Abstract
The role of the plasminogen activation system (PAS) was investigated during the course of infection of a relapsing fever Borrelia species in plasminogen-deficient (plg –/–) and control (plg +/+ and plg +/–) mice. Subcutaneous inoculation of 104 spirochetes resulted in a peak spirochetemia five days after infection with 20–23 × 106 organisms per milliliter of whole blood in all mice, indicating that the PAS had no effect on the development of this phase of the infection. Anemia, thrombocytopenia, hepatitis, carditis, and splenomegaly were noted in all mice during and immediately after peak spirochetemia. Fibrin deposition in organs was noted in plg –/– mice but not in controls during these stages. Significantly greater spirochetal DNA burdens were consistently observed in the hearts and brains of control mice 28–30 days after infection, as determined by PCR amplification of this organism's flagellin gene (flaB), followed by quantitative densitometry. Furthermore, the decreased spirochetal load in brains of plg –/– mice was associated with a significant decrease in the degree of inflammation of the leptomeninges in these mice. These findings indicate a role for the PAS in heart and brain invasion by relapsing fever Borrelia, resulting in organ injury.
Introduction
Tick-borne relapsing fever Borrelia and Borrelia burgdorferi are two worldwide groups of spirochetes transmitted by soft ticks (family Argasidae) and hard ticks (family Ixodidae), respectively. Relapsing fever Borrelia, including old-world and new-world groups, cause an illness characterized by periods of fever and spirochetemia, separated by afebrile intervals without evidence of organisms in the blood. Subsequent organ invasion can lead to cardiac, neurologic, and other manifestations (1). Lyme disease is a chronic illness caused by several genospecies within the Borrelia burgdorferi sensu lato group, but is not as rapidly evolving as relapsing fever. The clinical manifestations of Lyme disease include cutaneous, neurologic, cardiac, and arthritic disorders that develop as B. burgdorferi disseminates through the skin and vasculature to invade secondary organ sites. Lyme disease lacks the marked and recurrent spirochetemia of relapsing fever, but the organ-specific clinical manifestations can be similar, particularly those involving the nervous system (2, 3). Lymphocytic meningitis and facial nerve palsy are neurologic manifestations shared by a similar percentage of patients in both diseases (2, 3). After initial infection, relapsing fever Borrelia reach high levels in the blood (first peak), followed by subsequent smaller peaks. As the host responds with specific antibodies, the spirochetemia drops precipitously. Antigenic variation is responsible for the spirochetemic episodes of B. hermsii, with multiphasic antigenic diversity achieved through somatic mutations in rearranged variable major protein genes (reviewed in 4; 5–7). Recently, a similar, but not identical, antigenic variation strategy has been reported for B. burgdorferi (8).
Plasmin, a broad spectrum serine protease, is responsible for fibrin degradation during thrombolysis (9–11). Other roles for plasmin have been proposed in both physiological and pathological processes. These include skin, corneal, and arterial wound repair (12–14), tumor progression (15–17), and ischemic and excitotoxic brain damage (18–22). Recently, use of the plasminogen activation system (PAS) by pathogenic bacteria has received much attention. A growing number of studies have shown that gram-negative and gram-positive bacteria can interact with the host's PAS to increase their invasiveness and enhance their ability to cross tissue barriers (reviewed in 23). Some gram-positive cocci and Yersinia can activate plasminogen to enzymatically active plasmin with their endogenous plasminogen activators (PA) (24–27). Most other bacteria, including B. burgdorferi and B. hermsii, can incorporate plasminogen onto their surfaces, where it can be activated to plasmin by the host's PA (23). In either case, the incorporated proteolytic activity on the surface of pathogenic bacteria may be particularly important for organisms that must disseminate from their site of entry in the skin to the blood and other organs and avoid fibrin-based immobilization. Specifically, the use of the host's PAS by B. burgdorferi has been extensively studied and has demonstrated greater invasiveness in in vitro assays, degradation of extracellular matrix components, and a role in dissemination, with enhanced penetration across biological barriers (28–34).
Through early studies in the first half of this century, the brains of animals infected with various species of relapsing fever Borrelia were shown to remain infectious after the spirochetemic phases (3). Residual brain infection, but not gross central nervous system (CNS) abnormalities, was demonstrated recently in a mouse model of B. hermsii (35) and in a severe combined immunodeficiency (SCID) mouse model of B. turicatae (36). The latter study also demonstrated that CNS infection was serotype-specific (37). Another murine model using a blood isolate from a patient with relapsing fever (38) showed that meningitis developed shortly after the third peak of spirochetemia (39). This model, as is the case with other murine models of relapsing fever, closely resembles the human infection (1, 3). The model relies on cutaneous inoculation of the organisms to produce the characteristic relapsing spirochetemia, reticuloendothelial system activation, and meningitis. That this Borrelia sp. is capable of a consistent course of illness in several strains of immunocompetent mice presented the opportunity to ask, using plasminogen-deficient mice (10), whether the PAS has a role in the development of the recurrent spirochetemia of relapsing fever, and in the organ invasion and organ injury characteristic of this disease.
