Platelet endothelial cell adhesion molecule-1 (PECAM-1, or CD31) is a 130-kDa member of the immunoglobulin (Ig) superfamily that is expressed on the surface of circulating platelets, monocytes, neutrophils, and particular T-cell subsets. It is also a major constituent of the endothelial cell intercellular junction, where up to an estimated one million molecules are concentrated. Because of this cellular expression pattern, PECAM-1 is implicated in several functions, including transendothelial migration of leukocytes, angiogenesis, and integrin activation (for a review of the biology of PECAM-1, see ref. 1). When the cDNA sequence for PECAM-1 was determined more than eight years ago (2), it was originally assigned to the growing family of Ig-like cell adhesion molecules (Ig-CAMs). This classification was the result of the sequence similarity of PECAM-1's extracellular Ig domains to those of other Ig-CAMs characterized at that time, including carcinoembryonic antigen (CEA), intercellular adhesion molecule-1 (ICAM-1), and neural cell adhesion molecule (NCAM). Recent observations however, suggest that we may have been following the wrong half of the molecule and that PECAM-1 may not be a member of the Ig-CAM family after all.
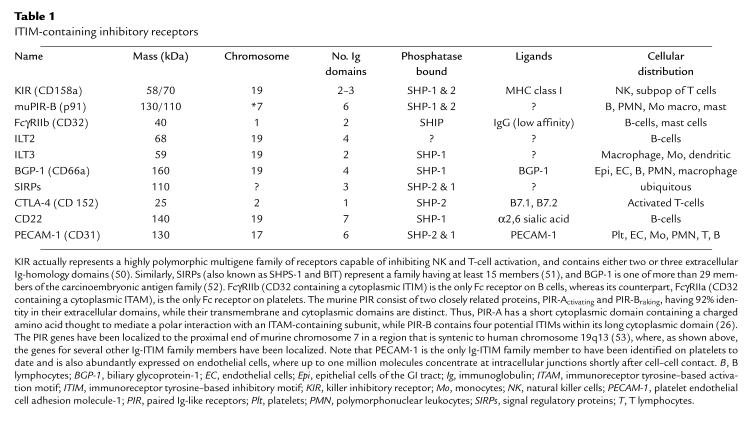
Ig-superfamily members have been known for some time to mediate cell adhesion (e.g., NCAM, ICAM-1, and vascular cell adhesion molecule-1 [VCAM-1]) or antigen recognition (e.g., immunoglobulins, T-cell receptors, and MHC molecules). However, a subgroup comprising 30 members characterized by the presence of one or more immunoreceptor tyrosine–based inhibitory motifs (ITIMs) within their cytoplasmic domain has recently emerged (3, 4). Currently recognized members of the Ig-ITIM family are listed in Table 1. They include members of the killer inhibitory receptor (KIR) family, the murine paired Ig-like receptor-braking protein (PIR-B), a low-affinity receptor for IgG (FcγRIIb), at least two members of the Ig-like transcript (ILT) family, a transmembrane domain–containing member of the CEA family known as biliary glycoprotein–1 (BGP-1), cytotoxic T lymphocyte–associated protein-4 (CTLA-4), and the B cell–specific antigen, CD22. When members of the Ig-ITIM family are appropriately engaged, they become phosphorylated on distinct tyrosine residues located within their cytoplasmic ITIM resulting in the creation of specific docking sites for Src-homology 2 (SH2) domain–containing intracellular lipid- and protein-tyrosine phosphatases, such as SHIP, SHP-1, or SHP-2. These catalytic enzymes, once localized to their cytoplasmic anchors and activated, are then able to effect a wide range of cellular events, most notably inhibition of tyrosine kinase–mediated signaling, proliferation, and cellular activation.
Table 1.
