Abstract
Novel agents have provided new a foundation for multiple myeloma therapies. When combined with other anti-myeloma agents, these compounds significantly enhance clinical efficacy. High-dose steroids are frequently used in anti-myeloma combination regimens; however, the doses employed are often poorly tolerated, especially in patients with concurrent comorbid conditions. We hypothesized that a steroid-independent combination regimen could be developed without significant compromise of efficacy. The availability of such a regimen will be important for patients whose concurrent ailments make them poor candidates for steroid containing anti-myeloma regimens.
A phase II single institute, non-randomized clinical trial was conducted to investigate a novel steroid-free three-drug combination of bortezomib (V), pegylated liposomal doxorubicin (D), and thalidomide (T), the VDT regimen. Forty-three newly diagnosed multiple myeloma patients requiring treatment were enrolled on this study.
The overall response rate and complete response (CR) + near complete response (nCR) rate was 78% and 35%, respectively. Median time to progression was 29.5 months. Fatigue, rash, neuropathy, constipation and infections were the most common side effects.
We concluded that VDT is a tolerable and an effective regimen capable of inducing high response rates and can be employed in patients considered to be poor candidates for steroid-based treatment regimens.
Keywords: multiple myeloma, clinical trials, steroids, thalidomide, newly diagnosed multiple myeloma
INTRODUCTION
Multiple myeloma (MM) is the most common plasma cell cancer and the second most common hematological malignancy for which cure remains an elusive goal.(Jemal, et al 2008) Conventionally, high-dose steroids have been one of the most effective anti-myeloma agents with the ability to control disease rapidly. However, in MM, steroid resistance is rapid and early relapses are frequent.(Alexanian, et al 1992)
Over the past decade, several novel compounds have been approved for the treatment of patients with MM.(Orlowski, et al 2007, Rajkumar, et al 2006, Richardson, et al 2005, Weber, et al 2007) These include the immunomodulatory agents (IMiDs; thalidomide and lenalidomide), the proteasome inhibitor, bortezomib, and pegylated liposomal doxorubicin (PLD). These agents have demonstrated single-agent efficacy comparable to high-dose steroids, with particular benefit observed when administered in combination regimens. It is important to note that present treatment regimens demonstrating higher overall response rate (ORR) and complete response (CR) rates often incorporate these compounds with other anti-myeloma agents (such as melphalan, cyclophosphamide); however, high-dose steroids remain an important and consistent component of these regimens.
As the median age at diagnosis of MM is 70 years, traditional steroid dosing is often poorly tolerated due to concurrent comorbid medical conditions, such as diabetes mellitus, hypertension or other cardiovascular diseases. Also, high-dose steroid treatment can increase susceptibility to infectious complications due to their immunosuppressive effects. Recent translational studies have noted an inhibitory effect of steroids on lenalidomide-mediated natural killer (NK) cell stimulation, suggesting that steroids may compromise the efficacy of immunomodulating agents in patients with MM.{Schafer, 2008 }(Hsu, et al 2011) This hypothesis is substantiated by a recent large phase III study that demonstrated improved survival in newly diagnosed MM patients treated with the combination of lenalidomide and low dose dexamethasone as compared to lenalidomide and high dose dexamethasone.(Rajkumar, et al 2010) Taken together, these observations suggest that the development of an effective, steroid-sparing regimen can have a substantial clinical benefit in patients with MM; especially in the context of regimens incorporating immunomodulatory agents.