Methods
Spirochetes.
An uncultivable Borrelia species isolated from a patient with relapsing fever was used for all of the experiments and was maintained in the laboratory by mouse-to-mouse passage (38) or from frozen (liquid N2) citrated whole blood in 20% glycerol.
Experimental procedures.
Transgenic mice of a mixed 129/Black Swiss background were reared at the Division of Developmental Biology at the Children's Hospital Research Foundation in Cincinnati, Ohio, USA (10). Littermate pairs of 2–2.5-month-old plasminogen-deficient (plg–/–) and heterozygous (plg+/–) and homozygous (plg+/+) control mice of both sexes were used for all the experiments. The genotype of the mice was determined by PCR as described (11). The phenotype of the mice was confirmed by immunoblotting the plasma (34).
The mice were inoculated subcutaneously with 104 spirochetes that had been harvested from a donor mouse at the time of the first peak of spirochetemia. Citrated (0.11 M sodium citrate), infected blood from the donor mouse was centrifuged for 8 s at 800 g in an Eppendorf Model 5415C (Eppendorf North America, Inc.,Madison, Wisconsin, USA) (Hamburg, Germany) to remove the plasma with the majority of the spirochetes. The erythrocyte pellet was subsequently washed three times with HBSS supplemented with 2% BSA (Sigma Chemical Co., St. Louis, Missouri, USA), and the procedure was repeated to recover spirochetes in the medium. Spirochetes harvested in HBSS (without BSA) were used for the inocula to minimize contamination of plasma proteins in the inoculum. Spirochetemia was monitored by direct blood examination under darkfield microscopy. The number of organisms in 5 ml of a 1:10 dilution of whole blood in HBSS was counted to derive the numbers of spirochetes/ml of whole blood. Mice were weighed and sacrificed by CO2 inhalation at the time of the first peak of spirochetemia, 1–2 days after the first peak, and at 28–30 days after inoculation. Blood was collected in citrate by cardiac puncture immediately after euthanasia and divided in half. Half the blood was used for PCR, and the other half was used to obtain hematologic values. Total numbers of erythrocytes, hematocrit, hemoglobin, and numbers of platelets and leukocytes were obtained with a Coulter STKS Hematology Flow Cytometer (Beckman Coulter Inc., Miami, Florida, USA). The spleens were weighed and divided in half. Hearts, kidneys, liver, and brains were also divided in half for PCR and for histology. Uninfected controls of all three plg genotypes were treated in the same manner.
Detection of Borrelia species’ DNA in murine tissues using the PCR.
Genomic DNA was extracted from organs as described (40). Genomic DNA from 200 μl of whole blood was isolated with Easy-DNA (Invitrogen Corp., San Diego, California, USA) according to the manufacturer's instructions. DNA was quantitated by spectrophotometry, aliquoted, and stored at –20°C. A standard curve was generated by serial dilution of a known number of spirochetes in mouse blood. This curve was used as a positive control in all PCR experiments.
The flaB gene (flagellin), used in an earlier study to demonstrate the phylogenetic difference between this new Borrelia isolate and other known relapsing-fever organisms, was the target for its detection in murine tissues (38). Oligonucleotide primers fla-1 5′-GCTCAAATTAGAGGATTATCCCAAGC-3′ and fla-2 5′-GCATCTGAATATGTACCATTACCAG-3′ generated an efficiently amplified DNA fragment (EMBL/GenBank accession No. U28499). Each PCR reaction tube contained 60 pmol of each primer, 2.5 U of Native Taq DNA Polymerase (Roche Molecular Systems, Branchburg, New Jersey, USA), 10 ml of 10× PCR buffer (100 mM Tris-HCl with pH 8.3, 500 mM KCl), 20 ml of MgCl2 (25 mM), 2.8 ml each of dATP and dTTP (10 mM), and 1.2 ml each of dCTP and dGTP (10 mM) (Promega Corp., Madison, Wisconsin, USA). The optimal amount of template DNA (450 ng) for detection of Borrelia in infected murine tissues had been determined as described previously (34). Sterile distilled water (dH2O) was used to bring the final volume of each PCR reaction to 100 ml. PCR was carried out using the GeneAmp PCR System 9600 (Perkin-Elmer Corp., Norwalk, Connecticut, USA). PCR conditions consisted of 15 s of initial denaturation at 94°C, followed by 45 cycles of 94°C (15 s), 67°C (30 s), and 72°C (15 s). At the end of 45 cycles, samples were held at 72°C for 7 min and stored at 4°C, concentrated by SpeedVac (Savant Instruments Inc., Holbrook, New York, USA), and loaded onto a 2% agarose gel. Negative controls included dH2O and DNA from uninfected mouse tissues for experimental template DNA. Gels were electrophoresed at 70 V and stained with ethidium bromide. Images were scanned into a computer imaging program with a GS-670 Imaging Densitometer (Bio-Rad Laboratories, Hercules, California, USA). Densitometric data given as OD of the amplimers allowed for comparisons of relative spirochetal burdens.