ITIM-containing inhibitory receptors
ITIMs are identifiable by the consensus sequence L/I/V/S–x–Y–x–x–L/V and have been found to exist alone or in pairs within the cytoplasmic domain of an increasingly recognized number of inhibitory receptors. For example, the inhibitory Fcγ receptor, FcγRIIb, harbors a single ITIM within its cytoplasmic domain and signals predominantly through SHIP, an inositol phosphatase that binds to the ITIM via its single amino-terminal SH2 domain. On the other hand, Ig-ITIM family members that bind SHP-1 and SHP-2 most commonly contain two or more ITIMs separated by at least 20 residues (50 Å) each — a feature that no doubt contributes specificity to their recruitment and activation of these tandem SH2 domain–containing protein-tyrosine phosphatases (5). The distance separating ITIMs is in sharp contrast to the much shorter spacing between the bisphosphotyrosyl sequences present in immunoreceptor tyrosine–based activation motifs (ITAMs; consensus = Y-x-x-L-x6–8-Y-x-x-L), which are located within the cytoplasmic domains of stimulatory receptors such as T-cell receptor ζ chain, the Fc receptor γ chain (a 14-kDa signaling subunit that is associated with FcγRI, FcγRIII, FcεRI and platelet GPVI), all three CD3 subunits (ε, γ, and δ), the Igα/Igβ dimer that is associated with the μ chain of the B-cell receptor, and FcγRIIa (for recent reviews of the biology of ITAMs, see refs. 6 and 7). As a consequence of the close proximity of their phosphotyrosine residues, ITAMs appear to have a much higher affinity for the SH2 domains of protein-tyrosine kinases like ZAP-70, Syk, and phosphatidylinositol-3-kinase (8) than they do for the more widely spaced SH2 domains of protein-tyrosine phosphatases (5). This fact has only been appreciated of late, however, resulting in the inadvertent assignment of a number of proteins containing canonical ITIMs to the ITAM family of stimulatory receptors (9–11).
PECAM-1 appears to be one such example. Several years ago, Modderman et al. (12) found that PECAM-1 could become phosphorylated on tyrosine residues after treatment of platelets with pervanadate, a protein-tyrosine phosphatase inhibitor. Subsequently, Jackson et al. (13, 14) showed that two specific tyrosine residues, Y663 and Y686, located within the 118–amino acid PECAM-1 cytoplasmic domain, formed a specific docking site for the protein-tyrosine phosphatase, SHP-2, and that this signaling molecule bound avidly to PECAM-1 after platelet aggregation. This has since been confirmed by several investigators (15, 16), and the number of cellular activation events that can lead to PECAM-1 tyrosine phosphorylation and SHP-2 binding has been expanded to include shear (17) or oxidative (18) stress, osmotic shock (17), exposure to lysophosphatidyl choline (19), and monoclonal antibody–induced cross-linking of the T-cell receptor (20), the Fcε receptor (20), or PECAM-1 itself (21). Thus, PECAM-1 appears to fulfill one of the criteria established for inclusion in the Ig-ITIM family (3): it recruits one or more SH2 domain–containing phosphatases after phosphorylation. In addition, the paired ITIMs within its cytoplasmic domain are remarkably similar to those present in other well-established members of the Ig-ITIM family (Fig. 1 and Table 2). Taken together, it would appear that PECAM-1 is, in fact, a member of the Ig-ITIM family, and may not necessarily have cell adhesion as its primary function after all. Rather, like other members of this family, PECAM-1 may function as an inhibitory receptor, serving to moderate or attenuate tyrosine kinase–mediated signaling pathways, or to set thresholds for cellular activation in the vascular cells that express it.
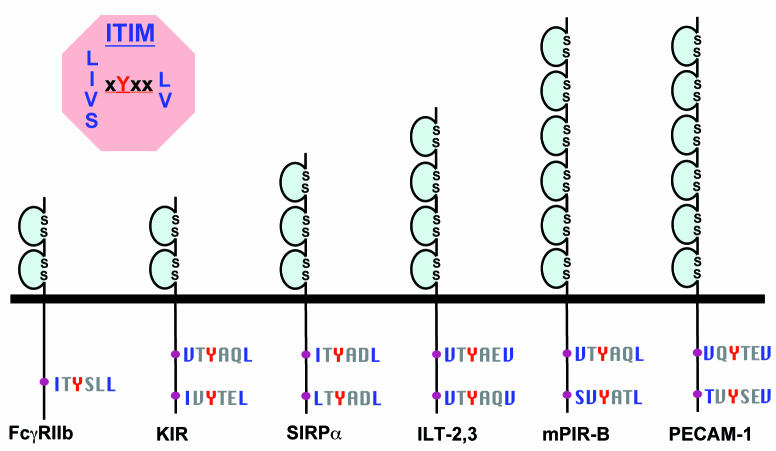
Figure 1.
