+ splenocytes and a 20% reduction in CD45+/CD3+ splenocytes 5 days after therapy (data not shown). Cell counts returned to 95% of normal by 14 days after initiation of treatment. Based on these studies, the therapeutic dosing of chemoradiation used for the experiment was fractionated radiation to the tumor flank at 3 Gy/day for 5 days (15 Gy total) and 2 consecutive days of 4mg/kg cisplatin and 50 mg/kg 5-fluorouracil. Similar to the human clinical experience of concurrent chemoradiation on locally advanced squamous cell carcinoma of the head and neck, this concurrent chemoradiation schedule resulted in a significant slowing of tumor growth and improvement in survival, but no complete and durable tumor regression was seen in our model (Figure 1). However, the dosing of chemotherapy and radiation was not designed to be curative.
Figure 1. Intratumoral dendritic cell vaccination augments the anti-tumor effects of chemoradiation.
Mice bearing 7-day subcutaneous SCCVII tumors (2.5 × 105 cells) were treated with 2 days of systemic cisplatin (4 mg/kg) and 5-fluorouracil (50 mg/kg) and 5 days of fractionated radiation to the flank tumor site (3 Gy/day; 15 Gy total). Mice were given three weekly intratumoral injections of dendritic cells (1 × 106/injection) beginning on the first day of chemoradiation. The number of long-term surviving mice with complete tumor regression are indicated. (*) p < 0.05 compared to HBSS.
Three weekly intratumoral injections of dendritic cells alone (1 × 106/injection) had no significant antitumor effects on 7-day SCCVII flank tumors (Figure 1). This indicated that the presence of a high number of naïve dendritic cells at the tumor site was insufficient to induce antitumor immunity and tumor regression in this poorly immunogenic tumor. However, the injection of intratumoral dendritic cells along with concurrent chemoradiation resulted in the complete, long-term regression in approximately one-third of treated mice and significantly slowed tumor growth and improved survival when compared to untreated animals (Figure 1).
Local intratumoral dendritic cell injection after chemoradiation induces systemic antitumor immunity
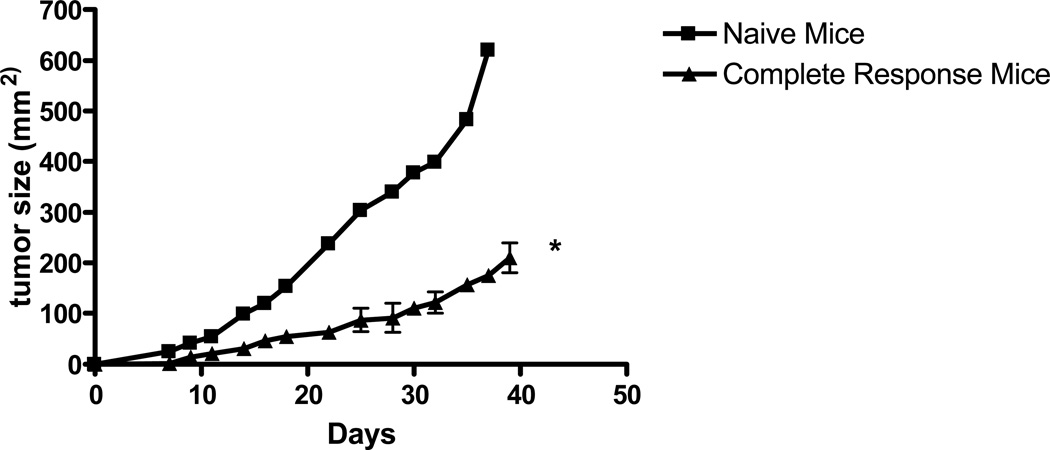
The observation that untreated tumor-bearing mice succumb to local tumor progression in 3 to 5 weeks and the combination of chemoradiation and dendritic cells results in long-term surviving mice (>3 months) suggests that intratumoral dendritic cell vaccination may have a systemic antitumor effect. To test this observation, mice with flank tumors that were found to have a complete response after chemoradiaton and dendritic cell vaccination were re-challenged with 2.5 × 105 SCCVII squamous carcinoma cells in the contralateral flank with age-matched naïve mice used as controls. Chemoradiation and dendritic cell-treated mice had significantly slower tumor growth compared to naïve mice despite the absence of any current treatment, suggesting an immune-mediated memory response (Figure 2).
Figure 2. Mice with a complete tumor regression after chemoradiation and dendritic cell injection are resistant to subsequent tumor challenge.
Mice with complete tumor regression after chemoradiation and dendritic cell injection were rechallenged in the contralateral flank with SCCVII tumor (2.5 × 105 cells) 60 days after the initial treatment. The results are representative of 5 mice in the complete response treatment arm. (*) p < 0.05 compared to naïve mice.