Histology.
Peripheral blood smears were fixed in absolute methanol and stained with Giemsa (Sigma Chemical Co.). Organs were immediately fixed in 10% buffered formalin and embedded in paraffin. Hearts, livers, spleens, and kidneys were sectioned at 8 μm for staining with hematoxylin and eosin, with Dieterle's stain to view spirochetes, and with the Martius-scarlet-blue method to view fibrin deposition. Brains were also sectioned at 15 μm to use with antibody F4/80 (41, 42), a rat monoclonal antibody to a murine mature macrophage-microglia antigen (Harlan Bioproducts for Science Inc., Indianapolis, Indiana, USA). After quenching with H2O2 and blocking with BSA, the thick sections were incubated with the antibody to F4/80, followed by an incubation with biotinylated secondary anti–rat IgG (Vector Laboratories, Burlingame, California, USA). The avidin-biotin-peroxidase complex was developed with diaminobenzidine and H2O2 (18). Morphometric analysis to determine the extent of meningitis was carried out using OPTIMAS 5.0 imaging analysis software (Optimas Corp., Bothell, Washington, USA). Statistical analyses were done with the InStat 2.01 Statistical Software Package (Graph-Pad Software, San Diego, California, USA).
Results
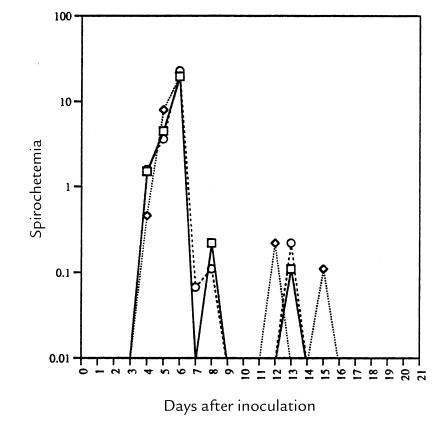
The mouse PAS has no effect on spirochetemia and on relapses. Subcutaneous inoculation of 104 Borrelia species into plg +/+, plg +/–, and plg –/– mice resulted in peak spirochetemias that reached similar numbers of organisms per milliliter of blood (Fig. 1). The prepatent period in the plg –/– mice was slightly, but not significantly, delayed relative to the control mice. All mice reached levels of 20–23 × 106 spirochetes per milliliter of blood within 5–6 days of inoculation. The timing of the second and third peaks was not influenced by the mouse phenotype; nor were the levels of spirochetemia in the subsequent peaks. At ∼20–21 days, spirochetes were no longer evident in the blood of any mice, indicating that the PAS had no effect on the duration of the spirochetemia (Fig. 1). These organisms, as is the case with other relapsing fever borreliae (29, 30), bind plasmin(ogen) specifically under experimental conditions; they also bind plasminogen in mice in in vivo conditions, as detected by immunofluorescence (data not shown).
Figure 1.
The mean course of the spirochetemia in plg–/– (circles), control plg+/– (diamonds), and control plg+/+ (squares) mice (n = 10 mice of each genotype) in a logarithmic scale after subcutaneous inoculation of 104 organisms. Error bars for each point were omitted for clarity; the highest SD at peak was 1.4 × 106 organisms.
Murine relapsing fever leads to anemia, thrombocytopenia, and organ pathology.