Schematic diagram of selected Ig-superfamily members bearing one or more ITIMs (adapted from ref. 48). See Table 2 for a complete list of Ig-ITIM family members that bind SHP-1, SHP-2, or both, as well as the detailed amino acid sequence separating the paired ITIMs. Ig, immunoglobulin; ILT, Ig-like transcript; ITIM, immunoreceptor tyrosine–based inhibitory motif; KIR, killer inhibitory receptor; PECAM-1, platelet endothelial cell adhesion molecule-1; PIR-B, paired Ig-like receptor-braking protein; SIRP, signal regulatory protein.
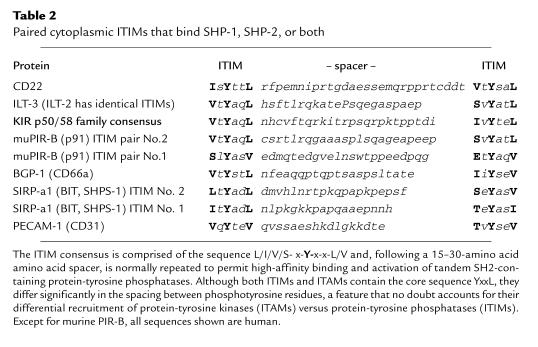
Table 2.
Paired cytoplasmic ITIMs that bind SHP-1, SHP-2, or both
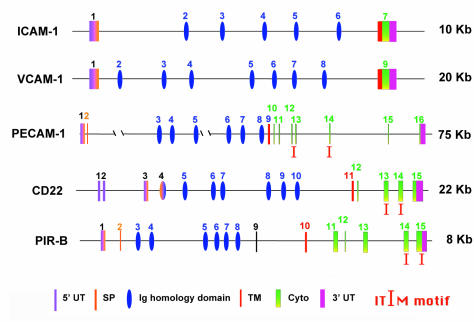
Further support for inclusion of PECAM-1 within the Ig-ITIM, rather than the Ig-CAM, family can be found by comparing the genomic organization of PECAM-1 with genes that encode two representative Ig-CAMs (ICAM-1 and VCAM-1) and two Ig-ITIM family members (CD22 and PIR-B). As shown in Fig. 2, the Ig-homology domains that comprise the extracellular region of each of these genes are encoded by individual exons — a feature common among members of the Ig superfamily (22); one notable exception is NCAM (23). However, whereas the transmembrane domain, the cytoplasmic domain, and the 3′ untranslated region are normally encoded by a single exon for Ig-CAMs, these domains are often split into six or more small exons in genes encoding members of the Ig-ITIM family. Notably, the ITIMs themselves are commonly encoded by separate exons (coincidentally, exons 13 and 14 for both PECAM-1 and CD22), providing the potential to generate alternatively spliced isoforms that differ in both structure and function (24, 25). Moreover, the signal peptide in several Ig-ITIM family members, including KIR, PIR-B, and CD22 (26), is split between two exons, with the second exon being a mini-exon 25–40 bp in length. PECAM-1 shares this unusual genomic organization.
Figure 2.
Organization of the genes encoding selected Ig-CAMs and Ig-ITIM family members. Note that Ig-ITIM family genes often contain a mini-exon encoding all or part of the signal peptide, and their cytoplasmic domains are encoded by multiple exons. Both of these features differ significantly from that of Ig-CAMs, in which the transmembrane and cytoplasmic domains, as well as the 3′-untranslated region, are often encoded by a single, fused exon. ICAM-1, intercellular cell adhesion molecule-1; Ig-CAMs, Ig-like cell adhesion molecules; VCAM-1, vascular cell adhesion molecule-1.