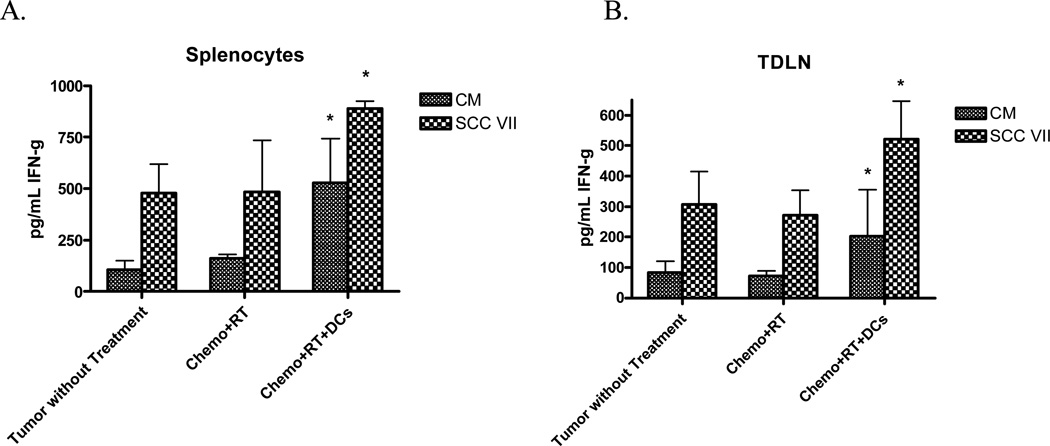
To further assess the systemic immune response, IFN-γ secretion was measured from splenocytes and lymphocytes from the tumor-draining lymph node (TDLN) of chemoradiation and dendritic cell-treated mice that were co-cultured with SCCVII tumor cells (Figure 3). Splenocytes and TDLN from treated mice had significantly higher basal secretion of IFN-γ than untreated mice. When co-cultured with relevant SCCVII tumor, IFN-γ secretion increased significantly when compared to unstimulated cells. These findings support the notion that the local administration of dendritic cells along with chemoradiation has a systemic antitumor effect.
Figure 3. Chemoradiation and intratumoral DC vaccination results in elevated IFN-γ secretion.
Chemoradiation and DC vaccination results in an elevated baseline and stimulated IFN-γ level in both splenocytes and tumor draining lymph node. ELISA was performed to evaluate IFN-γ and reported as the mean ± SEM of triplicate samples. 3 animals were individually analyzed for each treatment group. (*) p < 0.05 compared to tumor without treatment
CD4+, CD8+, and NK cells are involved in tumor regression
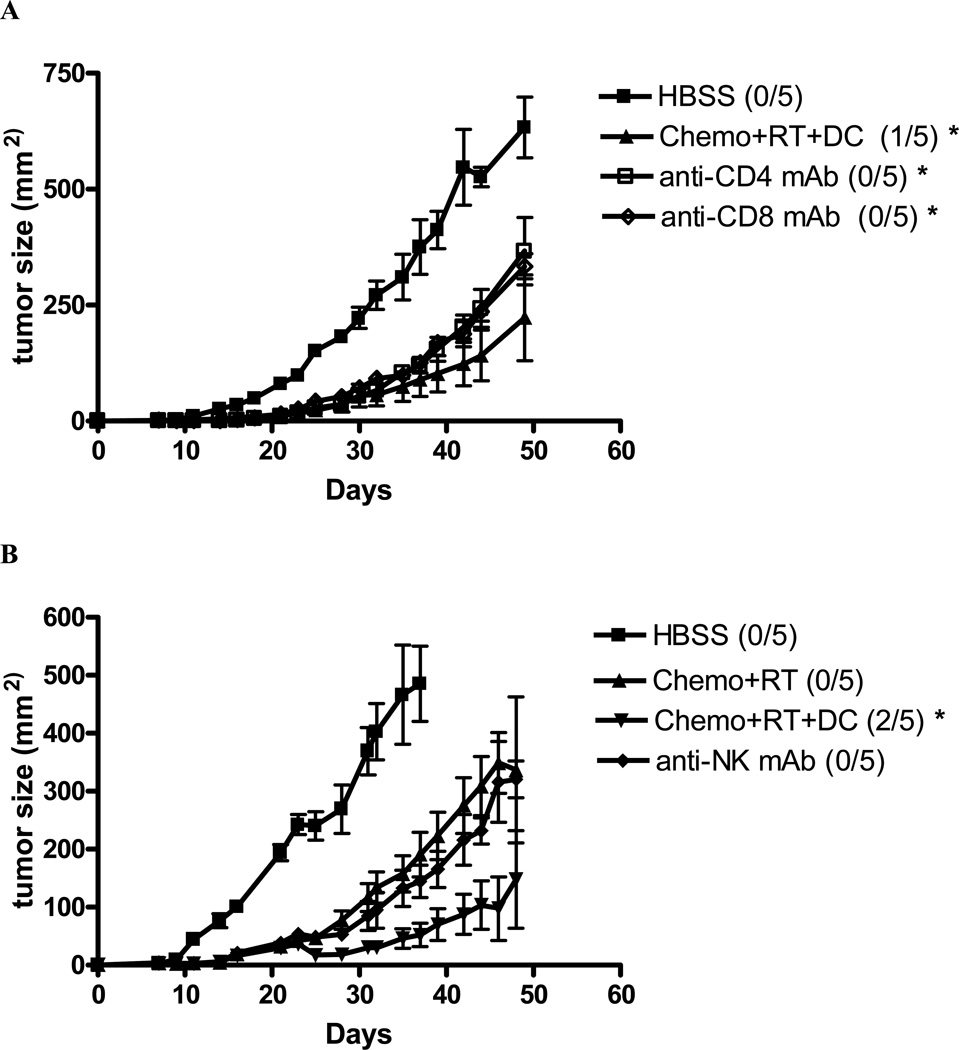
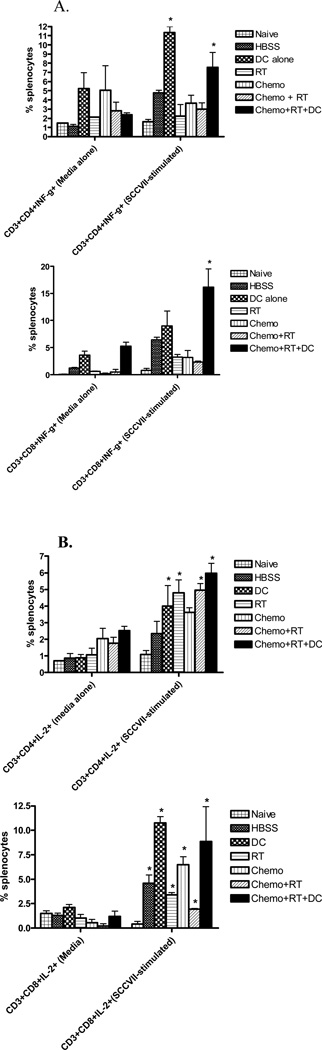
To determine the role of CD4+ T cells, CD8+ T cells, and NK cells in the antitumor response of concurrent chemoradiation and intratumoral dendritic cell treatment, mice were selectively depleted of these cells by monoclonal antibodies. Antibodies were given 1 week post-tumor injection at the beginning of chemoradiation and dendritic cell injection and was repeated weekly to maintain T cell and NK cell depletion. Both T cell and NK cell depletion was greater than 98% at 