Abstract
Highly active antiretroviral therapy (HAART) has been shown to be effective in different populations, but data among injection drug users are limited. Human immunodeficiency virus-infected injection drug users recruited into the Acquired Immunodeficiency Syndrome Link to Intravenous Experiences (ALIVE) Study as early as 1988 were tested semiannually to identify their first CD4-positive T-lymphocyte cell count below 200/μl; they were followed for mortality through 2002. Visits were categorized into the pre-HAART (before mid-1996) and the HAART eras and further categorized by HAART use. Survival analysis with staggered entry was used to evaluate the effect of HAART on acquired immunodeficiency syndrome-related mortality, adjusting for other medications and demographic, clinical, and behavioral factors. Among 665 participants, 258 died during 2,402 person-years of follow-up. Compared with survival in the pre-HAART era, survival in the HAART era was shown by multivariate analysis to be improved for both those who did and did not receive HAART (relative hazards = 0.06 and 0.33, respectively; p < 0.001). Inferences were unchanged after restricting analyses to data starting with 1993 and considerations of lead-time bias and human immunodeficiency viral load. The annual CD4-positive T-lymphocyte cell decline was less in untreated HAART-era participants than in pre-HAART-era participants (— 10/μl vs. —37/μl, respectively), suggesting that indications for treatment may have contributed to improved survival and that analyses restricted to the HAART era probably underestimate HAART effectiveness.
Keywords: antiretroviral therapy, highly active, HIV, substance abuse, intravenous, substance-related disorders, survival, treatment outcome
Highly active antiretroviral therapy (HAART) significantly improves the prognosis of human immunodeficiency virus (HIV)-infected persons by reducing HIV load, increasing cell levels of CD4-positive T lymphocytes (CD4+), delaying progression to acquired immunodeficiency syndrome (AIDS), and reducing mortality (1–7). Most clinical efficacy studies have been conducted in predominantly White male study populations; there are fewer data among minorities and injection drug users (8, 9). Data from cohort and surveillance studies have suggested less benefit of HAART among minorities and injection drug users (10, 11). While earlier studies have shown that lack of or delayed access was a major problem in these populations (12, 13), the proportion gaining access has improved over time (14). Also, because injection drug users have an excess mortality risk over their population peers related to their drug use (15), the extent to which HAART improves survival in these populations is unclear.
Among the predictors of HIV disease progression while on HAART, the CD4+ cell level is the most important (5–7). Although current clinical guidelines recommend initiating HAART when CD4+ cell levels are less than 200/ll, and possibly when 200–350/ll (16, 17), use of antiretroviral therapy for those with advanced immunosuppression has been constant since the introduction of such therapy. We therefore sought to evaluate survival among persons with CD4+ cell counts below 200/μl, stratified by the availability and use of HAART, within a large cohort of HIV-infected, predominantly African-American injection drug users followed for up to 14 years.
MATERIALS AND METHODS
Population
Participants were part of the AIDS Link to Intravenous Experiences (ALIVE) Study in Baltimore, Maryland, already described in detail elsewhere (18). The study recruited injection drug users through community outreach starting in 1988–1989, with additional recruitments in 1994 and 1998. Semiannual follow-up visits included comprehensive interviews (sexual behavior, drug use, and medical history including HAART use during the preceding 6 months), a clinical examination, and phlebotomy. The current study included 665 participants who were HIV seropositive and had at least one visit with a CD4+ cell count below 200 cells/μl. Follow-up for this analysis started with the date of the index visit, when the CD4+ cell count for each participant first dropped below 200/μl, and continued through December 31, 2002. This censoring date was determined on the basis of the last update (in 2004) of death information obtained through the National Death Index to permit lags in reporting.
Ascertainment of death and cause of death
Study staff were notified of deaths during routine follow-up contact with family members or partners of participants with missed visits. Death certificates were obtained from the Maryland State Vital Records Office for confirmation. For all participants including those lost to follow-up, records were requested from the National Death Index Plus. Confirmation of correct matches was done with the requested death certificates, medical examiner reports, and medical record review.
Cause of death was categorized (19) as HIV related if the participant was HIV infected and if the primary cause of death was AIDS/HIV or included an AIDS-defining opportunistic infection using the 1993 Centers for Disease Control and Prevention definition (20). Otherwise, death was categorized as non-AIDS related. Cause of death was classified as unknown if there was no death certificate available, or if the cause of death was not provided.