Reexamination of the literature provides still further evidence that PECAM-1 functions as an inhibitory receptor in vascular cells, although much of this evidence is circumstantial. First, Schimmenti et al. (27) found more than eight years ago that transfecting full-length PECAM-1 into NIH/3T3 cells causes them to migrate more slowly — a feature that was at that time attributed to the putative adhesive properties of the molecule, but in retrospect could easily have been due to introduction of an inhibitory receptor. In this regard, it is notable that NIH/3T3 cells expressing PECAM-1Y686F, a non–SHP-2–binding variant of PECAM-1 (14), migrate almost normally (28, 29), suggesting that inhibition of cell migration by PECAM-1 is a function of its cytoplasmic ITIM rather than its extracellular domain. Second, PECAM-1 is lost from the surface of the majority of CD4+ (30–33) and half of CD8+ (30, 31) T lymphocytes as they make the transition from naive to memory cells. Furthermore, PECAM-1–negative CD4+ T cells respond better to recall antigens, secrete more interleukin-4 (IL-4), and provide better help for B-cell immunoglobulin production than do PECAM-1–positive CD4+ T cells (33). This is consistent with PECAM-1 playing a suppressive role in T-cell effector function. Third, although several laboratories, including my own, have reported that anti–PECAM-1 monoclonal antibodies, when bound to the cell surface, seemingly cause activation of β1 (30, 34), β2 (35–37), and β3 (21) integrins, it is possible that this phenomenon is simply due to antibody-mediated sequestration of an inhibitory receptor (PECAM-1/SHP-2) away from activatory receptors that it normally regulates, resulting in a cell with increased adhesive properties. A similar explanation has been proposed for the action of anti-CD22 monoclonal antibodies, which activate B-cells, even though CD22 is now known to be an ITIM-containing negative regulator of B cell function (reviewed in ref. 38). Finally, we have recently found that PECAM-1 inhibits T-cell receptor–mediated release of calcium from intracellular stores (39) and may also function to shut down the mitogen-activated protein kinase pathway in cells after serum stimulation (Wang, R., and Newman, P.J., unpublished observations). Given these observations and the structural similarities of the PECAM-1 protein and gene to other ITIM-bearing inhibitory receptors, we propose that PECAM-1 be reassigned to the Ig-ITIM family.
What might be the implications for the presence of a newly recognized inhibitory receptor that is expressed on the surface of circulating platelets and leukocytes, and expressed abundantly at the intercellular junctions of endothelial cells after cell–cell contact? A number of testable predictions come to mind.
First, like other members of the Ig-ITIM family, PECAM-1 may function to feedback inhibit or set thresholds for cellular activation resulting from signaling cascades that emanate from ITAM-containing receptors. For example, Fc receptors (40) and a newly recognized platelet collagen receptor (41) signal via ITAMs. Might their activating and/or adhesive functions be modulated by PECAM-1 in cells that express them? The T-cell receptor complex also contains multiple ITAM-bearing subunits (the ζ chain and the CD3 ε, δ, and γ subunits), the signaling patterns of which are thought to be regulated, in part, by the CTLA-4 and KIRs, each of which belong to the Ig-ITIM family (Table 1). Why should T cells express still another inhibitory receptor? The answer may lie within the extracellular domain: the homophilic nature of the PECAM-1 extracellular domain (42, 43) may serve to target phosphatase activity to specific sites within the cell that are not served by these other inhibitory receptors. It is interesting to note that CTLA-4–deficient mice have constitutively activated T cells and a lymphoproliferative disorder (44, 45). It might be instructive to see, via the generation of double knockouts, whether the additional loss of PECAM-1 might lead to an even more severe phenotype.