24 hours after injection and persisted for greater than 1 week (data not shown). As shown in Figure 4a and 4b, both CD4+ and CD8+ T cells as well as NK cells are involved in the tumor regression seen with concurrent chemoradiation and dendritic cell injections. These findings suggest that both innate and adaptive immune responses are important in tumor rejection after treatment with concurrent chemoradiation and dendritic cell injections. It is likely, based on these data, that both CD4+ and CD8+ T cells are equally important for the maintenance of adaptive immunity. In support of the observation that CD4+ and CD8+ T cells are important in this animal model, intracellular staining of Th1 cytokines IFN-γ and IL-2 in both CD4+ and CD8+ T cells are increased in the splenocytes of mice treated with chemoradiation and intratumoral dendritic cells after the in vitro stimulation with relevant tumor (Figure 5a and b).
Figure 4. CD4+ and CD8+ Tcells as well as NK cells are involved in the antitumor response of concurrent chemoradiation and intratumoral dendritic cell injections.
Mice treated with chemoradiation and intratumoral dendritic cell injections were given depleting ascites from hybridomas producing monoclonal Abs against murine CD4, CD8, and NK cells. The numbers of long-term surviving mice with complete tumor regression in each group are indicated. (*) p < 0.05 compared to HBSS.
Figure 5. Chemoradiation and intratumoral dendritic cell administration increase Th1 cytokines IFN-γ and IL-2 in both CD4+ and CD8+ T cells after stimulation with SCCVII tumor.
