INTRODUCTION
Sleep difficulties have long been recognized in children with attention-deficit/hyperactivity disorder (ADHD), to the point that in the past, restless sleep was a diagnostic criterion.1 Complaints of significant sleep problems are often reported on parent-report questionnaires.2–5 Problems include delayed sleep onset, night wakings, increased movement during sleep, and increased bedtime resistance. Consistent abnormalities have not been documented on polysomnography and actigraphy.5 It has been suggested that disconnects between parent-report instruments and objective measures are not contradictory, but that questionnaire measures are sensitive to problems not detected by polysomnography or actigraphy (e.g., bedtime resistance), and vice versa.5 Associations between sleep problems and poorer quality of life measures for both children with ADHD and their families have been documented.2
While approximately a third of ADHD cases have no comorbidity,6, 7 33% have at least one, including anxiety (25%) or mood disorders (15–75%).8, 9 Combinations of comorbidities are also noted, with 19%, 8%, 3% and 1% of one cohort having two, three, four and five additional comorbid diagnoses besides ADHD respectively.7 Although neuropsychiatric conditions including depression and anxiety are known to adversely affect sleep,10 it is unclear whether sleep problems in ADHD are attributable to ADHD itself, or to associated comorbidities.
Our main objective was to test the separate associations of comorbid anxiety and depression with overall sleep disturbance scores on a pediatric sleep questionnaire in children with ADHD. Our secondary objective was to identify specific subscales that differed between subjects with and without psychiatric comorbidities. We hypothesized that children with ADHD and comorbidities would have higher overall sleep disturbance scores than those without comorbidities.
METHOD
Study Subjects
This study consisted of a cross-sectional ancillary analysis of an established cohort of children with ADHD enrolled in a genetics study.7, 11, 12 Subjects were recruited from specialty and general pediatric clinics in the greater Philadelphia area. This protocol was approved by the Institutional Review Boards of The Children’s Hospital of Philadelphia and the University of Pennsylvania School of Medicine. Parents and 18-year-old subjects provided consent; children under 18 years, assent. The protocol for the present ancillary study was approved by the Institutional Review Board of Johns Hopkins Medical Institutions, where the first author subsequently moved.
The inclusion criteria for the Genetics of ADHD Study included ages 6–18 years, symptoms of ADHD, and availability of both biological parents to participate in genetics testing. The study was limited to Caucasians of European descent to assemble a more genetically homogenous study population.7
Exclusion criteria for the Genetics of ADHD Study included < 36 weeks’ gestational age; major medical conditions (except asthma), neurologic disorders (e.g., epilepsy); and major psychiatric disorders (psychosis, bipolar disorder and pervasive developmental disorders). Children with onset of major depressive symptoms prior to onset of ADHD symptoms, or ADHD symptoms primarily during episodes of depression, were excluded. Children with documented IQ scores < 75, history of cognitive impairment, or inability to understand and complete the Schedule for Affective Disorder and Schizophrenia for School-Age Children-P-IVR (K-SADS), were also excluded. The K-SADS was administered by a single experienced investigator (JE).
For inclusion in this ancillary sleep analysis, subjects had to have been diagnosed with ADHD via the K-SADS administered in the parent study. In addition, we required completion of the Children’s Sleep Habits Questionnaire (CSHQ). This study refers only to diagnoses classified on the K-SADS as present within the last 12 months. Subjects with one or more anxiety diagnoses (generalized anxiety disorder, overanxious disorder, panic disorder, separation anxiety, social anxiety, specific phobias) on the K-SADS and without mood disorder diagnoses qualified for membership in the anxiety group; likewise subjects with one or more mood disorder diagnoses (major depression, minor depression, dysthymic disorder) without anxiety diagnoses qualified for membership in the depression group. Subjects with both anxiety and mood disorders were excluded from analysis. We further excluded children identified by the K-SADS as having psychiatric disorders with effects on sleep that are integral to their diagnostic criteria; namely, post-traumatic stress disorder and cyclothymia.13
Assessment
Each subject and parent(s) were separately administered the K-SADS, a semi-structured interview, to identify ADHD and psychiatric comorbidities.14 The K-SADS was originally developed for use in research on depression in children and is one of the most widely used and best researched neuropsychiatric diagnostic structured interviews for children and adolescents.14 It has been widely used in ADHD research in the U.S. and across the world, including as a gold standard comparator.15–23 Diagnoses were keyed to the Research Diagnostic Criteria for syndromes similar in children and adults24; other diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised, and 4th Edition (DSM IIIR/IV) criteria.13
There are several versions of the K-SADS, and the version used for this study (IVR) rates each symptom on a graded severity basis rather than using dichotomous variables as in other versions. This allowed for a composite severity rating score of ADHD as well as the other neuropsychiatric disorders. To meet diagnostic criteria for ADHD, in addition to having six out of nine symptoms in the inattentive and/or hyperactive-impulsive categories as per DSM-IV criteria, an overall severity threshold also had to be met, including impairment in two or more settings. Subtypes of ADHD were also assigned via the K-SADS.