Definition of HAART groups
All observations prior to June 30, 1996, were considered pre-HAART; observations after that date were considered as being in the HAART era. Persons in the pre-HAART era could have received mono- or dual nucleoside antiretroviral therapy, anti-opportunistic infection medications, or no anti-retroviral treatment. In the HAART era, we also distinguished between individuals who reported using HAART (coded as “treatment” group) and the others (coded as “no HAART treatment”) who reported receiving other anti-retroviral or anti-opportunistic infection medications or no antiretroviral treatment (“no treatment”). The definition of HAART was based on the International AIDS Society–US panel and the US Public Health Service guidelines relevant to the study period (16, 17). Therapy use was updated at each follow-up visit and based on self-reports.
Laboratory assays
HIV antibodies were assayed using commercial tests interpreted with standard criteria. T-cell subsets were determined by flow cytometry. HIV plasma viral load was determined using reverse transcriptase polymerase chain reaction (Amplicor HIV-1 Monitor Test, version 1.5; Roche Molecular Systems, Inc., Branchburg, New Jersey) on specimens stored at —70°C prior to viral load determinations.
Statistical analysis
Mortality rates were calculated using person-time techniques. Kaplan-Meier survival curves and log-rank tests were used to compare time to death among three groups: pre-HAART era, HAART era-treated, and HAART era-untreated drug users. The time origin was defined as the study visit when CD4+ cell counts first fell below 200/μl (index visit). Group membership was updated as a time-dependent covariate, but the survival time for each person was evaluated relative to the origin. Thus, for example, a person could have an index visit in the pre-HAART era, then move to the HAART era, and sequentially contribute time to both groups, with a delayed entry (21, 22). In the HAART era, a person could switch between the treatment and no treatment groups, depending on the reported anti-HIV medications received at each visit. As there might be residual benefit for survival among those not on HAART but who had prior HAART, analyses were repeated with HAART defined as a step function, being fixed at 1 following initiation. To examine potential survivor (or frailty) bias, we also repeated the Kaplan-Meier analyses, restricting the analyses of mortality to those whose first CD4+ count of less than 200 cells/μl was in or after 1993 instead of 1988.
For the Kaplan-Meier analysis, censoring time was defined as the earliest date of the following events: 1) if the person was known to be alive at the end of the study, December 31, 2002; 2) if the person was lost to follow-up, the date of the last visit plus 9 months (or interval to the end of the study if shorter); 3) if the person died of non-AIDS-related causes, the date of death; and 4) if the person's death was AIDS related and the interval between the last visit and death was longer than 2 years, the observation was censored at the date of the last visit plus 2 years. This was necessary, as the relevance of participant data measured more than 2 years before death with regard to mortality was questionable.
Survival was examined using Cox regression models with time-dependent covariates. First, univariate associations were explored, adjusted for “lead-time bias,” using a proxy lead time variable, defined as the length of time on the study at the index visit, that is, the time interval (in years) between the first follow-up visit in the ALIVE Study and the first visit with a CD4 count of less than 200 cells/ll. From this, multivariable models were constructed to adjust simultaneously for potential confounding factors and to formally test for interactions. These factors included other HIV-related medications, clinical status, sociodemographic characteristics, alcohol and drug use, insurance status, and hospitalizations. Evaluation of the significance of associations between the various factors and survival was based on estimates of robust variances that accounted for the correlation among observations from the same participants.
To summarize longitudinal trends in CD4+ cells, in each of the three groups, we used linear regression with generalized estimating equations to adjust for intraperson correlation. Estimates of slopes are given with robust standard errors and confidence intervals.
RESULTS
From cohort recruitment, there were 917 HIV-seropositive participants at baseline, of whom 505 had a visit with a CD4 T-lymphocyte count of less than 200/μl at least once during follow-up. There were also 330 HIV sero-converters, of whom 160 had a visit with a CD4 count of less than 200/μl at least once during follow-up. Of the 665 total participants with initial CD4+ cell counts of less than 200/μl at baseline (n 93) or anytime during ALIVE Study follow-up (n = 572), the overall demographics at entry into the study were as follows: median age, 39.2 years; Black, 94.6 percent; and male, 74.6 percent. In the 6 months before the initial CD4+ cell count of less than 200/μl, 18.6 percent were homeless, and 63.7 percent injected illicit drugs, of whom 34.1 percent injected at least daily and 18.2 percent smoked crack or snorted cocaine.