Spleens from naïve mice and mice with 29-day tumor after completion of the various treatment regimens were harvested. Splenocytes were cultured for 24 hours with either media alone or with SCCVII tumor cells (5:1). Intracellular cytokine staining for IFN-γ (Figure 5A) and IL-2 (Figure 5B) and analysis by flow cytometry were performed. (*) p < 0.05 compared to media-cultured (control) cells.
Chemoradiation results in intratumoral apoptosis and Treg cell depletion
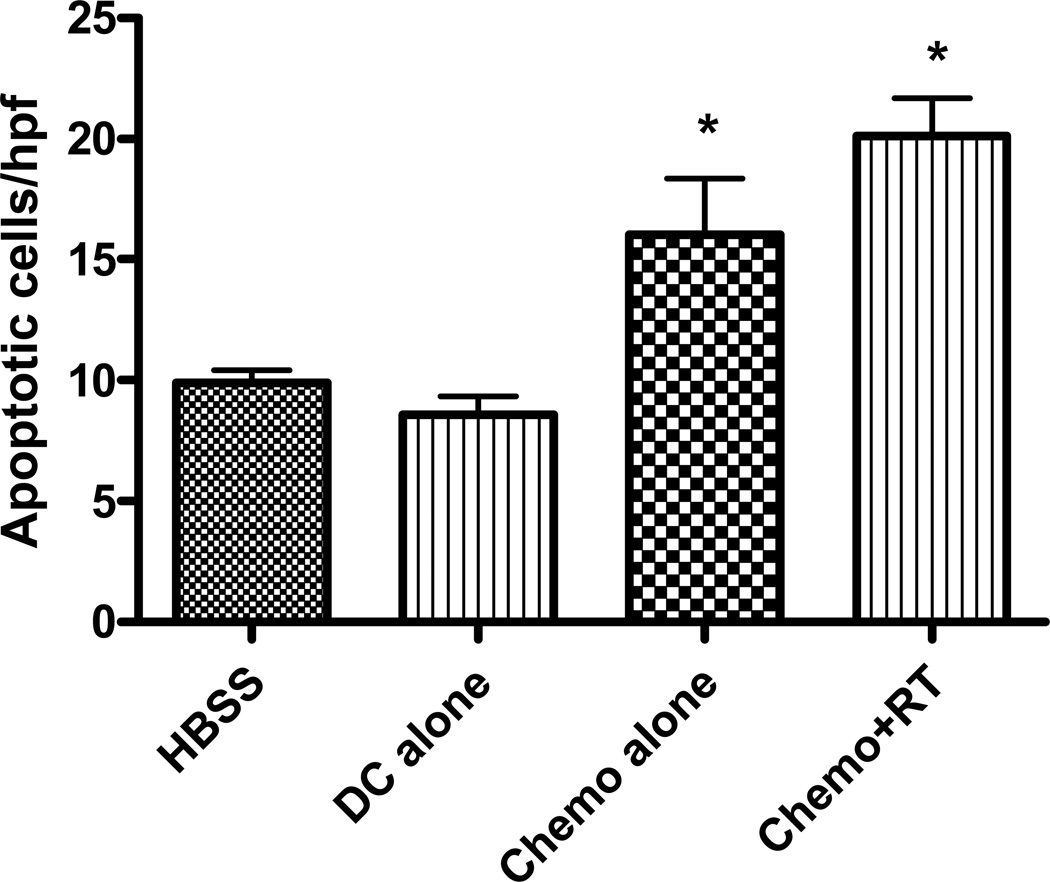
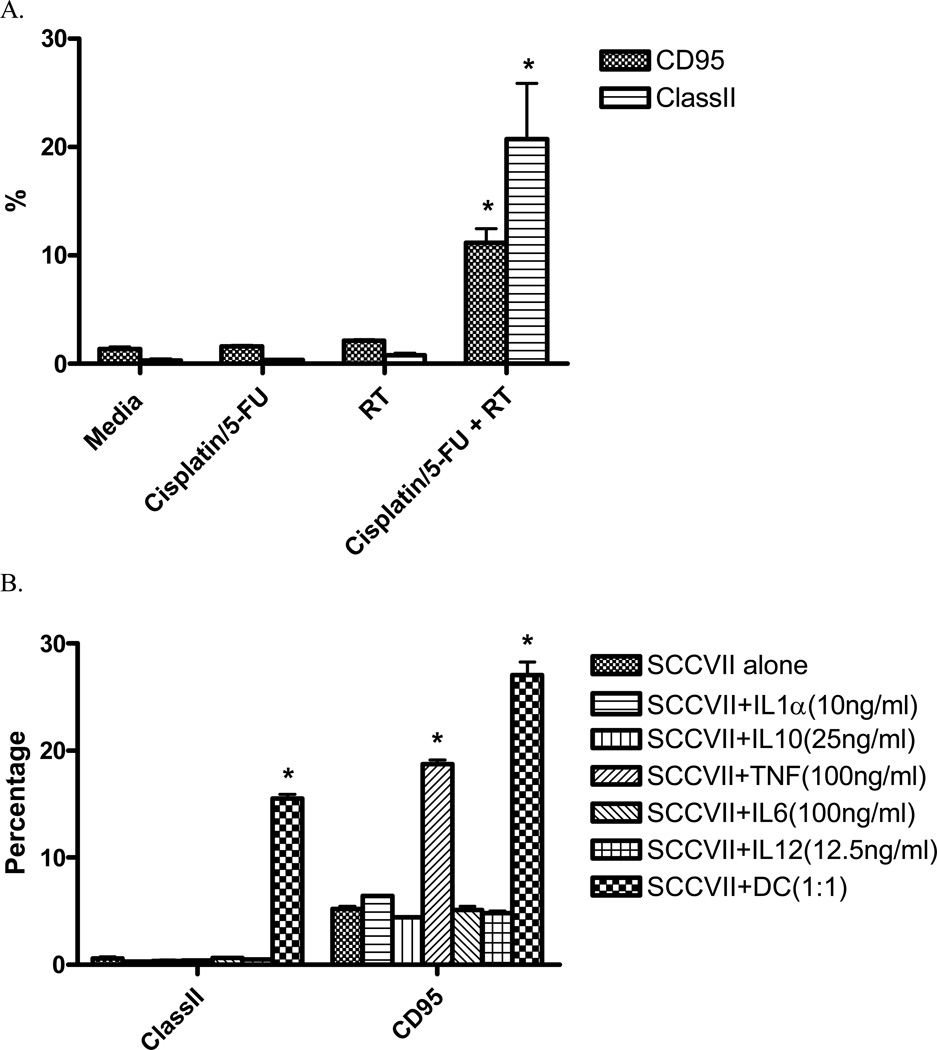
Dendritic cells have the ability to uptake and process apoptotic cells to generate an effective immune response 28 and have been shown to inhibit tumor growth when given i.t. to tumors with high levels of baseline apoptosis 29. To determine whether or not chemoradiation results in increased apoptosis, in situ TUNEL staining was performed on tumors at the completion of concurrent chemoradiation. As can be seen in Figure 6, chemotherapy and radiation results in a roughly 100% increase in apoptotic cells within the tumor microenvironment. Cisplatin and 5-Flourouracil alone also results in a significant increase in apoptosis relative to untreated tumors. The increase in apoptosis seen in this model may be related to the increase in CD95 (Fas) expression seen on the cell surface when SCCVII cells are treated with combined chemoradiation (Figure 7a). In addition, the presence of LPS-activated DC and TNF-α ( but not other DC cytokines) also increases CD95 expression on the surface of tumor cells (Figure 7b), suggesting another mechanism for increased apoptosis in this model. Interestingly, chemoradiation as well as LPS-activated DC also increase the expression of MHCII on the surface of SCCVII tumor cells, perhaps augmenting the antitumor immune response when chemoradiation is combined with intratumoral dendritic cells.