Bortezomib is one of the most effective single-agent therapies for MM, with the ability to induce CR in 11% of patients with relapsed or relapsed/refractory MM. This has resulted in the use of bortezomib as a critical component of most of the currently employed anti-myeloma regimens. Preclinical evaluations supported combining bortezomib with doxorubicin. This synergism is mediated, at least in part, through interruption of survival pathways involving nuclear factor (NF)-κB and P44/42 mitogen-activated protein kinase, among others, which are activated in myeloma cells in response to genotoxic stress. These findings led to the successful clinical evaluation and US Food and Drug Administration (FDA) approval of the bortezomib-PLD (VELCADE® and Doxil®) combination. (Orlowski, et al 2007, Orlowski, et al 2005)
Thalidomide has made an important impact in MM therapy. Its exact mechanism remains undetermined despite its approval and widespread use; most investigators agree that it exerts its effects on the MM microenvironment. Thus, components of the tumour microenvironment (cellular and cytokine) that support MM cell survival and foster drug resistance (Urashima, et al 1997) can be targeted using thalidomide.(D’Amato, et al 2001)
Here we report the clinical efficacy of a novel triple-drug combination that is steroid-independent. We hypothesized that concurrent targeting of the MM cell microenvironment with thalidomide may enhance the anti-myeloma effect of the bortezomib/PDL combination. We previously investigated this triple-drug combination (bortezomib, PDL and thalidomide; VDT) in MM patients with relapsed or refractory disease and demonstrated its feasibility and safety.(Chanan-Khan, et al 2009) We observed impressive efficacy in heavily pretreated and refractory patients, which encouraged us to investigate this novel steroid-independent regimen in treatment-naïve MM patients. The final results of this phase II clinical trial are presented here.
PATIENTS AND METHODS
Patient eligibility
Patients with newly diagnosed, symptomatic MM who were ≥ 18 years of age were eligible for enrollment. Patients who received steroids or local radiation for spinal cord compression were eligible for the study. Additional eligibility criteria included a Karnofsky performance status of ≥ 60%, compliance with the S.T.E.P.S (System for Thalidomide Education and Prescribing Safety) program, adequate hepatic and renal function, left ventricular ejection fraction (LVEF) of ≥ 50% and adequate bone marrow function (haemoglobin ≥ 80 g/l, platelets ≥ 75 × 109/l, and absolute neutrophil count ≥ 1 × 109/l). Pregnant and/or lactating females were excluded from the study. Additionally, patients with grade ≥ 2 peripheral neuropathy with in two weeks of enrollment, patients with hypersensitivity to boron or mannitol, and patients with uncontrolled cardiovascular disease or infections were also excluded from the study. The study was conducted at the Roswell Park Cancer Institute and was approved by the Institutional Review Board. Written informed consent was obtained from all patients.
Study design
This open-label, non-randomized, phase II study was registered at www.clinicaltrials.gov as # NCT00523848. Patients were enrolled between June 2006 and December 2008. The primary objective of the study was to determine ORR (CR+ partial response, PR) of the VDT regimen. Secondary objectives included determination of CR, time to progression (TTP) and toxicity of the VDT regimen. Using an exact one-stage design it was determined that 40 evaluable patients would need to be enrolled for the study to have at least 80% statistical power to detect 20% difference in objective response rate with the VDT regimen.
During treatment, patients were evaluated after completion of each cycle and each visit included recording of medical history, physical examination, complete blood count (CBC), serum creatinine, blood urea nitrogen, blood glucose, electrolytes, lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, billirubin, uric acid, β2-microglobulin, C-reactive protein (CRP), serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE), quantitative immunoglobulin levels, 24-h urine for creatinine clearance and urine protein electrophoresis (UPEP) along with IFE. Bone marrow aspiration and biopsy was conducted to confirm the CR status of patients and at the time of disease progression. Patients who had disease progression at any time were taken off study. These evaluations were also conducted at baseline prior to treatment initiation. In addition, skeletal surveys were performed and left ventricular ejection fraction (LVEF) was evaluated, either by echocardiogram or multi gated acquisition (MUGA) scan.