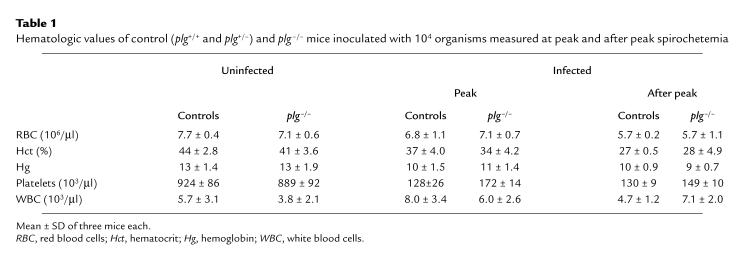
Anemia was detected in all mice at peak spirochetemia and became more marked during the period after peak (Table 1). Thrombocytopenia was a major finding in this infection in all mice, and unlike the anemia, the levels of platelets remained low at 28–30 days after inoculation (Table 1). Leukocytosis was an erratic finding (with a range of 2.5–13 × 103/μl in all infected mice), as can be seen by the standard deviations of the measurements (Table 1). The differentials consisted uniformly of absolute increases in the numbers of lymphocytes, atypical lymphocytes, and monocytes in all mice (data not shown).
Table 1.
Hematologic values of control (plg+/+ and plg+/–) and plg–/– mice inoculated with 104 organisms measured at peak and after peak spirochetemia
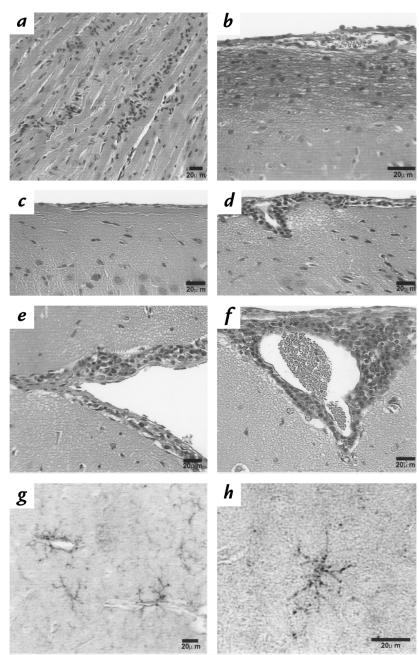
The histopathology of the liver and spleen at peak and after peak of this infection has been described (38) and, apart from fibrin deposition in the plg –/– mice, was not markedly different among the three groups. As has also been described for plg –/– mice, extensive hepatic fibrin deposition was also noted in the uninfected mice (10) but was qualitatively more pronounced in the infected animals at peak and after peak than in the uninfected controls (not shown). Splenomegaly is a feature of this infection (38), but the weight of the spleens was not significantly different among the three groups of mice. There was no significant pathology associated with the kidneys in any of the animals. The hearts of infected mice, regardless of genotype, showed perivascular mononuclear cell infiltrates in the pericardium during peak spirochetemia. Focal myocarditis with mononuclear cell infiltrates, interstitial edema, and isolated muscle fiber necrosis developed in all mice after peak and persisted for 28–30 days after inoculation (Fig. 2a). Morphometric assessment of the hearts did not yield convincing data regarding differences in inflammation among the plg –/– and control mice.
Figure 2.
Histopathology of organs. (a–f) Shown are 8-μm sections. All except b were stained with hematoxylin and eosin. (a) Heart of a mouse at peak spirochetemia with mononuclear cell infiltration of the myocardium. (b) Fibrin deposition in the submeningeal region of the brain of a plg–/– mouse after peak, stained with the Martius-scarlet-blue method. (c) Normal meninges of an uninfected control mouse. (d) Perivascular mononuclear cell infiltrate and submeningeal edema corresponding to the one-layer (+1) value of the inflammatory index at 28–30 days after inoculation in the brain of a plg–/– mouse. (e) Two layers of inflammatory cells in the meninges, corresponding to a +2 value in the inflammatory index, extending into the fissure at 28–30 days in a control mouse. (f) A +3 inflammatory index value with submeningeal edema in a control mouse. (g–h) Shown are 15-μm sections of brains from infected mice at 28–30 days. Each was stained with monoclonal antibody F4/80 to murine activated macrophage/microglia and developed with diaminobenzidine. (g) Cluster of perivascular F4/80 reactivity in the brain of a plg +/– control mouse. (h) Solitary microglia illustrating the morphology and large size of these cells.
The PAS has an effect on the histopathology of the brain as a result of the infection.
Submeningeal fibrin deposition was seen in the brains of plg –/– mice at peak and after peak, and persisted to 28–30 days after inoculation (Fig. 2b). Fibrin deposition in brain tissue was not noted in infected control mice or in uninfected plg –/– mice. A meningitis with mononuclear cell infiltrates is an outcome of this infection in mice at ∼25–30 days after inoculation (39). All infected mice, irrespective of genotype, developed meningitis of variable severity, submeningeal edema, and plexitis, but inflammatory cell infiltrates in the brain parenchyma were not found. An initial qualitative assessment indicated that the degree of meningeal inflammation was greater in control than in plg –/– mice. To verify this observation, an inflammatory index was devised.