Figure 6. Chemoradiation results in increased intratumoral apoptosis.
Intratumoral apoptosis was measured by Apoptag assays. Ten randomly selected high powered fields were analyzed and the number of positively stained tumor cells were tabulated on 24 day tumors. Two mice per group were analyzed. (*) p < 0.05 compared to HBSS-treated mice.
Figure 7. Chemoradiation and LPS-stimulated dendritic cells seperately increase Class II and CD95 (Fas) expression on the surface of SCCVII tumor cells.
A. SCCVII tumor cells were cultured for 24 hours after treatment with a single 3Gy fraction of radiation and/or cisplatin (5µg/mL)/5-Flurouracil (400ng/mL). Tumor cells were stained for the presence of MHC class II and CD95 and analyzed by flow cytometry. (*) p < 0.05 when compared to media-cultured tumor cells B. SCCVII tumor cells were cultured fro 24 hours with a subset of cytokines known to be secreted by dendritic cells as well as LPS-stimulated DC. Tumor cells were stained for the presence of MHC class II and CD95 and analyzed by flow cytometry. (*) p < 0.05 when compared to SCCVII cultured alone.
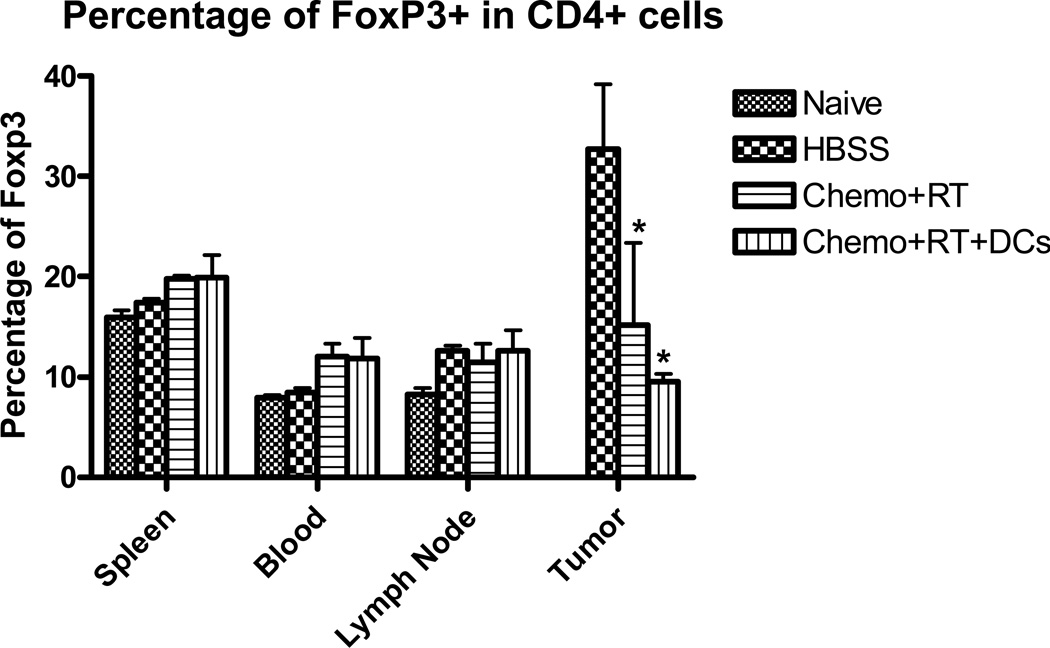
Regulatory T (Treg) cells inhibit T cell activity through soluble inhibitory mediators such as TGF-β and IL-10 30, 31 and exert suppressive activity upon CD8+ effector and CD4+ helper T cells. Recent evidence has demonstrated that T reg cell-mediated immunosuppression is one of the main obstacles to successful tumor immunotherapy32. To test the hypothesis that chemoradiation results in a decrease in tumor Treg cells, tumor was harvested and analyzed by flow cytometry for the presence of CD4+FoxP3+ cells. In addition, to examine the contributions of various biologic compartments to Treg levels in vivo, single cell suspensions from spleen, blood and tumor draining lymph nodes were also analyzed by flow cytometry. Figure 4 demonstrates an approximately 50% decrease in the presence of intratumoral Treg cells with chemoradiation. There were no significant differences in Treg cell numbers in the other compartments that were analyzed, but there was a trend towards fewer Treg cells in the blood of untreated tumor-bearing mice.