Because the parent genetics study’s goal was to establish phenotypes encompassing not only ADHD diagnosis but associated comorbidities, the K-SADS was chosen as it incorporates DSM diagnostic criteria for affective, anxiety, and behavioral disorders. The total interview time is approximately three hours, with subject and parent interviewed separately. In the case of major discrepancies, parent and subject are interviewed together for clarification.
Thus, the semi-structured nature of the K-SADS ensures both that DSM diagnostic criteria are addressed, and that the trained clinician conducting the interview integrates information from various settings thus providing a more rigorous assessment as well as the flexibility to investigate symptoms beyond the limits of a scripted interview or questionnaire.
Sensitivity and specificity of diagnostic measures can be challenging to assess for behavioral and psychiatric diagnoses which do not have tests which are truly gold standards. Concurrent validity of K-SADS has been documented with the CBCL (both internalizing and externalizing scales), Conners Parent Rating Scale, and SCARED-P.25 Test-retest reliability compared favorably with other structured interviews such as the DISC-2 and DICA-C.25
The K-SADS interview incorporates information from both parents and children to identify disorders occurring within the past year and the week prior to administration.14 It has been validated in children ages 6–17 years.25 The K-SADS has been compared with the Connors Parent Rating Scale, Internalizing and Externalizing subscales of the Child Behavior Checklist, Screen for Child Anxiety Related Emotional Disorders, Beck Depression Inventory, and Hamilton Depression Rating Scale.14, 25, 26 Information regarding stimulant use within the week prior to the evaluation was collected. Intraclass correlations (ICCs) were calculated for a 344 subject subset of the original genetics cohort comparing diagnoses by the investigator administering the K-SADS with another clinician experienced in the K-SADS who reviewed videotaped interviews. All ICC values were significant (P < 0.05).12
The child psychiatrist (JE) who originally administered the K-SADS records subsequently reevaluated the results to determine if subjects initially meeting anxiety or mood disorder criteria still met criteria if sleep symptoms were excluded from their comorbidity diagnoses.
Parents were instructed to self-administer the CSHQ, a 33 item parent-response instrument that screens for sleep complaints and has been validated in children four to ten years of age.27, 28 However, it has previously been used in older children, such as in the multisite Autism Treatment Network (ATN).29 The rationale for use of the CSHQ with this cohort was similar to that expressed by Goldman, et al., in analysis of sleep issues in the ATN cohort: “Although not validated in ages eleven and older, to maintain consistency of questionnaires within the ATN (i.e., avoid using a different questionnaire for the ATN adolescents), the CSHQ was also administered to adolescents with ASD to ascertain the extent of sleep problems in that group.”29 Similarly our intent was to compare children and adolescents with ADHD with each other, rather than with an external standard.
The original genetics study only implemented the CSHQ after 75 of the 500 participants had enrolled. CSHQ items were rated on a 3-point Likert scale reflecting frequencies of “rarely”, “sometimes”, and “usually”. Eight subscales focus on symptoms associated with sleep disorders categories: Bedtime Resistance, Sleep Onset Delay, Sleep Duration, Sleep Anxiety, Night Wakings, Parasomnias, Sleep Disordered Breathing, and Daytime Sleepiness. These parallel symptom clusters associated with the revised International Classification of Sleep Disorders (ICSD) classifications most common in children.30 Higher subscale scores indicate more complaints. Individual items were summed to obtain a Total Sleep Disturbance Score (TSDS). Previously published internal consistency was adequate for both community and clinical samples. Alpha coefficients of CSHQ subscales for the community sample ranged from 0.36–0.70, and for the clinical sample, 0.44 to 0.83.27 Acceptable test-test reliability was obtained in a subset of the community sample. A TSDS score of 41 yielded a sensitivity of 0.80 and specificity of 0.72 to correctly identify a clinical sample of children diagnosed with sleep disorders in a pediatric sleep clinic.27
The study asked parents to complete the Sleep Related Breathing Disorder scale of the Pediatric Sleep Questionnaire (PSQ) to screen children for obstructive sleep apnea (OSA).31 This scale consists of 22 items representing symptoms of OSA, divided into three subscales: snoring, sleepiness, and behavior. A cutoff score for abnormality yielded a sensitivity of 0.85 and specificity of 0.87 for polysomnographically diagnosed obstructive sleep-related breathing disorders in the original sample.31
DATA ANALYSIS
Subject characteristics were compared across three groups: ADHD with anxiety, ADHD with depression, and ADHD without anxiety or depression. A non-parametric equality of medians test was used to test for differences in ages among groups. Chi-squared tests were used to test for differences in binary or categorical variables: sex, ADHD subtype, and use of stimulants in the week prior to K-SADS administration. Wilcoxon rank sum tests were used to compare PSQ scores for anxious and depressed groups to the no comorbidity group.