The 665 participants contributed a total of 2,401.6 person-years of observation, including 977.3 person-years before and 1,424.4 person-years after the introduction of HAART. Among those in follow-up after 1996, 62.1 percent ever received HAART (of whom 48.2 percent were anti-retroviral naive before HAART). Among the 427 participants restricted to the HAART era, those who received HAART (n = 265) contributed 507.8 person-years, and those not on HAART contributed 916.6 person-years of observation.
Causes of death for the entire study period, by numbers of participants, included the following: AIDS, 222; infection, 48; drug related/overdose, 39; trauma, 7; cancer (non-AIDS), 11; and systemic, 48. “Systemic” causes included renal (including end-stage renal disease), pulmonary, cardiac (including cardiovascular/neurologic, i.e., stroke), and other neurologic (including encephalopathy) causes, as well as gastrointestinal (including pancreatic, liver, diabetes) causes and chronic alcoholism. For survival analyses, nine AIDS-related deaths were censored, since their last follow-up visit was more than 2 years before death (see Materials and Methods), and thus 213 deaths appear in the analyses. Causes of death were similar in the pre-HAART and HAART eras (data not shown).
The all-cause mortality rate in the cohort overall between study inception in 1988 and 2002 was 15.7/100 person-years (95 percent confidence interval (CI): 14.1, 17.3). The HIV-related mortality rate was 8.9/100 person-years (95 percent CI: 7.7, 10.2). Mortality rates were significantly (p < 0.001) lower during the HAART era (5.7/100 person-years) than in the pre-HAART era (15.9/100 person-years), as was the relative hazard of 0.24 (95 percent CI: 0.18, 0.32).
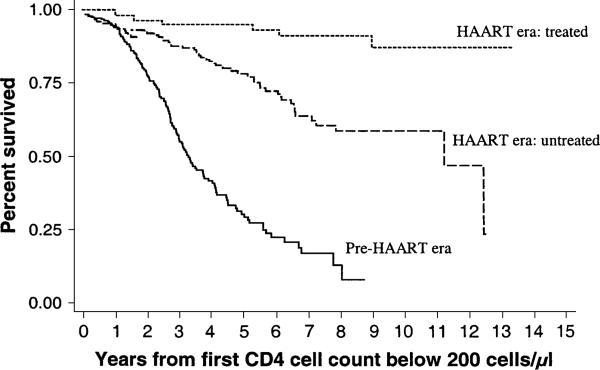
Figure 1 shows the Kaplan-Meier curves for three groups: HAART era treated with HAART; HAART era not treated with HAART; and the pre-HAARTera. Mortality was significantly (log-rank test: p < 0.001) lower in both the HAART era-treated group (relative hazard 0.05, 95 percent CI: 0.02, 0.12) and the HAART era-untreated group (relative hazard = 0.27, 95 percent CI: 0.19, 0.38) than in the pre-HAART referent group (table 1). When observations were restricted to the HAART era, mortality was lower among those who received treatment than among those who did not (relative hazard = 0.42, 95 percent CI: 0.24, 0.73).
FIGURE 1.
Kaplan-Meier curve of survival by availability and utilization of highly active antiretroviral therapy (HAART) among human immunodeficiency virus (HIV)-seropositive injection drug users, ALIVE Study, 1988–2002. ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences.
TABLE 1.
Incidence rate and relative hazards of survival by utilization of HAART* among injection drug users, ALIVE* Study, 1988–2002
| Category | No. | Person-years | Incidence rate/100 person-years | Relative hazard | 95% confidence interval |
|---|---|---|---|---|---|
| Pre-HAART | 453 | 977 | 15.86 | 1.00 | Referent |
| Post-HAART, untreated | 408 | 917 | 5.67 | 0.27 | 0.19, 0.38 |
| Post-HAART, treated | 242 | 508 | 1.18 | 0.05 | 0.02, 0.12 |
HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences.