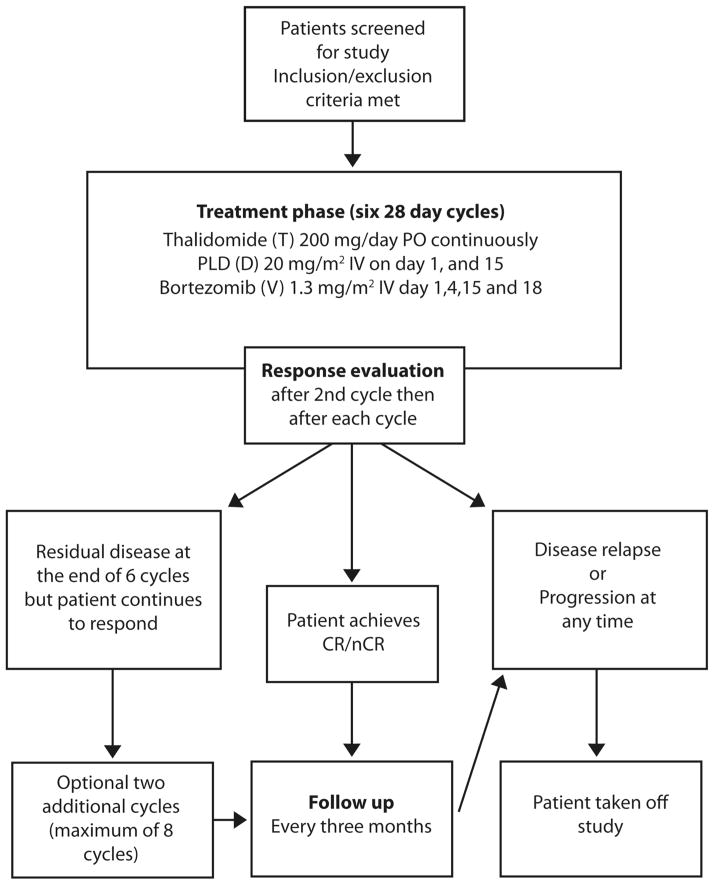
The study design is shown in Figure 1. Eligible patients were scheduled to receive six treatment cycles; however, patients with residual disease who were responding to therapy at the end of six cycles were allowed to receive two additional cycles. Patients were followed until disease relapse, progression or initiation of subsequent therapy, whichever came first. All patients had the option to proceed with high dose therapy (HDT) and autologous haematopoietic stem cell rescue after six cycles of VDT. However, since this study evaluated the efficacy of VDT as initial therapy the patients who elected to proceed with HDT (n=2) were censored at the time of HDT.
Figure 1. Consort diagram.
Clinical trial design.
PO, orally; IV, intravenously; CR, complete response; nCR, near complete response.
Response evaluation
Response was evaluated according to modified Blade’s criteria.(Blade, et al 1998) Briefly, CR was defined by absence of M-protein on SPEP, as well as negative IFE, and less than 5% plasma cells in bone marrow; near CR needed all criteria of CR except that IFE was positive. PR was defined as ≥ 50% reduction in serum M-protein and ≥ 90% reduction in urine M-protein. Patients who had < 50% but ≥ 25% reduction in serum M-protein and < 90% but ≥ 50% reduction in urine M-protein were designated as having a minimal response. The responses required confirmation by a repeat evaluation at least 6 weeks apart. Disease progression was defined as > 25% increase in serum or urine M-protein (in urine there has to be an absolute increase of > 200 mg/24 h of protein) confirmed on two occasions. For patients with CR, disease progression was defined by re-appearance of M-protein as detected by IFE and confirmed on a second occasion.
Statistical methods
Descriptive statistics such as frequencies and relative frequencies were computed for categorical variables. Numeric variables were summarized using simple descriptive statistics such as the mean, median, standard deviation, and range. Exact 95% confidence interval (95% CI) estimates of the ORR and CR rate were derived using the Clopper Pearson method. The TTP was estimated using the Kaplan-Meier method. A 0.05 nominal significance level was used in all testing. All statistical analyses were done using SAS (version 9.1).
Treatment schema
The VDT regimen comprised of bortezomib (V; at 1.3 mg/m2 given intravenously on days 1, 4, 15, and 18), PLD (D; 20 mg/m2 given intravenously on days 1, and 15), and thalidomide (T; 200 mg orally every day throughout the study) given on a 4-week treatment cycle. All patients received prophylaxis for herpes zoster with acyclovir (400 mg orally twice a day). Weight-adjusted low-dose warfarin (1 mg for patients ≤ 70 kg; 2 mg for patients > 70 kg body weight) was used, as described previously, for venous thromboembolism prophylaxis.(Miller, et al 2006) Patients with myeloma bone disease were allowed to receive standard bisphosphonate therapy.