Wilcoxon rank sum tests were used to compare CSHQ total and subscale scores for the anxious and depressed groups to the no comorbidity group, because scores were not normally distributed. Subjects with completion of ≥ 31/33 items on CSHQ were included in analysis of TSDS. Subjects with any individual subscales for which all items were completed were included in analysis of those subscales. Similar post-hoc analyses were performed for subjects with both anxiety and mood diagnoses comparing them with the no comorbidity group.
Sensitivity analysis was performed to determine the contribution of sleep symptoms to diagnoses of comorbidities by K-SADS. We determined whether subjects in the depression and anxiety groups would retain their classifications if sleep symptoms that contributed to diagnoses were disregarded. Subjects who no longer met criteria for an anxiety or mood comorbidity without the contribution of sleep symptoms were removed from their comorbid groups, and analyses repeated.
Multivariate regression modeling was performed to evaluate predictors of sleep disturbance in this cohort.
Many subscales on the CSHQ were highly correlated with each other and TSDS. For example, Spearman correlations between TSDS for subjects who completed the entire CSHQ and each of the subscales ranged from 0.2 to 0.70 (P < 0.001 for all). This is expected, as in several cases, certain items were used to calculate >1 subscale, and all 33 items are summed for TSDS. Therefore we did not correct for multiple comparisons, because the typical correction for multiple comparisons (Bonferroni) assumes independent outcomes. Rather, we examined results in terms of internal consistency.
RESULTS
Study Group
500 subjects with ADHD were recruited for the parent genetics study. Of the 425 enrolled after addition of the CSHQ to the genetics study protocol, 10 failed exclusion criteria and an additional 60 with both anxiety and depression were excluded from planned analyses. Among the 355 remaining, 38 did not complete ≥ one CSHQ subscale. Thus, 317 subjects were analyzed for ≥ one CSHQ subscale: 195 without comorbidities, 60 anxious and 62 depressed. Median age (range) was 8.9 (6–18.7) years. 78% of subjects were male. 264 of these completed ≥ 31 CSHQ items: 161 without comorbidities, 50 anxious and 53 depressed.
Those 38 subjects who enrolled after addition of the CSHQ to the protocol who had no other reason for exclusion yet failed to complete ≥ one CSHQ subscale, had a higher proportion of girls (45% vs. 22%, P = 0.002) and older age (11.3 ± 3.2 years vs. 9.7 ± 3.1, P = 0.003) than subjects who completed ≥ one subscale. They also had a larger proportion with anxiety (39% vs. 19%, P = 0.003). However, non-completers did not differ significantly from the 317 in proportion diagnosed with mood disorder or neither comorbidity; ADHD subtype; or stimulant use (P > 0.05 for all).
Table 1 details characteristics of the three groups who completed ≥ one subscale of the CSHQ: subjects without psychiatric comorbidity, with anxiety and with depression. There were no significant differences among the groups in terms of age, sex, ADHD subtypes or stimulant use in the week prior to K-SADS administration. Characteristics of subjects who completed ≥ 31 CSHQ items were similar.
Table 1.
Subject Characteristics.
| No Comorbidities |
Anxiety | Depression | P values | |
|---|---|---|---|---|
| N | 195 | 60 | 62 | |
| Age, years [median (range)] | 8.8 (6–18.7) | 8.9 (6–17.8) | 10.3 (6–17.8) | 0.1 |
| Sex, n (% male) | 153 (78) | 41 (68) | 53 (85) | 0.1 |
|
ADHD subtype, n (%) Inattentive Hyperactive Combined Not Otherwise Specified |
55 (28) 19 (10) 120 (62) 1(1) |
19 (32) 6 (10) 34 (57) 1 (2) |
17 (27) 4 (6) 41 (66) 0 (0) |
0.9 |
| Stimulant use in past week, n (%) | 26 (13) | 10 (17) | 15 (24) | 0.1 |
| Disruptive behavior disorder, n (%) | 65 (33) | 15 (25) | 46 (74) | <0.001 |
Psychiatric Comorbidities and Sleep
Median TSDS for subjects without comorbidities was 44 (inter-quartile range = 40–49); for subjects with anxiety, 48 (inter-quartile range = 43–54); and for subjects with depression, 46 (inter-quartile range = 41–52). Compared with subjects without comorbidities, TSDS in anxious subjects was greater (P = 0.008). TSDS in depressed subjects was not significantly different from those without comorbidities. Post-hoc analysis of TSDS in subjects with both anxiety and mood diagnoses also was not significantly different from those without comorbidities.