DISCUSSION
Chemotherapy or radiation alone has been shown in several studies to significantly improve the efficacy of intratumoral dendritic cell vaccination strategies.21, 22, 33–37 In contrast, the role of concurrent chemoradiation in augmenting intratumoral dendritic cell vaccinations is less clear. In light of the increasing reliance on concurrent chemoradiotherapy for the first-line treatment of SCCHN, we undertook studies to investigate the benefit of adjuvant intratumoral dendritic cell vaccination during concurrent chemotherapy and local tumor irradiation in a murine model of squamous cell carcinoma. Our studies demonstrated that the combination of concurrent chemoradiation with the intratumoral injection of naïve dendritic cells resulted in a reduction in tumor burden that was associated with complete tumor regression in 30% of mice that was partially dependent on CD4+ and CD8+ T cells, as well as NK cells. Mice with complete tumor regression after treatment were resistant to a subsequent tumor challenge, suggesting systemic immunity. The ability of concurrent chemoradiation to increase the tumor cell surface expression of CD95 (Fas) and MHCII and increase intratumoral apoptosis may partially explain the synergistic effects with intratumoral dendritic cell administration. Upregulation of MHCII may also improve immunogenicity in this model. In addition, concurrent chemoradiation was shown to decrease intratumoral Treg cells which may augment intratumoral DC therapy by decreasing immunosuppression in the tumor microenvironment.
Patients with SCCHN have profound deficiencies in both the innate and adaptive immune responses that are believed to play a major role in the continued poor survival rates in this population despite significant advances in surgery, chemotherapy, and radiotherapy.3 Patients with SCCHN present with reduced CD3+, CD4+, and CD8+ T cell counts and patients with active disease have much lower levels of CD4+ T cells that persist long after curative therapy. 9, 38 Along with lower absolute numbers of T cells, T-cell priming in the tumor microenvironment is dysfunctional.39 SCCHN, along with other solid tumor malignancies, have the capacity to skew T cell immune responses towards the Th2 phenotype.40–43 The Th2 phenotype favors immune tolerance through the elevation of plasma levels of IL-4, IL-6, and IL-10 in SCCHN.43, 44 Importantly, there is also an imbalance of antigen-presenting cell subsets in the tumor microenvironment that favor immune tolerance. Plasmacytoid dendritic cells (DCs) from SCCHN tumors have impairments in maturation 11 and are defective in their ability to generate IFN-α 10, a cytokine important in NK cell activity and antitumor reactivity.45 In ovarian tumors, a large number of plasmacytoid DCs, but not functional mature myeloid DCs, accumulate in the tumor microenvironment 46 and are able to induce CD4+ suppressive Treg cells.47 Similarly, elevated levels of Treg cells have been demonstrated in patients with SCCHN and this finding may be contribute to tumor tolerance.12 It is these immune defects, among others, that allow tumors to actively escape innate and adaptive tumor immunity as well as standard immunotherapeutic approaches.
Chemotherapy and radiation have been shown to have the capacity to change the tumor microenvironment to create a background that is more suitable for tumor rejection both by the native immune system as well as through immunotherapeutic treatment approaches.48 The apoptosis-inducing death receptor CD95 (Apo-1/Fas) is thought to play a key role in apoptosis and is upregulated with chemotherapeutic drugs 49–51 and radiation. 52 In our studies, the use of chemoradiation was also found to upregulate CD95 and may be one mechanisms by which apoptosis is increased in this model. Recent work53, 54 has shown that another mechanism for the immunoadjuvant effects of radiotherapy and chemotherapy is the toll-like receptor (TLR) 4-dependent antigen processing on dendritic cells that is dependent upon calreticulin exposure (the “eat me signal”) and HMGB1 release (the “danger signal”) by dying tumor cells. This specific mechanism of apoptosis, however, does not seem to be the primary mechanism in this model since tumor growth in mice lacking a functional TLR-4 treated with chemoradiation did not significantly differ from mice with a functional TLR-4 (data not shown). In our study, the use of intratumoral dendritic cells alone without concurrent chemoradiation did not have a significant antitumor effect. A significant antitumor effect, however, was only seen with chemoradiation alone or chemoradiation with intratumoral dendritic cell vaccination. MHCII was also upregulated in tumor cells treated with chemoradiation. MHC antigen down-regulation is a presumed tumor growth promoting mechanism and the effects of chemoradiation may improve immunogenicity in this model. Interestingly, LPS-stimulated DC also increased the CD95 and MHCII on tumor cells. It is plausible that both chemoradiation and intratumoral DC administration may augment a pro-apoptotic environment as well as increased immunogenicity resulting in increased therapeutic efficacy.
In addition to the effects on the in situ immune system, chemotherapy and radiation have been shown to augment ex vivo adoptive immunotherapy and dendritic cell vaccinations strategies. One of the key observations has been the finding that both chemotherapy and total body irradiation (TBI) enhance immunotherapy through the depletion of Treg cells. In our study, we found a significant decrease in the intratumoral Treg cells of mice treated with chemotherapy and fractionated radiation to the tumor. The effects of chemoradiation on T cell counts in the spleen were only modest with a 10–20% decrease in CD3+ cells 5 days after chemoradiation and a return to normal levels by 14 days after treatment (data not shown). Levels of Treg cells in the spleen, blood, and lymph node of treated animals did not differ significantly with controls. This finding would suggest, though does not prove, that the elimination of Treg cells in the tumor may be do to the local effects of chemoradiation in the tumor microenvironment. This is contrast to the lymphodepletion seen with TBI or lymphodepleting regimens of chemotherapy where there is a systemic depletion of the T cell pool. However, the lack of differences seen outside the tumor microenvironment may be due to trafficking to compartments that were not studied.