Toxicity assessment and dose modifications
All patients who received any treatment were assessed for toxicity. The NCI common toxicity criteria for adverse events (version 3.0) were used for toxicity assessment.
Thalidomide was discontinued for any patient who experienced grade 4 non-haematological toxicity. For any associated grade 3 non-haematological toxicity (excluding constipation, somnolence and fatigue), thalidomide was held until resolution to ≤ grade 2 and restarted at a 50% reduced dose. The specific toxicities of palmar plantar erythrodysesthesia syndrome (PPE) and bortezomib-associated neuropathy were managed according to predefined dose modification criteria as described below.
Palmar plantar erythrodysesthesia
The dose of PLD was held for grade ≥ 3 PPE occurring ≤ 5 weeks after last infusion. Treatment could be resumed at a 25% decreased dose upon complete resolution of PPE. Patients in whom grade 3 or 4 PPE persisted beyond 6 weeks of the last PLD dose were removed from study.
Neuropathy
Bortezomib dose modification for sensory neuropathy, with or without neuropathic pain, was conducted as per standard prescribing guidelines provided in the package insert.
RESULTS
Forty-three patients were enrolled, of whom 40 were evaluable for response (three patients were not response-evaluable: one patient had grade 3 PPE and generalized rash during cycle 2, one patient withdrew consent within the first week of therapy, and one patient experienced pneumonia and prolonged recovery from infection). Baseline characteristics of response-evaluable patients are summarized in Table 1. The median age was 60.5 years (range 40–81). The majority of patients had intermediate to high disease burden as determined by Durie Salmon staging (88% stage II or more; 58% stage III). The median β2-microglobulin was 3.7 mg/l (range 1.6–16.5).
Table 1.
Demographics and baseline clinical characteristics of patients enrolled.
| Patients (n=40) | ||
|---|---|---|
|
| ||
| Characteristic | No. | % |
| Age, years | ||
| Median | 60.5 | |
| Range | 40–81 | |
|
| ||
| Sex | ||
| Female | 14 | 35 |
| Male | 26 | 65 |
|
| ||
| Durie Salmon stage | ||
| I | 5 | 12 |
| II | 12 | 30 |
| III | 23 | 58 |
|
| ||
| International Staging System stage* | ||
| I | 18 | 45 |
| II | 13 | 32 |
| III | 7 | 18 |
|
| ||
| Bone disease | 30 | 75 |
|
| ||
| Karnofsky Performance Score | ||
| ≤ 80% | 7 | 18 |
| > 80% | 33 | 82 |
|
| ||
| M-protein isotype | ||
| IgG | 27 | 67 |
| IgA | 12 | 30 |
| Light chain only | 1 | 3 |
|
| ||
| β2-microglobulin, mg/l | ||
| Median | 3.7 | |
| Range | 1.6–16.5 | |
|
| ||
| Lactate dehydrogenase, iu/l | ||
| Median | 444 | |
| Range | 152–1229 | |
|
| ||
| C-reactive protein, mg/l | ||
| Median | 2.2 | |
| Range | 0.3–38 | |
|
| ||
| Serum albumin, g/l | ||
| Median | 42 | |
| Range | 31–48 | |
|
| ||
| Haemoglobin, g/l | ||
| Median | 113 | |
| Range | 77–152 | |
|
| ||
| Platelet count, 109/l | ||
| Median | 230 | |
| Range | 92–565 | |
|
| ||
| Cytogenetics | 21 | 53 |
| High risk** | 8 | 20 |
| Standard risk | 13 | 33 |
Post-hoc calculation; data missing in two patients.
Among the high-risk group, all 8 patients had deletion of the long arm of chromosome 13 (13 q-) by fluorescence in-situ hybridization (FISH ); two patients also had deletion of the short arm of chromosome 17 (17 p-) by FISH; three patients had translocation between chromosome 4 and 14 (t 4; 14) by FISH, and two patients had translocation between chromosome 14 and 16 (t 14; 16) by FISH.