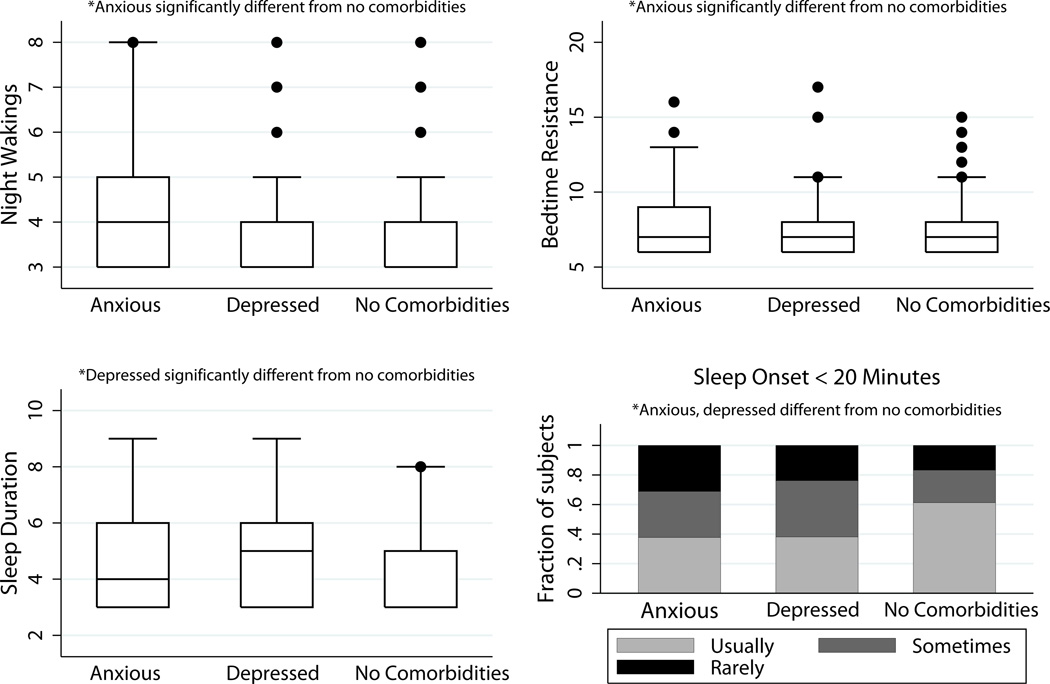
Comparisons of subscores are summarized in Table 2. Compared with subjects without comorbidities, Bedtime Resistance, Sleep Onset Delay and Night Wakings subscales were higher in anxious subjects (P = 0.03, 0.007, & 0.007 respectively). Sleep Onset Delay and Sleep Duration subscales were higher in depressed subjects (P = 0.003 & 0.01 respectively). These differences are illustrated in Figure 1. The Sleep Onset Delay subscale consists of a single item: whether a child falls asleep within 20 minutes after going to bed. There was a higher proportion without comorbidities who usually had a sleep onset of ≥ 20 minutes (61%) versus anxious (38%, P = 0.007) or depressed (37%, P = 0.003). The remaining subscales did not differ between groups. Post-hoc analyses of subscales in subjects with both anxiety and mood diagnoses also had a lower proportion of subjects with sleep onset of < 20 minutes (28%, P < 0.0001), while other subscales did not differ for this group.
Table 2.
Children’s Sleep Habits Questionnaire subscale scores. P-values refer to the comorbidity group as compared with the no comorbidity group.
| Subscale [Median (range) (P- value)] |
Anxiety | Depression | No Comorbidities |
|---|---|---|---|
| Bedtime Resistance | 7.5 (6–16) (0.03) | 7 (6–17) (0.8) | 7 (6–15) |
|
Sleep Onset Delay % Rarely asleep ≤ 20 minutes % Sometimes % Usually (P-value) |
30 32 38 (0.007) |
24 39 37 (0.003) |
17 22 61 |
| Sleep Duration | 4 (3–9) (0.4) | 5 (3–9) (0.01) | 3 (3–8) |
| Sleep Anxiety | 5 (4–11) (0.1) | 4 (4–10) (0.3) | 4 (4–11) |
| Parasomnias | 9 (7–12) (0.2) | 9 (7–13) (0.4) | 8.5 (7–15) |
| Sleep- Disordered Breathing | 3 (3–5) (0.8) | 3 (3–8) (0.9) | 3 (3–7) |
| Night Wakings | 4 (3–8) (0.007) | 3 (3–8) (1) | 3 (3–8) |
| Daytime Sleepiness | 13 (8–22) (0.3) | 14 (8–22) (0.2) | 13 (8–22) |
Figure 1. CSHQ subscales differing between comorbidities and no comorbidities.