In our mouse model of squamous cell carcinoma, CD8+ and CD4+ T cells as well as NK cells appear to be important for the antitumor effects of chemoradiation and intratumoral dendritic cell injections. No animals treated with monoclonal antibodies against CD4+ T cells, CD8+ T cells, or NK cells were cured with chemoradiation and dendritic cell injections and the growth of tumor was accelerated in these animals. In addition, IFN-γ and IL-2 secretion were found to be elevated in both CD8+ and CD4+ T cells of treated mice when challenged with relevant tumor. CD8+ effector T cells have a central role in the elimination of tumor by recognizing tumor antigens in the context of major histocompatibility complex class I molecules on antigen presenting cells.29, 55 The observation that IFN-γ secretion was elevated from splenocytes and lymphocytes from tumor draining lymph nodes of treated mice challenged with relevant tumor supports the importance of CD8+ effector T cells. However, CD4+ T cells also play an important role in antitumor immunity by helping to initiate and maintain the antitumor immune response.56, 57 The finding that mice with a complete response after chemoradiation and dendritic cell treatment were resistant to a late tumor challenge is consistent with the relative importance of CD4+ T cells in this model.
From a quantitative standpoint, NK cells in our model may play a more significant role in the antitumor response as demonstrated in Fig 4c. It is known that activated NK cells stimulate the maturation of dendritic cells and can facilitate innate and adaptive immunity.58 Human NK cells express some members of the TLR family and have the ability to produce IFN-γ and TNF as well as acquire cytolytic activity against immature DCs.58, 59 DC-NK cell interactions are highly dependent on the stimuli received, the site where the interactions occur, and cytokine microenvironment. Our data would suggest that chemoradiation and intratumoral dendritic cell injections have a significant effect on NK cell activity that is eliminated after the addition of anti-NK monoclonal antibody. It is possible that the danger signals elicited by chemoradiation may activate NK cells in a manner similar to dendritic cells.
In addition to the immunologic effects of chemoradiation and intratumoral dendritic cell injections, chemoradiation also has a direct cytoreductive effect that can be potentially curative when treatments are given in sufficient doses. However, significant toxicity can be associated with curative doses of chemoradiation and high rates of complete, durable responses remain elusive.1 The doses of chemotherapy and radiation given in this tumor model were not curative, but the addition of intratumoral dendritic cell injections resulted in significantly slower tumor growth as well as complete tumor regression in 30% of mice. Our findings suggest the possibility that intratumoral dendritic cell injections could be used with standard chemotherapeutic regimens to improve cure rates as well as decrease the likelihood of local, regional, and distant recurrences given the potential for systemic antitumor immunity. Alternatively, the use of intratumoral dendritic cell injections may allow the use of lower doses of chemotherapy and radiation for less advanced tumors, thus decreasing the side effects of aggressive chemoradiation.
In this study, we presented data that indicate chemoradiation and intratumoral dendritic cell injections have significant antitumor effects that are associated with increased tumor apoptosis and decreased intratumoral Treg cells. These findings are at least partially due to the complex interplay between CD4+ T cells, CD8+ T cells, and NK cells in this tumor model. This treatment strategy may serve as a useful adjuvant to current chemotherapeutic regimens in patients with squamous cell carcinoma of the head and neck that are immunosuppressed and relatively refractory to immunotherapeutic interventions.
Figure 8. Intratumoral Treg cells are decreased in chemoradiation-treated mice.
Single-cell suspensions of spleen, blood , tumor draining lymph node (TDLN) and tumor from mice 24 days after tumor inoculation were generated and lymphocytes were isolated using a Ficoll gradient. Lymphocytes were stained with CD4 and FoxP3 and FACS results are reported as a percentage of FoxP3+ cells that are CD4+. These results are representative of at least 3 separate experiments. (*) p < 0.05 compared to HBSS-treated mice.