Toxicities
All enrolled patients (n=43) were assessed for toxicity (Table 2). No treatment-related mortality was observed. Two deaths were recorded; one patient died from rapid disease progression and one patient died of causes unrelated to disease or treatment. At least one adverse event was seen in all patients. Only five patients had grade 4 adverse events that included neutropenia, hyperuricaemia, confusion, pulmonary embolism and respiratory failure; each occurred in five different patients.
Table 2.
Incidence and severity of adverse events.
| Patients (n=43)
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
|
| ||||||||
| No. | % | No. | % | No. | % | No. | % | |
| Haematological | ||||||||
| Anaemia | 8 | 19 | 9 | 21 | 2 | 5 | 0 | 0 |
| Neutropenia | 1 | 2 | 10 | 23 | 8 | 19 | 1 | 2 |
| Lymphopenia | 8 | 19 | 9 | 21 | 11 | 26 | 0 | 0 |
| Thrombocytopenia | 6 | 14 | 3 | 7 | 2 | 5 | 0 | 0 |
|
| ||||||||
| Constitutional | ||||||||
| Fatigue | 19 | 44 | 5 | 12 | 3 | 7 | 0 | 0 |
| Dizziness | 16 | 37 | 1 | 2 | 0 | 0 | 0 | 0 |
| Confusion | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
|
| ||||||||
| Cardiovascular | ||||||||
| CHF | 0 | 0 | 0 | 0 | 2 | 5 | 0 | 0 |
| Arrhythmias | 5 | 12 | 1 | 2 | 0 | 0 | 0 | 0 |
| Oedema | 9 | 21 | 1 | 2 | 1 | 2 | 0 | 0 |
|
| ||||||||
| Gastrointestinal | ||||||||
| Nausea | 9 | 21 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhoea | 10 | 23 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 27 | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Pulmonary | ||||||||
| Dyspnea | 4 | 9 | 1 | 2 | 1 | 2 | 0 | 0 |
| Pleural effusions | 0 | 0 | 0 | 0 | 4 | 9 | 0 | 0 |
| IP | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Resp. failure | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
|
| ||||||||
| VTE | ||||||||
| DVT/PE | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 |
|
| ||||||||
| Infections | ||||||||
| Pneumonias/URI | 0 | 0 | 5 | 12 | 7 | 16 | 0 | 0 |
| Neutropenic fever | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Bacteraemia | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Others | 1 | 2 | 2 | 5 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Skin | ||||||||
| Rash | 21 | 46 | 5 | 12 | 3 | 7 | 0 | 0 |
| PPE | 5 | 12 | 3 | 7 | 3 | 7 | 0 | 0 |
|
| ||||||||
| Neuropathy | 15 | 35 | 5 | 12 | 3 | 7 | 0 | 0 |
|
| ||||||||
| Metabolic | ||||||||
| Hyperglycaemia | 12 | 23 | 3 | 7 | 2 | 5 | 0 | 0 |
| Hyperuricaemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
Abbreviations: CHF, congestive heart failure; IP, interstitial pneumonitis; VTE, venous thromboembolism; DVT/PE, deep venous thrombosis/pulmonary embolism; URI, upper respiratory tract infections; PPE, palmar plantar erythrodysesthesia.
Non-haematological toxicity
Fatigue (63%), rash (67%), and constipation (63%) were the most common non-haematological adverse events. Most of these common toxicities were mild. Only three patients (7%) with grade 3 rash required dose modifications or treatment interruptions. Venous thromboembolic events occurred in two patients (5%; one DVT and one PE). One patient developed interstitial pneumonitis after 6 cycles of therapy and was empirically treated with steroids and antibiotics. Two patients (5%) developed clinical congestive heart failure (CHF); both had stable pre-existing hypertension and coronary artery disease. Bradycardia was found to be the precipitating event for CHF in one patient and responded to thalidomide dose reduction. Neither of these two patients had a reduction in LVEF and both went on to receive additional anthracycline doses after passing cardiology evaluation.