The inflammatory index was determined by examination using a blinded investigation of three sagittal 8-μm sections of brains stained with hematoxylin and eosin (with each of the three sections separated by 24 μm of tissue). Three areas of meningeal infiltrates were classified according to their depth: a value of 0 was assigned to normal meninges (Fig. 2c); a value of 1 was assigned to areas with one layer of infiltrating cells (Fig. 2d); a value of 2 was assigned to areas with two layers (Fig. 2e); and a value of 3 was assigned to meningeal areas of three or more infiltrating cell layers (Fig. 2f). Means were obtained for these values for the three sections examined, and added to a value of 1 for each of the following: infiltrates of the brain parenchyma, plexitis, and submeningeal edema. According to this scheme, the mean (± SD) inflammatory index for 11 control mice (2.9 ± 0.4) was significantly different (P < 0.01, two-tailed Student's t test) from the mean for 11 plg –/– mice (1.4 ± 0.4). There were no differences between plg +/+ and plg +/– mice.
Focal, perivascular microgliosis (F4/80 positive cells) was a prominent response in this infection at 28–30 days after inoculation. Foci were unevenly distributed throughout the brain sections in all mice and formed tight clusters around blood vessels (Fig. 2g). Although rare, solitary microglia were noted (Fig. 2h), the tight clustering of these large cells around the vessels did not permit accurate enumeration. Mono-cyte/macrophages infiltrating the leptomeninges and the choroid plexus also reacted with F4/80 (not shown). Spirochetes were not detected in any brain sections (Dieterle's stain) at 28–30 days.
The PAS has a role in heart and brain invasion.
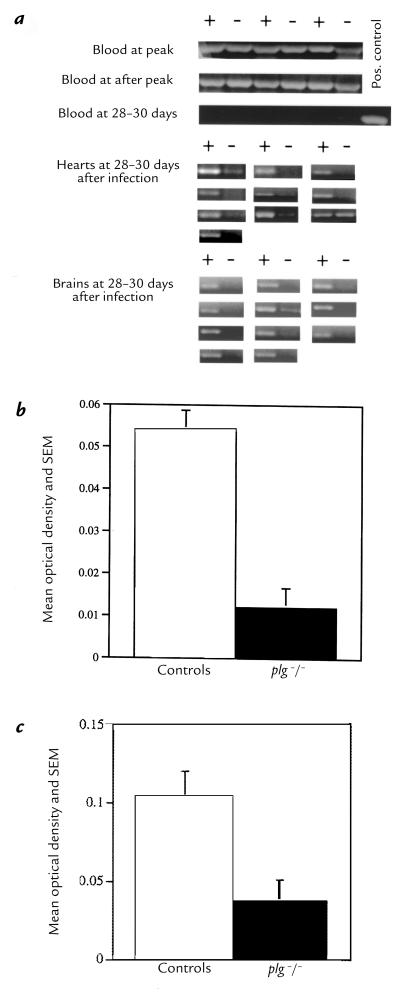
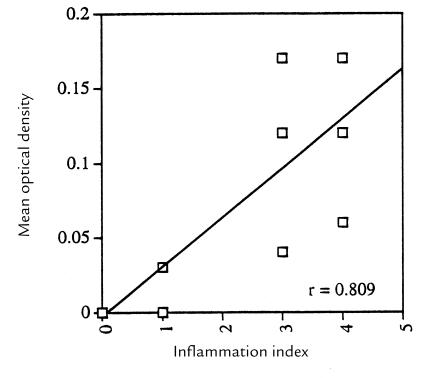
PCRs of blood at peak and after peak of spirochetemia were strongly positive. This was expected, because at peak the organisms are readily visible (by microscope) in the blood, and after peak the blood is laden with granular material (possibly debris from lysed organisms) or with spirochetes whose numbers may be below the limits of microscopic detection (Fig. 3a). In contrast, amplification of flaB in blood at 28–30 days was virtually undetectable. Of 11 pair of bloods from infected mice examined, only two mice (one control and one plg –/–) had optical density (OD) values of the amplimers greater than those obtained as background values from uninfected mice (Fig. 3a). The lack of reactivity of the blood at 28–30 days permitted the amplification of spirochetal DNA in tissues without possible contamination from blood-derived organisms. PCRs were performed on the hearts of 10 pair of control and plg –/– mice (Fig. 3a), and scanned using a densitometer. The mean OD of the controls (0.054 ± 0.022) was significantly higher (P = 0.0007, two-tailed Mann-Whitney test) than the mean OD of the plg –/– mice (0.012 ± 0.014) (Fig. 3b). PCRs performed on the brains of 11 pair of control and plg –/– mice (Fig. 3a) and scanned by densitometer had a mean OD of the controls (0.104 ± 0.017) that was significantly higher (P = 0.0087, two-tailed Student's t test) than the mean OD of the plg –/– mice (0.037 ± 0.013) (Fig. 3c). A significant (P < 0.0015) positive correlation was obtained between the OD of the amplimers and the values of the inflammatory index (Fig. 4).