Several Children’s Sleep Habits Questionnaire (CSHQ) subscales differ between subjects with and without comorbidities, as shown in these box plots. In box plots, the line within the box marks the median. The boundary of the box closest to zero indicates the 25th percentile and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. Outlying points are also marked. Anxious subjects had higher scores compared with those without comorbidities on Night Wakings and Bedtime Resistance subscales (P = 0.007 and 0.03, respectively). Depressed subjects had significantly higher scores compared with those without comorbidities on Sleep Duration subscale (P = 0.01). Sleep Onset Delay subscale results are displayed in histograms. Both anxious and depressed subjects differed significantly from those without comorbidities on the single question Sleep Onset Delay subscale, with higher proportions reporting longer sleep onset (P = 0.007 and 0.003, respectively).
When subjects meeting anxiety criteria were reevaluated to determine whether they would still meet criteria if sleep symptoms were excluded from diagnoses, only three subjects with anxiety who completed the CSHQ lost their comorbidity categorization. Medians and ranges for subscales were largely unchanged. This sensitivity analysis yielded similar results for TSDS and subscales: anxious subjects still had higher TSDS scores, Bedtime Resistance, and Sleep Onset Delay subscale scores (P < 0.05 for all). Night Wakings subscale scores for anxious subjects retained the same median and range as before sensitivity analysis but were no longer significantly different from those without comorbidity (P = 0.054).
Pediatric Sleep Questionnaire
There was no difference in PSQ scores between anxious subjects and those without comorbidities who completed the PSQ (medians 0.29 vs.0.31, P = 0.5). Although total scores were greater in depressed subjects compared to those without comorbidities (medians 0.36 vs. 0.31; P = 0.007), post hoc analysis indicated no differences in the snoring subscale (P = 0.8) indicative of OSA; behavior and sleepiness subscales were significantly higher (P < 0.05). This pattern did not suggest that OSA was genuinely more frequent in depressed subjects.
Multivariate Regression Analysis
Multivariate regression analysis looking at factors potentially contributing to TSDS (Table 3), revealed that anxiety and PSQ > 0.33 were independently associated with higher TSDS (P = 0.002 and 0.001 respectively; adjusted R2 = 0.27). Age, sex, depression, ADHD subtype, and stimulant use in the last week were not associated with TSDS.
Table 3.
Multivariate regression variables potentially contributing to Children’s Sleep Habits Questionnaire total scores.
| Variable | β-coefficient | 95% confidence interval |
P-value |
|---|---|---|---|
| Anxiety | 3.4 | 1.2 to 5.7 | 0.002 |
| Abnormal PSQ score | 7.6 | 5.8 to 9.4 | <0.001 |
| Age (per year) | 0.1 | −0.3 to 0.4 | 0.7 |
| Male Sex | 1.2 | −0.8 to 3.2 | 0.3 |
| Depression | −0.2 | −2.4 to 2.1 | 0.9 |
|
ADHD Subtype vs. inattentive subtype ADHD-hyperactive subtype (compared with inattentive) ADHD-combined subtype (compared with inattentive) |
−0.6 1.1 |
−3.8 to 2.5 −1 to 3.2 |
0.7 0.3 |
| Stimulant use in last week | −1.3 | −3.5 to 1 | 0.3 |
DISCUSSION
In this study, anxious children with ADHD had higher overall sleep disturbance scores on CSHQ than children with ADHD without psychiatric comorbidities. Specific subscales that drove these higher scores, Bedtime Resistance, Sleep Onset Latency and Night Wakings, can be summarized as involving sleep initiation and maintenance. Depressed children with ADHD did not have higher overall sleep disturbance scores than children without comorbidities, but they had higher subscale scores in Sleep Onset Latency as well as Sleep Duration. Sleep Onset Latency scores were associated with the presence of psychiatric comorbidity in our sample with ADHD.
Several studies have addressed effects of comorbidities on sleep in children with ADHD. Corkum and colleagues evaluated multiple groups of subjects ages 6–12: unmedicated ADHD, medicated ADHD, subjects with other psychiatric diagnoses, and healthy nonclinical subjects.4 Similar to our subjects, they underwent a structured diagnostic interview. This influential publication evaluated potential confounders for sleep difficulties in children with ADHD. It found that Generalized Anxiety Disorder and Separation Anxiety Disorder were associated with sleep-related involuntary movements rather than dyssomnias (a factor including difficulty arising and sleep onset problems). Our study identified differences in anxious children with ADHD not only in constructs similar to their dyssomnias factor, but also in night wakings (which Corkum et al. classed with parasomnias).