PPE, a recognized complication of liposomal doxorubicin, was observed in 11 patients (26%). Three patients (7%) had grade 3 PPE, of whom one patient discontinued study because of persistent PPE before being evaluable for response. Sensory neuropathy, a well-known side effect of thalidomide and bortezomib, was manageable and was seen in 23 patients (53%); only three patients (7%) had grade 3 neuropathy
Haematological toxicity
Haematological toxicities were mild-to-moderate, with lymphopenia being the most common toxicity experienced by 28 patients (66%). Only one patient (2%) experienced grade 4 neutropenia, while grade 3 neutropenia and thrombocytopenia were present in eight (19%) and two (5%) patients, respectively. All cases of thrombocytopenia were asymptomatic.
Response
Of the 40 evaluable patients, 31 patients achieved PR or better for an ORR of 78% (95% CI: 61.6–89.2%; Table 3). Complete and near complete response (CR+nCR) was seen in 14 patients (35%; 95% CI: 20.6–51.7%) while nine (23%) patients achieved a CR. Although the number of patients with available cytogenetic data was small, no difference in ORR or CR rate was observed between the groups with standard (n=13) or high- risk (n=8) cytogenetic features.
Table 3.
Response to VDT among evaluable patients
| Best confirmed response | Patients (n=40) | |
|---|---|---|
| No. | % | |
| ORR (≥ PR) | 31 | 78 |
|
| ||
| CR+nCR | 14 | 35 |
|
| ||
| CRIFE- | 9 | 23 |
|
| ||
| Minimal response | 1 | 3 |
|
| ||
| Stable disease | 7 | 18 |
|
| ||
| Progressive disease | 1 | 3 |
Abbreviations: VDT, Bortezomib, pegylated liposomal doxorubicin, thalidomide; CR, complete response; nCR, near-complete response; CRIFE- immunofixation negative complete response.
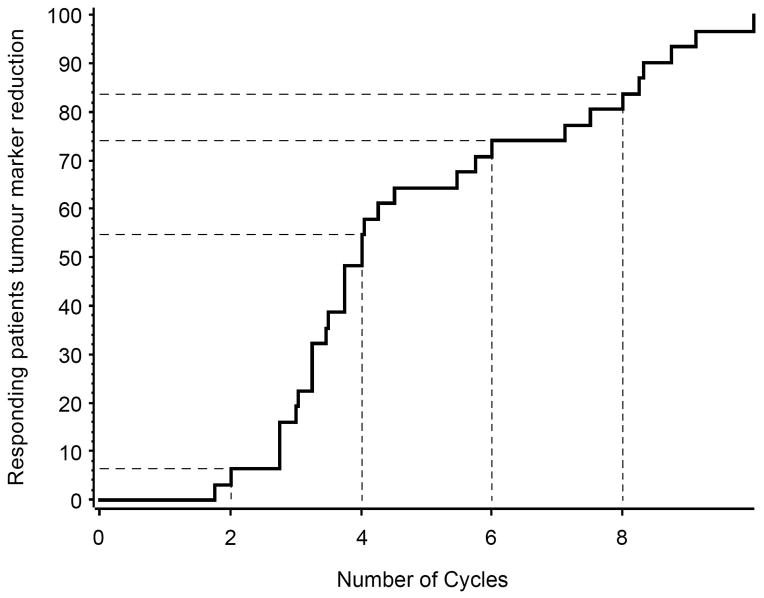
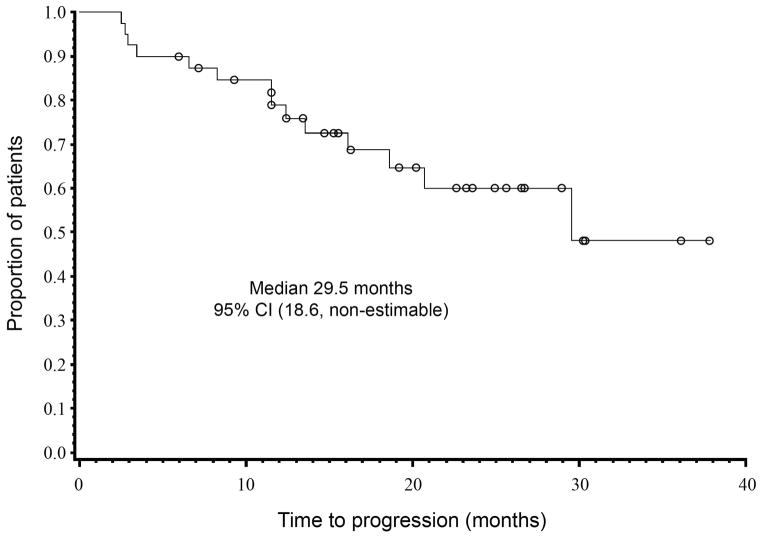
The median time to best response in patients achieving CR (n=9) was 7.4 months (range 3.4–9.2). Responding patients continued to have a decline in tumour markers even after completing treatment (Figure 2). The durability of response was reflected by a median TTP of 29.5 months (95% CI 18.6–upper limit not available; Figure 3).