We considered whether the improved mortality after 1996 might be due to different methodological concerns. When we repeated the analyses shown in figure 1, restricted to those whose first CD4+ count of less than 200 cells/μl was in 1993 or later, the results were unchanged. With pre-HAART as the referent, relative hazards for the HAART era treated and the untreated were 0.13 and 0.31, respectively. We also performed stratified analyses to consider the length of time in the study (“lead time”) as a covariate; adjustment for this did not alter the relations shown in figure 1. We examined the median initial CD4+ cell count by the intervals used for lead time; no significant differences were observed (data not shown). We also restricted analysis to persons among whom we observed the first drop in CD4+ cell count below 200/μl (i.e., excluding those already at less than 200/μl at baseline), and the results did not change (data not shown). Finally, repeating Kaplan-Meier analyses using HAART as a fixed variable showed results similar to those using HAART as a time-dependent variable (data not shown).
Table 2 shows the univariate associations between mortality and select sociodemographic factors by HAART status, as well as participant numbers per group (allowing for crossover to other groups), person-years, and mortality rates/ 100 person-years. Across each of these sociodemographic variables, mortality was higher in the pre-HAART than in the HAART era. Across treatment eras and categories, age, gender, race, and homelessness in the prior 6 months were each not significantly associated with mortality.
TABLE 2.
Univariate associations of HIV* mortality with sociodemographic factors and mortality rates/100 person-years by HAART* strata, ALIVE* Study, 1988–2002
| Variables | Pre-HAART era | HAART era (1996–2002) |
Overall relative hazard† | p value | |
|---|---|---|---|---|---|
| No treatment‡ | Treatment‡ | ||||
| Race | |||||
| Caucasian | |||||
| No.§ | 24 | 21 | 11 | ||
| Person-years | 43 | 50 | 24 | ||
| Rate | 18.54 | 0 | 4.19 | 1.00 | |
| African American | |||||
| No. | 429 | 387 | 231 | ||
| Person-years | 934 | 867 | 484 | ||
| Rate | 15.74 | 6.00 | 1.03 | 0.98 | 0.956 |
| Gender | |||||
| Male | |||||
| No. | 345 | 296 | 176 | ||
| Person-years | 726 | 664 | 367 | ||
| Rate | 15.84 | 6.03 | 1.36 | 1.00 | |
| Female | |||||
| No. | 108 | 112 | 66 | ||
| Person-years | 251 | 253 | 141 | ||
| Rate | 15.91 | 4.74 | 0.71 | 0.91 | 0.543 |
| Age (years) | |||||
| <30 | |||||
| No. | 47 | 18 | 3 | ||
| Person-years | 79 | 27 | 2 | ||
| Rate | 16.36 | 0 | 0 | 1.00 | |
| 30–40 | |||||
| No. | 259 | 162 | 61 | ||
| Person-years | 538 | 306 | 89 | ||
| Rate | 14.88 | 5.88 | 1.13 | 0.83 | 0.558 |
| >40 | |||||
| No. | 206 | 295 | 201 | ||
| Person-years | 361 | 583 | 417 | ||
| Rate | 17.21 | 5.83 | 1.20 | 0.91 | 0.760 |
| Homeless | |||||
| No | |||||
| No. | 424 | 377 | 233 | ||
| Person-years | 828 | 773 | 467 | ||
| Rate | 15.58 | 5.56 | 1.07 | 1.00 | |
| Yes | |||||
| No. | 155 | 121 | 47 | ||
| Person-years | 149 | 144 | 41 | ||
| Rate | 17.41 | 6.27 | 2.43 | 1.28 | 0.157 |
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences.
Relative hazards are for overall associations, adjusted for lead time (see text).
“Treatment” and “no treatment” refer to receipt of the HAART regimen (see text).
Number of participants in each category for the fixed factors and the number contributing information to each category of time-dependent factors.
Across each of the drug use variables presented (table 3), mortality was lower in the HAART era than in the pre-HAART era. In univariate analysis, mortality was inversely associated with the use of alcohol (relative hazard = 0.64), although this effect was much weaker or reversed in the HAART era. Snorting cocaine was also associated with lower mortality (relative hazard = 0.32), a finding that persisted in the HAART era. No differences in mortality were noted by drug detoxification or any methadone maintenance (table 2), by injection frequency and type of drug injected (i.e., heroin, cocaine, or both), or by cigarette smoking (data not shown).
TABLE 3.