Mick et al. looked at a sleep questionnaire in children with ADHD vs. typically developing controls.32 Following adjustment for age, pharmacotherapy, and psychiatric comorbidity as defined by K-SADS, this group found little residual association of ADHD with problem sleep. Multiple comorbid anxiety disorders carried the highest risk of sleep problems. The identification of risk associated with anxiety is consistent with our findings. Our use of an instrument which parallels ICSD pediatric sleep diagnoses provides additional information about areas of sleep difficulty. Relatively few of our subjects endorsed stimulant or other psychotropic medication. We speculate that the parents of subjects who enrolled often sought an initial clinical evaluation for ADHD. Therefore our sample may have been underpowered evaluate differences in sleep based on medication use.
Mayes et al. compared a 10-item sleep problems subscale of a parent-report instrument in a mixed anxiety/depression group with ADHD, a group with ADHD without anxiety or depression, and a control group without ADHD.33 They found higher combined subscale scores in the anxiety/depression subgroup and higher scores for certain individual items: difficulty falling asleep, restless sleep, waking too early, and sleeping less than normal. Our findings, too, highlight sleep onset as problematic in subjects with comorbidities, which our study was able to identify as associated with depression and anxiety separately, as well as in a mixed group.
Most recently, Hansen and colleagues in Norway compared children with ADHD with and without anxiety, children with anxiety only, and controls (N=25, 39, 41, and 36, respectively for a total of 141), with diagnoses based on K-SADS PL interviews.34 Interestingly this study of medication free children showed no differences in sleep onset associated with anxiety, but rather, differences in nighttime waking as identified in our study, bedtime resistance, and perhaps surprisingly, daytime sleepiness. This Norwegian study confined their subjects to a narrower age range, 7–13 years of age.
Neuropsychiatric studies of school-aged children indicate subjective sleep difficulties are frequently associated with depression, anxiety and behavioral problems such as ADHD.10 The relationship is complex and bidirectional since emotional and behavioral problems can affect sleep.35
The association between sleep and depression has been more clearly delineated in adults than children. In adults, mood disorders such as major depressive disorder have been associated with insomnia (with increased sleepiness in a subset), early morning awakening, and fatigue.36 Sleep phenotype in children is less defined, consistent with our study, in that few significant differences were found between children with and without depression. Depressed children and adolescents have reported insomnia, hypersomnia, night wakings, fatigue, and poor sleep quality.37
A sleep phenotype for anxiety is perhaps better defined in children than in adults. Clinical samples indicate significant sleep disturbances in children with generalized anxiety.38, 39 In one study, 88% of children with anxiety disorders had ≥ one sleep-related problem such as insomnia, nightmares, and reluctance to go to sleep. 55% had ≥ three problems, which decreased after pharmacological treatment of anxiety.40 It makes sense that hyperarousal and preoccupations associated with anxiety interfere with sleep, specifically initiation and maintenance.
There were several limitations to this study. Subjects in this study were evaluated using a research diagnostic instrument, and were not consistently diagnosed in accordance with the American Academy of Pediatrics guidelines for diagnosis of ADHD, which recommend use of classroom teachers as informants in addition to parents.41 Therefore, there may be differences between our cohort and children who were diagnosed with ADHD in the community. A control group without ADHD had not been recruited for the original study so was not available for analysis. Some subjects had incomplete data, and did not complete CSHQ, PSQ, or both. While there were many similarities between CSHQ completers and non-completers, non-completers were older and more likely to be female and anxious.
Differences in TSDS among subjects with ADHD and anxiety and subjects with ADHD and no comorbidity were relatively small in magnitude, although statistically significant. However, the CSHQ screens for multiple sleep issues, only some of which were hypothesized to differentially affect children with comorbidities. Detection of these differences affecting TSDS in anxious and depressed groups speaks to robust differences, primarily in the question of sleep onset latency, rather than random variation.
Because this cohort was assembled for a genetics study and deliberately limited to Caucasian children of European descent, generalizability of findings to other ethnic or racial groups may be limited.
Self-report sleep data was not obtained in older children and adolescents who would be able to provide it. Parents may be less aware of sleep issues in this older age group since they likely do not monitor teenagers’ sleep as much as that of younger children.42 A national poll of 1500 adolescents in 2006 found that fewer than half adolescents self-reported adequate sleep duration, while 90% of their parents believed their child was obtaining adequate sleep.43 Therefore, it is possible that parent reports of sleep duration problems such as insomnia and night wakings if anything represent an underestimation of issues involving sleep duration. At the same time, there are sleep disorders which manifest during sleep such as snoring and parasomnias which subjects including adolescents may not be able to report due to their own lack of awareness during the symptoms. In these cases, parental report may provide more accurate information.44
The CSHQ and PSQ are screening questionnaires rather than diagnostic instruments, so it is unknown how many in this cohort would meet criteria for diagnoses of sleep disorders. Objective measures such as actigraphy or polysomnography were not performed. Children were not screened for Restless Legs Syndrome, which is believed to be prevalent among children with ADHD and may affect sleep latency.45 It is unclear, however, that this would be differentially prevalent between children with ADHD with and without psychiatric comorbidities.