Figure 2. Time to response in responding patients.
The maximum reduction in serum M-protein occurred between cycle 2 and 4 with continued after completing 8 cycles of therapy.
Figure 3. Time to progression for evaluable patients.
The time to progression (TTP) was estimated using Kaplan-Meier method and was defined as the time from start of therapy to the first evidence of disease relapse, progression or death from disease progression, whichever came first.
95% CI, 95% confidence interval; NA, not achieved.
DISCUSSION
The introduction of novel agents, such as IMiDs, bortezomib, and PLD, has evidently transformed myeloma therapy. This has resulted in substantial improvements in clinical outcomes of myeloma patients, including prolongation of survival. Although initial clinical investigations focused on the use of novel compounds as single agents, it has become clear that their incorporation into combination regimens is indispensable for the achievement of robust and durable clinical responses. Steroids have excellent activity in MM and, therefore, have been at the forefront of combination regimens. While intermittent use of steroids is relatively safe, the doses and schedule used in conventional myeloma regimens is associated with significant cardiovascular, metabolic, musculoskeletal and infectious morbidity as the majority of patients are elderly (median age 70 years). For the aforementioned reasons, efforts to develop a steroid-sparing regimen capable of delivering “deep” and durable responses seem logical.
Orlowski et al led the way by first demonstrating the feasibility and then the efficacy of bortezomib/PLD, a steroid-free regimen.(Orlowski, et al 2007, Orlowski, et al 2005) In the phase III study, it was noted that, although there was no significant difference in ORR or the CR rate between the bortezomib/PLD combination and bortezomib alone, the improvement in progression-free survival between the two groups favoured the combination regimen.(Orlowski, et al 2007) We attempted to improve the clinical benefit of this regimen by altering the dose schedule and adding the IMiD, thalidomide. As preclinical investigations have demonstrated synergism between PLD and bortezomib(Ma, et al 2003), in the VDT regimen we modified bortezomib and PLD dosing to allow the maximum overlap of these two synergistic agents, i.e. twice per treatment cycle (day 1, and 15). Moreover, this strategy permitted us to administer an increased dose of PLD per cycle (40 mg/m2 vs. 30 mg/m2). In our experience with relapsed/refractory MM patients, the VDT regimen delivered high response rates (CR rate 22%), which were durable (median progression-free survival of 10.9 months, median overall survival of 15.7 months).(Chanan-Khan, et al 2009) In the present study, the same regimen generated response rates that are comparable to some of the steroid-inclusive regimens used in frontline setting. The CR/nCR rate of 35% is a strong indicator of the regimen’s potency. We note that it took relatively longer for patients treated with VDT to achieve the best response (median time to CR of 7.4 months) as compared to steroid-inclusive regimens (San Miguel, et al 2008), which may reflect the exquisite sensitivity of myeloma to steroids. For the same reason, we believe that by employing reliable steroid-free regimens, such as VDT for frontline treatment of myeloma, the steroid sensitivity of this incurable cancer can be exploited in emergency situations, such as spinal cord compression and renal failure.