Univariate associations of HIV* mortality with drug use factors and rates/100 person-years by HAART* strata, ALIVE* Study, 1988–2002
| Time-dependent factors during last 6 months | Pre-HAART era | HAART-era (1996–2002) |
Overall relative hazard† | p value | |
|---|---|---|---|---|---|
| No treatment‡ | Treatment‡ | ||||
| Alcohol (any) | |||||
| No | |||||
| No.§ | 264 | 272 | 181 | ||
| Person-years | 374 | 435 | 333 | ||
| Rate | 23.02 | 5.75 | 0.60 | 1.00 | |
| Yes | |||||
| No. | 334 | 271 | 133 | ||
| Person-years | 604 | 482 | 175 | ||
| Rate | 11.43 | 5.60 | 2.28 | 0.64 | 0.002 |
| Crack (any) | |||||
| No | |||||
| No. | 424 | 379 | 225 | ||
| Person-years | 822 | 776 | 453 | ||
| Rate | 17.27 | 5.28 | 1.10 | 1.00 | |
| Yes | |||||
| No. | 131 | 110 | 51 | ||
| Person-years | 155 | 141 | 55 | ||
| Rate | 8.39 | 7.82 | 1.82 | 0.72 | 0.117 |
| Snort cocaine (any) | |||||
| No | |||||
| No. | 448 | 401 | 240 | ||
| Person-years | 930 | 881 | 501 | ||
| Rate | 16.35 | 5.90 | 1.20 | 1.00 | |
| Yes | |||||
| No. | 60 | 44 | 12 | ||
| Person-years | 48 | 36 | 7 | ||
| Rate | 6.29 | 0 | 0 | 0.32 | 0.039 |
| Any injection during past 6 months | |||||
| No | |||||
| No. | 263 | 254 | 184 | ||
| Person-years | 379 | 418 | 330 | ||
| Rate | 16.63 | 5.50 | 1.52 | 1.00 | |
| Yes | |||||
| No. | 342 | 294 | 125 | ||
| Person-years | 599 | 498 | 178 | ||
| Rate | 15.37 | 5.82 | 0.56 | 0.99 | 0.965 |
| Methadone maintenance (any) | |||||
| No | |||||
| No. | 447 | 391 | 218 | ||
| Person-years | 922 | 792 | 385 | ||
| Rate | 16.06 | 5.81 | 1.30 | 1.00 | |
| Yes | |||||
| No. | 71 | 110 | 77 | ||
| Person-years | 55 | 125 | 122 | ||
| Rate | 12.62 | 4.81 | 0.82 | 0.80 | 0.430 |
| Detoxification | |||||
| No | |||||
| No. | 439 | 391 | 234 | ||
| Person-years | 866 | 828 | 463 | ||
| Rate | 16.51 | 5.67 | 1.08 | 1.00 | |
| Yes | |||||
| No. | 112 | 99 | 55 | ||
| Person-years | 111 | 88 | 44 | ||
| Rate | 10.80 | 5.67 | 2.25 | 0.74 | 0.216 |
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences.
Relative hazards are for overall associations, adjusted for lead time (see text).
“Treatment” and “no treatment” refer to receipt of the HAART regimen (see text).
Number of participants in each category for the fixed factors and the number contributing information to each category of time-dependent factors.
For most of the clinical factors evaluated (table 4), mortality was lower in the HAART-era than in the pre-HAART-era groups, although this distinction was less apparent with lower CD4+ cell counts at study entry and with the diagnosis of sepsis. Relative hazards for mortality by the clinical variables across the periods of observation showed that mortality was associated with a CD4+ cell count of less than 150/μl at entry (relative hazard = 2.32) or with other clinical outcomes, including anemia (relative hazard = 4.36), sepsis (relative hazard = 3.02), bacterial pneumonia (relative hazard = 3.03), oral candidiasis or thrush (relative hazard = 2.60), inpatient visits (relative hazard = 2.79), and out-patient or emergency room visits (relative hazard = 1.87).
TABLE 4.