Some evidence suggests that children with ADHD have a higher prevalence of OSA, which affects 1–3% of all children.46 Our study did not show a differential risk of OSA between children with ADHD with and without psychiatric comorbidities. Thus, OSA did not seem to be a confounder associated with both sleep problems and psychiatric comorbidity. In addition, OSA is unlikely to be associated with prolonged sleep latency.
The strengths of this study include use of a large, psychiatrically well-characterized cohort in which all subjects were diagnosed with ADHD, rather than inattentive or hyperactive traits that may or may not reach clinical significance. Psychiatric diagnoses were made by a single, experienced investigator, reducing interrater bias. Standardized semi-structured interview was used, compatible with DSM-III/IV criteria. CSHQ is widely used in pediatric sleep research and readily available to investigators. Sensitivity analysis was performed to ensure that sleep-related criteria for comorbidities did not significantly affect results concerning sleep.
Conclusions
In summary, children with ADHD and psychiatric comorbidities had more parent-reported problems with sleep onset latency than children with ADHD without comorbidities. Further attention to relationships between psychiatric comorbidity and difficulties falling asleep in children with ADHD is merited. Behavioral treatments and medication management for anxiety and mood disorders may have beneficial effects on sleep as well as on comorbid conditions. Psychiatric comorbidities may represent targets for treatment of sleep problems in this vulnerable population.
Acknowledgements
The authors thank the families who participated in this study; referring clinicians; Tamika Scott, research coordinator; and Paul J. Ambrosini, M.D., for review of K-SADS interviews. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
This work was supported in parts by National Institute of Health grants R01 HL58585, K23 MH066275, K24 DK076808, T32 HL007713-14, U54 RR023567, and UL1 RR024134.
Dr. Marcus has received research funding for an investigator-initiated study, the subject of which is unrelated to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have no conflicts of interest to declare.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 2.Sung V, Hiscock H, Sciberras E, Efron D. Sleep problems in children with Attention-Deficit/Hyperactivity Disorder. Arch Pediatr Adolesc Med. 2008;162(4):336–342. doi: 10.1001/archpedi.162.4.336. [DOI] [PubMed] [Google Scholar]
- 3.Ball JD, Tiernan M, Janusz J, Furr A. Sleep patterns among children with attention-deficit hyperactivity disorder: a reexamination of parent perceptions. J Pediatr Psychol. 1997;22:389–398. doi: 10.1093/jpepsy/22.3.389. [DOI] [PubMed] [Google Scholar]
- 4.Corkum P, Moldofsky H, Hogg-Johnson S, et al. Sleep problems in children with attention-deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Wiggs L, Montgomery P, Stores G. Actigraphic and parent reports of sleep patterns and sleep disorders in children with subtypes of attention-deficit/hyperactivity disorder. Sleep. 2005;28(11):1437–1445. doi: 10.1093/sleep/28.11.1437. [DOI] [PubMed] [Google Scholar]
- 6.Jensen PS, Hinshaw H, Kraemer H, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Child Adolesc Psychiatry. 2001;40(2):147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Elia J, Ambrosini P, Berrettini W. ADHD characteristics: I. Concurrent co-morbidity patterns in children & adolescents. Child Adolesc Psychiatry Ment Health. 2008;2:15. doi: 10.1186/1753-2000-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety and other disorders. Am J Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- 9.Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry. 1997;36:1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Ivanenko A, Crabtree VM, Gozal D. Sleep in children with psychiatric disorders. Pediatric Clinics of North America. 2004;51:51–68. doi: 10.1016/s0031-3955(03)00181-0. [DOI] [PubMed] [Google Scholar]
- 11.Elia J, Capasso M, Zaheer Z, Lantieri F, Ambrosini P, Berrettini W, et al. Candidate gene analysis in an on-going genome-wide association study of attention-deficit hyperactivity disorder: suggestive association signals in ADRA1A. Psychiatr Genet. 2009;19(3):134–141. doi: 10.1097/YPG.0b013e32832a5043. [DOI] [PubMed] [Google Scholar]
- 12.Elia J, Takeda T, Deberardinis R, Burke J, Accardo J, Ambrosini PJ, et al. Nocturnal enuresis: a suggestive endophenotype marker for a subgroup of inattentive attention-deficit/hyperactivity disorder. J Pediatr. 2009;155(2):244.e239–244.e235. doi: 10.1016/j.jpeds.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM IV) 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 14.Ambrosini PJ. Historical development and present status of the Schedule for Affective Disorder and Schizophrenia for School-Age Children (K-SADS) J Am Acad Child Adolesc Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kessler R, Avenevolli S, Green J, et al. National Comorbidity Survey Replication Adolescent Supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry. 2009;48(4):386–399. doi: 10.1097/CHI.0b013e31819a1cbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa N, Grevet E, Salgado C, et al. Smoking and ADHD: an evaluation of self medication and behavioral disinhibition models based on comorbidity and personality problems. J Psychiatr Res. 2011;45(6):829–834. doi: 10.1016/j.jpsychires.