Overall, the VDT regimen was generally well tolerated, with no treatment-related mortality. Interestingly, the incidence of grade ≥ 3 neuropathy was low, thus demonstrating the feasibility of combining bortezomib and thalidomide. Although the incidence of PPE was 25%, most cases were mild and only 3 patients had grade 3 PPE. This may be related to the increased dose of PLD per cycle or due to an as yet uncharacterized pharmacokinetic interaction between the drugs. The incidence of venous thromboembolism was modest. We note that 19 patients (44%) in our study had an infectious episode with nine patients (20%) experiencing grade 3 infections. Pneumonia and/or upper respiratory infections were the most common infectious events and occurred in 11 patients (25%). Most grade 3 infectious events occurred within the first 3 months of treatment initiation, which, typically, is the time of severe immune compromise because of initial disease burden and therapy-related immune suppression.
In conclusion, we observed that VDT is a novel steroid-independent regimen for newly diagnosed MM patients that is capable of delivering high response rates. Furthermore, with the present schedule of administration, the combination of two potentially neurotoxic drugs does not appear to significantly increase the incidence of neuropathy. Given the high risk of infections observed in our study population, it appears reasonable to institute prophylactic antibiotics during the initial phase of treatment. We recommend that the clinical benefit of this promising regimen should be evaluated in a larger cohort of patients in phase III studies.
Acknowledgments
The research was in part supported by the Leukemia & Lymphoma Society (ACK) and the Jerra Barit Multiple Myeloma Research Fund (ACK).
Footnotes
AUTHOR CONTRIBUTIONS
Study design: Taimur Sher, Sikander Ailawadhi, Asher Chanan-Khan
Data analysis: Taimur Sher, Daniel Iancu, Kelvin Lee, Asher Chanan-Khan
Manuscript writing: Taimur Sher, Sikander Ailawadhi, Daniel Iancu, Aisha Masood, Kelvin Lee, Asher Chanan-Khan
Statistical analysis: Wei Tan and Gregory Wilding
Patient enrollment: Taimur Sher, Sikander Ailawadhi, Kena C. Miller, Debbie Manfredi, Margaret Wood, Myron S. Czuczman, Francisco J. Hernandez-Ilizaliturri, Frederick Hong, Raman Sood, Saif Soniwala, William Lawrence, Saad Jamshed
Final approval of manuscript: Taimur Sher, Sikander Ailawadhi, Daniel Iancu, Kelvin Lee, Asher Chanan-Khan
References
- Alexanian R, Dimopoulos M, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Musial L, Padmanabhan S, Yu J, Ailawadhi S, Sher T, Mohr A, Bernstein ZP, Barcos M, Patel M, Iancu D, Lee K, Czuczman MS. Bortezomib in combination with pegylated liposomal doxorubicin and thalidomide is an effective steroid independent salvage regimen for patients with relapsed or refractory multiple myeloma: results of a phase II clinical trial. Leuk Lymphoma. 2009;50:1096–1101. doi: 10.1080/10428190902912460. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Lentzsch S, Anderson KC, Rogers MS. Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma. Semin Oncol. 2001;28:597–601. doi: 10.1053/sonc.2001.28601. [DOI] [PubMed] [Google Scholar]
- Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, Smyth MJ, Neeson P, Ritchie DS. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117:1605–1613. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E, Neeser J, Mikail A, Adams J, Sjak-Shie N, Vescio RA, Berenson JR. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- Miller KC, Padmanabhan S, Dimicelli L, Depaolo D, Landrigan B, Yu J, Doran V, Marshal P, Chanan-Khan A. Prospective evaluation of low-dose warfarin for prevention of thalidomide associated venous thromboembolism. Leuk Lymphoma. 2006;47:2339–2343. doi: 10.1080/10428190600799631. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, Johri AR, Jones PE, Ivanova A, Van Deventer HW, Gabriel DA, Shea TC, Mitchell BS, Adams J, Esseltine DL, Trehu EG, Green M, Lehman MJ, Natoli S, Collins JM, Lindley CM, Dees EC. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Urashima M, Chen BP, Chen S, Pinkus GS, Bronson RT, Dedera DA, Hoshi Y, Teoh G, Ogata A, Treon SP, Chauhan D, Anderson KC. The Development of a Model for the Homing of Multiple Myeloma Cells to Human Bone Marrow. Blood. 1997;90:754–765. [PubMed] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]