Second, PECAM-1 is diffusely distributed on the surface of endothelial cells in culture but concentrates rapidly to cell–cell junctions once the cells have contacted each other (46). One could easily envision a scenario in which cell–cell contact leads not only to redistribution of PECAM-1 but also coincides with a transient tyrosine phosphorylation event that leads to localization of SHP-2 to cell borders. Might targeting phosphatase activity to the cell borders somehow contribute to the poorly understood phenomenon of contact inhibition? Further studies employing a constitutively active PECAM-1/SHP-2 chimeric protein may shed light on this question.
Third, PECAM-1 becomes rapidly tyrosine phosphorylated and binds SHP-2 after integrin-mediated adhesion of platelets and endothelial cells to a variety of extracellular matrix proteins (47), suggesting that PECAM-1 may function to limit the intensity and/or duration of integrin-mediated adhesion and signaling during such processes as thrombus formation and angiogenesis.
Finally, many members of the Ig-ITIM family have recently been shown to have closely related cousins, the extracellular domains of which share up to 96% sequence similarity, but whose cytoplasmic domains are devoid of ITIMs (48, 49). Thus, FcγRIIb (CD32), with its cytoplasmic ITIM, binds IgG and shuts down signaling, whereas its nearly identical but ITAM-containing cousin, FcγRIIa (paradoxically, also known as CD32), has the opposite effect. Similarly, the action of KIRs may be countered by killer activatory receptors(KARs), PIR-B by PIR-A, ILT-2 and ILT-3 by ILT-1, and SIRP-α by SIRP-β (48). Whether PECAM-1 has an antagonistic activatory counterpart (PECAM-2?) and how, in general, inhibitory receptors interact with activating receptors to regulate signaling pathways in blood and vascular cells during such events as thrombosis, inflammation, angiogenesis, and the immune response remain fruitful areas for future investigation.
Acknowledgments
I am grateful to Debra K. Newton-Nash for helpful discussions and for critically reading the manuscript, to Steven M. Albelda for his comments and for suggesting part of the title for this brief review, and to Ronggang Wang for allowing me to cite his unpublished data on the functional inhibitory activity of PECAM-1. PECAM-1 research in the author's laboratory is supported, in part, by grants HL-44612 and HL-40926 from the National Institutes of Health.
References
- 1.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman PJ, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 4.Cambier JC. Inhibitory receptors abound? [comment] Proc Natl Acad Sci USA. 1997;94:5993–5995. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 6.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 7.Isakov N. ITIMs and ITAMs. The Yin and Yang of antigen and Fc receptor-linked signaling machinery. Immunol Res. 1997;16:85–100. doi: 10.1007/BF02786325. [DOI] [PubMed] [Google Scholar]
- 8.Ottinger EA, Botfield MC, Shoelson SE. Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem. 1998;273:729–735. doi: 10.1074/jbc.273.2.729. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi H, Kubota M, Ohtake A, Sato A, Sano S. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J Biol Chem. 1996;271:25569–25574. doi: 10.1074/jbc.271.41.25569. [DOI] [PubMed] [Google Scholar]
- 10.Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- 11.Beauchemin N, et al. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- 12.Modderman PW, von dem Borne AEGKr, Sonnenberg A. Tyrosine phosphorylation of P-selectin in intact platelets and in a disulfide-linked complex with immunoprecipitated pp60c-src. Biochem J. 1994;299:613–621. doi: 10.1042/bj2990613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds PECAM-1 and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1 and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- 16.Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 17.Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- 18.Wang R, Paddock C, Augustine JA, Newman PJ. Oxidative stress triggers PECAM-1-mediated signaling pathways. Blood. 1998;92:548a. (Abstr.) [Google Scholar]
- 19.Ochi H, et al. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 induced by lysophosphatidyl choline in cultured cells. Biochem Biophys Res Commun. 1998;243:862–868. doi: 10.1006/bbrc.1998.8198. [DOI] [PubMed] [Google Scholar]
- 20.Sagawa K, Swaim W, Zhang J, Unsworth E, Siraganian RP. Aggregation of the high affinity IgE receptor results in the tyrosine phosphorylation of the surface adhesion protein PECAM-1 (CD31) J Biol Chem. 1997;272:13412–13418. doi: 10.1074/jbc.272.20.13412. [DOI] [PubMed] [Google Scholar]