Figure 3.
PCR amplifications of spirochetal DNA (flaB) from blood and brains. (a) Representative amplifications of spirochetal DNA of blood from control (+) and plg–/– mice (–) obtained at peak, after peak, and 28–30 days after inoculation. Control and plg–/– mice (10 pair) were used to detect spirochetal DNA in hearts at 28–30 days. Control and plg–/– mice (11 pair) were used to detect spirochetal DNA in brains at 28–30 days. (b) Mean OD of the amplimers from the PCRs of the hearts of 10 pair of controls and plg–/– mice. (c) Mean OD of the amplimers from the PCRs of the brains of 11 pair of controls and plg–/– mice. For b and c, the mean OD of the uninfected PCR tissue controls was subtracted from the OD of each infected mouse.
Figure 4.
Correlation between the mean OD of the PCR of brains and the corresponding inflammatory index value for each control and plg–/– mouse (11 pair; two of the points overlap).
Discussion
The murine model of relapsing fever used in this study is particularly suitable for characterizing the entire course of infection in the presence or absence of the PAS. In addition, the effects of this infection in immunocompetent mice were established from its natural site of entry in the skin, to the spirochetemic phases, and to organ invasion. This study has shown that the PAS has an effect on the invasion of the CNS by a relapsing fever Borrelia, and indirectly on the CNS response to the invading organisms. Similar effects on invasion of the heart were noted but without a noticeable difference in the inflammatory response in this organ. The PAS did not have a role in the establishment, duration, and levels of spirochetemia, nor did it affect the number and duration of the relapses, or the hematologic changes or other organ pathology that occurred as a result of the infection.
Entry to the CNS by microorganisms from the blood requires an alteration in the blood–brain barrier (BBB) (43). The interactions of the PAS with the tight junctions of the endothelium of the CNS vasculature that would permit increased permeability and access have not been studied in detail. The lack of spontaneous fibrin deposition in the brains of plg –/– mice, unlike the deposition of fibrin in other organs (10), suggests that the PAS alone does not have an effect on the integrity of the BBB. This leaves the possibility that CNS invasion from the blood by the relapsing fever Borrelia is facilitated by the use of the PAS by the organisms themselves. Plasmin-coated organisms could use the plasminogen receptors that have been reported in endothelium as a means of initial anchoring (44), and their enhanced proteolytic capacity could make it easier to move across the tight junctions of endothelium, basement membrane, and astrocytes. Our results showed significantly increased levels of spirochetal DNA, as well as increased inflammation, in the brains of control (plg +/+ and plg +/–) mice. The high level of correlation between these two measurements indicates that the greater inflammation in the brains of control mice is a response to the higher numbers of organisms. The mononuclear cell infiltrate in this infection is identical to what has been described for humans and for other animal models (1, 3). This infection also results in an obvious increase in perivascular microglia. Although the origin of the perivascular microglia (pericytes, F4/80-positive cells) is debated (41, 42, 45), their location around blood vessels, and the large numbers of F4/80-positive cells infiltrating the leptomeninges, suggest that these cells are recruited from the circulation, particularly because there was an absolute increase in the numbers of monocytes in the peripheral blood. In this regard, plg –/– mice exhibit normal activation of microglia, and these cells in mice can produce PAs (21). Under pathological conditions, neurons of mice can produce plasminogen (46). It is also known that human-infiltrating monocytes express plasminogen activators (47, 48). Invading spirochetes bound with plasminogen could rely on local activation in the CNS and start a proteolytic process that results in neurologic injury. Alternatively, the locally activated PAS could supply the components for plasmin acquisition by dividing organisms in the CNS. Relapsing fever Borrelia and B. burgdorferi can adhere to neural cells (49–51), and their capacity to cause injury may be enhanced by the PAS. It will be interesting to determine the ultimate role of the PAS, both inside and outside the CNS, in protecting against invading organisms. Specifically, does a PAS-mediated or PAS-enhanced inflammatory response in the CNS work to the advantage of the pathogen or its host?