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Bart O, Podoly T, Bar-Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Res Dev Disabil. 2010;31:1443–1447. doi: 10.1016/j.ridd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Karam R, Bau C, Salgado C, et al. Late-onset ADHD in adults: Milder, but still dysfunctional. J Psychiatr Res. 2009;43:697–701. doi: 10.1016/j.jpsychires.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Birmaher B, Ehmann M, Axelson D, et al. Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children-a preliminary psychometric study. J Psychiatr Res. 2009;43:680–686. doi: 10.1016/j.jpsychires.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dell'Agnello G, Maschietto D, Bravaccio C, et al. Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: A placebo-controlled trial. Eur Neuropsychopharmacol. 2009;19:822–834. doi: 10.1016/j.euroneuro.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Wehmeier P, Schacht A, Escobar R, Savill N, Harpin V. Differences between children and adolescents in treatment responseto atomoxetine and the correlation between health-related quality of life and Attention Deficit/Hyperactivity Disorder core symptoms: Meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health. 2010;4:30. doi: 10.1186/1753-2000-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle A, Ferreira M, Sklar P, et al. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: suggestive linkage to 3q13. Am J Med Genet Part B. 2008;147B:1399–1411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann B, Jones J, Dabbs K, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED); Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 28.Hart CN, Palermo TM, Rosen CL. Health-related quality of life among children presenting to a pediatric sleep disorders clinic. Behavioral Sleep Medicine. 2005;3(1):4–17. doi: 10.1207/s15402010bsm0301_3. [DOI] [PubMed] [Google Scholar]
- 29.Goldman SE, Richdale A, Clemons T, Malow B. Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- 30.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2 ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 31.Chervin R, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 32.Mick E, Biederman J, Jetton J, Faraone SV. Sleep disturbances associated with Attention Deficit Hyperactivity Disorder: the impact of psychiatric comorbidity and pharmacotherapy. J Child Adol Psychopharm. 2000;10:223–231. doi: 10.1089/10445460050167331. [DOI] [PubMed] [Google Scholar]
- 33.Mayes SD, Calhoun SL, Bixler EO, et al. ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: differences in sleep problems. J Ped Psychol. 2009;34:328–337. doi: 10.1093/jpepsy/jsn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen B, Skirbekk B, Oerbeck B, Richter J, Kristensen H. Comparison of sleep problems in children with anxiety and attention deficit/hyperactivity disorders. Eur Child Adolesc Psychiatry. 2011;20:321–330. doi: 10.1007/s00787-011-0179-z. [DOI] [PubMed] [Google Scholar]
- 35.Dahl RE, Ryan ND, Matty MK, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry. 1996;39(6):400–410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 36.Benca R. Sleep in psychiatric disorders. Neurol Clin. 1996;14:739–764. doi: 10.1016/s0733-8619(05)70283-8. [DOI] [PubMed] [Google Scholar]
- 37.Ivanenko A, Crabtree V, Gozal D. Sleep and depression in children and adolescents. Sleep Med Rev. 2005;9(2):115–129. doi: 10.1016/j.smrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Masi GMS, Mucci M, Poli P, Bertini N, Milantoni L. Generalized anxiety disorder in referred children and adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(6):752–760. doi: 10.1097/01.chi.0000121065.29744.d3. [DOI] [PubMed] [Google Scholar]
- 39.Pina AA, Silverman WK, Alfano CA, Saavedra LM. Diagnostic efficiency of symptoms in the diagnosis of DSM-IV: generalized anxiety disorder in youth. J Child Psychol Psychiatry. 2002;43:959–967. doi: 10.1111/1469-7610.00100. [DOI] [PubMed] [Google Scholar]
- 40.Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:224–232. doi: 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- 41.American Academy of Pediatrics. Clinical practice guideline: diganosis and evaluation of the chidl with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105(5):1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 42.Owens JA, Mindell J. Pediatric sleep medicine: priorities for research, patient care, policy and education. A report from the conference held February 19–20, 2005, Amelia Island, Florida. J Clin Sleep Med. 2005;2(1):77–88. [PubMed] [Google Scholar]
- 43.National Sleep Foundation. Sleep in America poll, 2006. www.sleepfoundation.org. [Google Scholar]
- 44.Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9:114–121. doi: 10.1016/j.prrv.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Picchietti D, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Medicine. 2007;9:770–781. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Redline S, Tishler PV, Schluchter M, et al. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]