- 21.Varon D, et al. Platelet/endothelial cell adhesion molecule-1 serves as a co-stimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood. 1998;91:500–507. [PubMed] [Google Scholar]
- 22.Williams AF, Barclay AN. The immunoglobulin superfamily. Domains for cell surface recognition. Ann Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham BA, et al. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 24.Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 (PECAM-1) reveals alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- 25.Baldwin HS, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during early mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 26.Alley TL, Cooper MD, Chen M, Kubagawa H. Genomic structure of PIR-B, the inhibitory member of the paired immunoglobulin-like receptor genes in mice. Tissue Antigens. 1998;51:224–231. doi: 10.1111/j.1399-0039.1998.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 27.Schimmenti LA, Yan H-C, Madri JA, Albelda SM. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol. 1992;153:417–428. doi: 10.1002/jcp.1041530222. [DOI] [PubMed] [Google Scholar]
- 28.Lu TT, Yan LG, Madri JA. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CS, Wang T, Madri JA. Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab Invest. 1998;78:583–590. [PubMed] [Google Scholar]
- 30.Tanaka Y, et al. CD31 expressed on distinctive T cell subsets is a preferential amplifier of β1 integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockinger H, et al. Phenotype of human T cells expressing CD31, a molecule of the immunoglobulin supergene family. Immunology. 1992;75:53–58. [PMC free article] [PubMed] [Google Scholar]
- 32.Ashman LK, Aylett GW. Expression of CD31-epitopes on human lymphocytes. CD31-monoclonal antibodies differentiate between naive (CD45RA+) and memory (CD45RA-) CD4-positive T-cells. Tissue Antigens. 1991;38:208–212. doi: 10.1111/j.1399-0039.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 33.Torimoto Y, Rothstein DM, Dang NH, Schlossman SF, Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992;148:388–396. [PubMed] [Google Scholar]
- 34.Leavesley DI, et al. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+ hemopoietic progenitor cells. J Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- 35.Piali L, et al. Murine platelet enbdothelial cell adhesion molecule (PECAM-1/CD31) modulates β2 integrins on lymphokine-activated killer cells. Eur J Immunol. 1993;23:2464–2471. doi: 10.1002/eji.1830231013. [DOI] [PubMed] [Google Scholar]
- 36.Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- 37.Berman ME, Xie Y, Muller WA. Roles of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and β2 integrin activation. J Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- 38.Neel BG. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- 39.Newton-Nash D, Oza S, Newman PJ. A new role for PECAM-1 (CD31): inhibition of T-cell receptor-mediated signal transduction. Blood. 1998;92:338a. (Abstr) [PubMed] [Google Scholar]
- 40.Ravetch JV. Fc receptors. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 41.Watson SP, Gibbins J. Collagen receptor signalling in platelets: extending the role of the ITAM. Immunol Today. 1998;19:260–264. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- 42.Sun Q-H, et al. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 44.Tivol EA, et al. Loss of CTLA-4 leads to a massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 46.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, et al. PECAM-1-mediated signal transduction can be initiated by cell adhesion to extracellular matrix. Blood. 1998;92:29a. (Abstr.) [Google Scholar]
- 48.Vely F, Vivier E. Conservation of structural features reveals the existence of a large family of inhibitory cell surface receptors and noninhibitory/activatory counterparts. J Immunol. 1997;159:2075–2077. [PubMed] [Google Scholar]
- 49.Colonna M. Unmasking the killer's accomplice. Nature. 1998;43:642–643. doi: 10.1038/35515. [DOI] [PubMed] [Google Scholar]
- 50.Steffens U, Vyas Y, Dupont B, Selvakumar A. Nucleotide and amino acid sequence alignment for human killer cell inhibitory receptors. 1998. Tissue Antigens. 1998;51:398–413. doi: 10.1111/j.1399-0039.1998.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 51.Kharitonenkov A, et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann U, Grunert F, Zimmerman W. Carcinoembryonic antigen family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 53.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]