The relapsing fever Borrelia invaded the heart with resulting histopathology that was similar to that described for B. turicatae infection in SCID mice (36) and for infection of B. burgdorferi in both SCID and C3H/He mice (52, 53). Unlike the brains, the levels of inflammation in the hearts of the control and plg –/– mice did not appear to be different. That both the hearts and brains of control mice had greater burdens of spirochetal DNA would suggest that the role of the PAS is in the invasion of these organs.
The PAS was not important in all phases of this multisystemic infection. Clearly it did not affect the penetration of the organisms from the cutaneous tissues to the blood, because the levels of spirochetemia were identical in all mice. The PAS presumably had no role in the production of the antibodies that are thought to mediate the abrupt end of the spirochetemia; thus, the subsequent peaks proceeded normally. Anemia and long-lasting thrombocytopenia are known features of human relapsing fever (1). We documented these features in this model, and their development was not affected by the lack of plasminogen. The extensive fibrin deposition in the brains of infected plg –/– mice suggests that fibrin accumulation in this tissue is a feature of the infection in these mice (10). Likewise, plg –/– mice appear to have normal numbers of platelets (10). The pathophysiology of the anemia and thrombocytopenia in this infection is not understood. The Borrelia species used in this study can bind platelets, as observed in fluid blood preparations under dark-field microscopy, and B. burgdorferi can bind platelets as well (54, 55). On the other hand, while binding to platelets by the spirochetes may partly explain the thrombocytopenia as a consumptive mechanism, we did not see any evidence of microthrombi in the tissues examined at peak spirochetemia. This organism did not exhibit the erythrocyte rosette formation that has been reported for a closely related species, B. crocidurae, which could partly explain the anemia seen here (56).
As the interactions of the host's PAS with pathogenic bacteria become a common feature of mechanisms of bacterial pathogenesis, it is apparent that use of the PAS is selective, even by the same organism at various stages of the infection. It is now becoming increasingly clear that the effect of the PAS is not uniform in dissemination or invasion. At the same time, the availability of transgenic mice for most of the known components of the fibrinolytic system is advancing our knowledge of this system in areas not been previously envisioned. Specifically, the role of the PAS in neurologic infection deserves further study.
Acknowledgments
We thank Alan G. Barbour of the University of California at Irvine for his review of this manuscript and for helpful discussions. We appreciate the help and guidance of Stella Tsirka of the State University of New York at Stony Brook. Supported by a grant from the United States Public Health Service (USPHS) (AR 40445), by a grant from the Mathers Foundation to J.L. Benach, and by a grant from the Commission for Cultural, Educational and Scientific Exchange between the United States of America and Spain to J. C. Garcia. Monco. J. L. Degen is supported by a grant from the USPHS (HL 47826) and by the American Heart Association (AHA), with funds contributed by the AHA Ohio affiliate (92-1103). T.H. Bugge is supported by the Danish Medical Research Council and by the Board of Trustees of the Children's Hospital Research Foundation.
References
- 1.Southern P, Garcia Monco JC, Sanford J. Relapsing fever. A clinical and microbiological review. Medicine. 1969;48:129–149. [Google Scholar]
- 2.Garcia JC Monco, Benach JL. Lyme neuroborreliosis. Ann Neurol. 1995;37:691–702. doi: 10.1002/ana.410370602. [DOI] [PubMed] [Google Scholar]
- 3.Cadavid D, Barbour AG. Neuroborreliosis during relapsing fever. Review of the clinical manifestations, pathology, and treatment of infections of humans and experimental animals. Clin Infect Dis. 1998;26:151–164. doi: 10.1086/516276. [DOI] [PubMed] [Google Scholar]
- 4.Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol. 1990;44:155–171. doi: 10.1146/annurev.mi.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 5.Coffey EM, Eveland WC. Experimental relapsing fever initiated by Borrelia hermsi. II. Sequential appearance of major serotypes in the rat. J Infect Dis. 1967;117:29–34. doi: 10.1093/infdis/117.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Meier JT, Simon MI, Barbour AG. Antigen variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo BI, Barbour AG. Antigen diversity in the bacterium B. hermsii through “somatic” mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 9.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 11.Bugge TH, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 12.Rømer J, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Moons L, Ploplis V, Plow E, Collen D. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J Clin Invest. 1997;99:200–208. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao WW, et al. Healing of corneal epithelial defects in plasminogen and fibrinogen deficient mice. Invest Ophthalmol Vis Sci. 1998;39:502–508. [PubMed] [Google Scholar]
- 15.Danø K, et al. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 16.Bugge TH, et al. Growth and dissemination of Lewis lung carcinoma in plasminogen deficient mice. Blood. 1997;90:4522–4531. [PubMed] [Google Scholar]