REFERENCES
- 1.Moyer JS, Wolf GT, Bradford CR. Current thoughts on the role of chemotherapy and radiation in advanced head and neck cancer. Current Opinion in Otolaryngology & Head & Neck Surgery. 2004;12:82–87. doi: 10.1097/00020840-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Young MRI. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head & Neck. 2006;28:462–470. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
- 4.Grandis JR, Falkner DM, Melhem MF, et al. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clinical Cancer Research. 2000;6:2794–2802. [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells.[erratum appears in Nat Med 1996 Nov;2(11):1267] Nature Medicine. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 6.Benefield J, Petruzzelli GJ, Fowler S, et al. Regulation of the steps of angiogenesis by human head and neck squamous cell carcinomas. Invasion & Metastasis. 1996;16:291–301. [PubMed] [Google Scholar]
- 7.Schroeder CP, Yang P, Newman RA, Lotan R. Eicosanoid metabolism in squamous cell carcinoma cell lines derived from primary and metastatic head and neck cancer and its modulation by celecoxib.[see comment] Cancer Biology & Therapy. 2004;3:847–852. doi: 10.4161/cbt.3.9.1037. [DOI] [PubMed] [Google Scholar]
- 8.Qin H, Valentino J, Manna S, et al. Gene therapy for head and neck cancer using vaccinia virus expressing IL-2 in a murine model, with evidence of immune suppression. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2001;4:551–558. doi: 10.1006/mthe.2001.0493. [DOI] [PubMed] [Google Scholar]
- 9.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Imbalance in absolute counts of T lymphocyte subsets in patients with head and neck cancer and its relation to disease. Advances in Oto-Rhino-Laryngology. 2005;62:161–172. doi: 10.1159/000082506. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann E, Wollenberg B, Rothenfusser S, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Research. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 11.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 12.Schaefer C, Kim GG, Albers A, et al. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer.[see comment] British Journal of Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Geiger J, Hutchinson R, Hohenkirk L, et al. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet. 2000;356:1163–1165. doi: 10.1016/S0140-6736(00)02762-8. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annual Review of Medicine. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 16.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annual Review of Immunology. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 17.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. Journal of Experimental Medicine. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature Medicine. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 19.Moyer JS, Maine G, Mule JJ. Early vaccination with tumor-lysate-pulsed dendritic cells after allogeneic bone marrow transplantation has antitumor effects. Biology of Blood & Marrow Transplantation. 2006;12:1010–1019. doi: 10.1016/j.bbmt.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Research. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 22.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Research. 2005;65:5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 23.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. Journal of Immunotherapy. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines.[see comment] Nature Medicine. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terando A, Mule JJ. On combining antineoplastic drugs with tumor vaccines. Cancer Immunology, Immunotherapy. 2003;52:680–685. doi: 10.1007/s00262-003-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khurana D, Martin EA, Kasperbauer JL, et al. Characterization of a spontaneously arising murine squamous cell carcinoma (SCC VII) as a prerequisite for head and neck cancer immunotherapy. Head & Neck. 2001;23:899–906. doi: 10.1002/hed.1130. [DOI] [PubMed] [Google Scholar]
- 28.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 29.Candido KA, Shimizu K, McLaughlin JC, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Research. 2001;61:228–236. [PubMed] [Google Scholar]
- 30.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. Journal of Immunology. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 31.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. Journal of Immunology. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 32.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 33.Ehtesham M, Kabos P, Gutierrez MAR, et al. Intratumoral dendritic cell vaccination elicits potent tumoricidal immunity against malignant glioma in rats. Journal of Immunotherapy. 2003;26:107–116. doi: 10.1097/00002371-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Research. 2001;61:7530–7535. [PubMed] [Google Scholar]
- 35.Tanaka F, Yamaguchi H, Ohta M, et al. Intratumoral injection of dendritic cells after treatment of anticancer drugs induces tumor-specific antitumor effect in vivo. International Journal of Cancer. 2002;101:265–269. doi: 10.1002/ijc.10597. [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Kusmartsev S, Cheng F, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clinical Cancer Research. 2003;9:285–294. [PubMed] [Google Scholar]
- 37.Shin JY, Lee SK, Kang CD, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histology & Histopathology. 2003;18:435–447. doi: 10.14670/HH-18.435. [DOI] [PubMed] [Google Scholar]
- 38.Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngology - Head & Neck Surgery. 1986;95:142–152. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 39.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Reviews Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 40.Pockaj BA, Basu GD, Pathangey LB, et al. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Annals of Surgical Oncology. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Smyth GP, Stapleton PP, Barden CB, et al. Renal cell carcinoma induces prostaglandin E2 and T-helper type 2 cytokine production in peripheral blood mononuclear cells. Annals of Surgical Oncology. 2003;10:455–462. doi: 10.1245/aso.2003.06.036. [DOI] [PubMed] [Google Scholar]
- 42.Reome JB, Hylind JC, Dutton RW, Dobrzanski MJ. Type 1 and type 2 tumor infiltrating effector cell subpopulations in progressive breast cancer. Clinical Immunology. 2004;111:69–81. doi: 10.1016/j.clim.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Lathers DMR, Achille NJ, Young MRI. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Human Immunology. 2003;64:1160–1166. doi: 10.1016/j.humimm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Lathers DMR, Young MRI. Increased aberrance of cytokine expression in plasma of patients with more advanced squamous cell carcinoma of the head and neck. Cytokine. 2004;25:220–228. doi: 10.1016/j.cyto.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Edwards BS, Merritt JA, Fuhlbrigge RC, Borden EC. Low doses of interferon alpha result in more effective clinical natural killer cell activation. Journal of Clinical Investigation. 1985;75:1908–1913. doi: 10.1172/JCI111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou W, Machelon V, Coulomb-L'Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells.[see comment] Nature Medicine. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 47.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. Journal of Immunology. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 48.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like Receptors in Tumor Immunotherapy. Clinical Cancer Research. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friesen C, Fulda S, Debatin KM. Cytotoxic drugs and the CD95 pathway. Leukemia. 1999;13:1854–1858. doi: 10.1038/sj.leu.2401333. [DOI] [PubMed] [Google Scholar]
- 50.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Medicine. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 51.Fulda S, Los M, Friesen C, Debatin KM. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. International Journal of Cancer. 1998;76:105–114. doi: 10.1002/(sici)1097-0215(19980330)76:1<105::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 52.Sheard MA. Ionizing radiation as a response-enhancing agent for CD95-mediated apoptosis. International Journal of Cancer. 2001;96:213–220. doi: 10.1002/ijc.1020. [DOI] [PubMed] [Google Scholar]
- 53.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 54.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death.[see comment] Nature Medicine. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 55.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annual Review of Immunology. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 56.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses.[comment] Journal of Experimental Medicine. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. Journal of Experimental Medicine. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nature Reviews Immunology. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 59.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]