- 17.Bugge TH, et al. Reduced metastasis of polyoma virus middle T antigen-induced mammary cancer in plasminogen deficient mice. Oncogene. 1998;16:3097–3104. doi: 10.1038/sj.onc.1201869. [DOI] [PubMed] [Google Scholar]
- 18.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 19.Tsirka SE, Bugge TH, Degen JL, Strickland S. Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci USA. 1997;94:9779–9781. doi: 10.1073/pnas.94.18.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 21.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YF, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 23.Boyle MDP, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 24.Reddy KNN, Markus G. Mechanism of activation of human plasminogen by treptokinase. J Biol Chem. 1972;247:1683–1696. [PubMed] [Google Scholar]
- 25.Sodeinde OA, et al. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 26.Lottenberg R, Des Jardin LE, Wang H, Boyle MDP. Streptokinase-producing streptococci grown in human plasma acquire unregulated cell-associated plasmin activity. J Infect Dis. 1992;166:436–440. doi: 10.1093/infdis/166.2.436. [DOI] [PubMed] [Google Scholar]
- 27.Kuusela P, Ulberg M, Saksela O, Kronvall G. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C and G streptococci. Infect Immun. 1992;60:196–201. doi: 10.1128/iai.60.1.196-201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klempner MS, et al. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1995;171:1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- 30.Coleman JL, et al. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs H, Simon MM, Wallich R, Bechtel M, Kramer MD. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect Immun. 1996;64:4307–4312. doi: 10.1128/iai.64.10.4307-4312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu LT, Perides G, Noring R, Klempner MS. Binding of human plasminogen to Borrelia burgdorferi. Infect Immun. 1995;63:3491–3496. doi: 10.1128/iai.63.9.3491-3496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perides G, Noring R, Klempner MS. Inhibition of Borrelia burgdorferi-bound fibrinolytic enzymes by α2-antiplasmin, PAI-1 and PAI-2. Biochem Biophys Res Commun. 1996;219:690–695. doi: 10.1006/bbrc.1996.0296. [DOI] [PubMed] [Google Scholar]
- 34.Coleman JL, et al. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 35.Cadavid D, Bundoc V, Barbour AG. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J Infect Dis. 1993;168:143–151. doi: 10.1093/infdis/168.1.143. [DOI] [PubMed] [Google Scholar]
- 36.Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadavid D, Pennington PM, Kerentseva TA, Bergström S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia uricatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anda P, et al. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. doi: 10.1016/s0140-6736(96)02332-x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia Monco JC, Miller NS, Backenson PB, Anda P, Benach JL. A mouse model of Borrelia meningitis after intradermal injection. J Infect Dis. 1997;175:1243–1245. doi: 10.1086/593681. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, et al. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch S, Austyn JM, Gordon S. Expression of the macrophage-specific antigen F4.80 during differentiation of bone marrow cells in culture. J Exp Med. 1981;154:713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 43.Garcia Monco JC, Fernandez Villar B, Calvo J, Benach JL. Borrelia burgdorferi in the central nervous system. Experimental and clinical evidence for early invasion. J Infect Dis. 1993;161:1187–1193. doi: 10.1093/infdis/161.6.1187. [DOI] [PubMed] [Google Scholar]
- 44.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- 45.Hickey WF, Kimura H. Perivascular microglial cells in the CNS are bone-marrow derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 46.Sappino A, et al. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassalli JD, Dayer JM, Wohlwend A, Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984;159:1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart PH, Vitti GF, Burgess DR, Singleton DK, Hamilton JA. Human monocytes can produce tissue-type plasminogen activator. J Exp Med. 1989;169:1509–1514. doi: 10.1084/jem.169.4.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia Monco JC, Fernandez Villar B, Benach JL. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989;160:497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- 50.Garcia Monco JC, Fernandez Villar B, Rogers R, Szczepanski A, Benach JL. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 51.Peters DJ, Benach JL. Borrelia burgdorferi adheres to and injures transformed neural cells. J Infect Dis. 1997;176:470–476. doi: 10.1086/514066. [DOI] [PubMed] [Google Scholar]
- 52.Schaible UE, et al. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 53.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 54.Galbe JL, Guy E, Zapatero JM, Peerschke EIB, Benach JL. Vascular clearance of Borrelia burgdorferi in rats. Microb Pathog. 1993;14:187–201. doi: 10.1006/mpat.1993.1019. [DOI] [PubMed] [Google Scholar]
- 55.Coburn J, Leong JM, Erban JK. Integrin aIIbb3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci USA. 1993;90:7059–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burman N, Shamaei-Tousi A, Bergström S. The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever borreliosis. Infect Immun. 1998;66:815–819. doi: 10.1128/iai.66.2.815-819.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]