Univariate associations of HIV* mortality and health factors and rates/100 person-years by HAART* strata, ALIVE* Study, 1988–2002
| Time-dependent factors during last 6 months | Pre-HAART era | HAART era (1996–2002) |
Overall relative hazard† | p value | |
|---|---|---|---|---|---|
| No treatment‡ | Treatment‡ | ||||
| CD4/μl | |||||
| >150 | |||||
| No.§ | 245 | 251 | 143 | ||
| Person-years | 555 | 619 | 311 | ||
| Rate | 11.17 | 3.88 | 0.32 | 1.00 | |
| ≤150 | |||||
| No. | 208 | 157 | 99 | ||
| Person-years | 422 | 298 | 197 | ||
| Rate | 22.02 | 9.40 | 2.54 | 2.32 | <0.001 |
| Anemia (hemoglobin, ≤11) | |||||
| No | |||||
| No. | 422 | 357 | 223 | ||
| Person-years | 768 | 714 | 427 | ||
| Rate | 9.37 | 2.24 | 0.70 | 1.00 | |
| Yes | |||||
| No. | 200 | 182 | 80 | ||
| Person-years | 209 | 203 | 81 | ||
| Rate | 39.71 | 17.73 | 3.71 | 4.36 | <0.001 |
| Sepsis | |||||
| No | |||||
| No. | 452 | 407 | 240 | ||
| Person-years | 961 | 909 | 503 | ||
| Rate | 15.40 | 5.50 | 1.00 | 1.00 | |
| Yes | |||||
| No. | 26 | 13 | 7 | ||
| Person-years | 16 | 8 | 4 | ||
| Rate | 43.66 | 26.10 | 22.95 | 3.02 | <0.001 |
| Any pneumonia | |||||
| No | |||||
| No. | 442 | 392 | 233 | ||
| Person-years | 856 | 835 | 470 | ||
| Rate | 12.27 | 4.91 | 0.90 | 1.00 | |
| Yes | |||||
| No. | 159 | 110 | 52 | ||
| Person-years | 122 | 82 | 37 | ||
| Rate | 41.11 | 13.48 | 5.34 | 3.03 | <0.001 |
| Any thrush | |||||
| No | |||||
| No. | 425 | 371 | 223 | ||
| Person-years | 727 | 774 | 432 | ||
| Rate | 11.14 | 4.13 | 0.70 | 1.00 | |
| Yes | |||||
| No. | 226 | 150 | 76 | ||
| Person-years | 250 | 142 | 76 | ||
| Rate | 30.00 | 14.04 | 3.94 | 2.60 | <0.001 |
| Inpatient during past 6 months | |||||
| No | |||||
| No. | 420 | 371 | 227 | ||
| Person-years | 698 | 749 | 427 | ||
| Rate | 10.75 | 4.28 | 0.50 | 1.00 | |
| Yes | |||||
| No. | 268 | 198 | 97 | ||
| Person-years | 279 | 168 | 81 | ||
| Rate | 28.63 | 11.89 | 4.94 | 2.79 | <0.001 |
| Outpatient/emergency room during past 6 months | |||||
| No | |||||
| No. | 246 | 220 | 48 | ||
| Person-years | 252 | 277 | 35 | ||
| Rate | 10.31 | 3.25 | 0 | 1.00 | |
| Yes | |||||
| No. | 407 | 363 | 233 | ||
| Person-years | 690 | 634 | 471 | ||
| Rate | 18.27 | 6.78 | 1.27 | 1.87 | 0.001 |
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences.
Relative hazards are for overall associations, adjusted for lead time (see text).
“Treatment” and “no treatment” refer to receipt of the HAART regimen (see text).
Number of participants in each category for the fixed factors and the number contributing information to each category of time-dependent factors.
In terms of antiretroviral treatment or use of prophylactic medicines (table 5), the univariate analysis showed that mortality was markedly reduced for those receiving HAART in contrast to those not receiving HAART (relative hazard = 0.04), but mortality was not significantly lower in persons who received antiretroviral therapy that did not meet HAART criteria. The use of other HIV-related medicines against the Mycobacterium avium complex and fungal diseases (predominantly candidiasis) was associated with higher mortality (relative hazard = 2.26 and 3.18, respectively). Similarly, Pneumocystis carinii pneumonia medication was associated with higher mortality, unless the person was also receiving HAART (table 5).
TABLE 5.
Univariate associations of HIV* mortality and medication use factors and rates/100 person-years by HAART* strata, ALIVE* Study, 1988–2002
| Time-dependent factors during last 6 months | Pre-HAART era | HAART era (1996–2002) |
Overall relative hazard† | p value | |
|---|---|---|---|---|---|
| No treatment‡ | Treatment‡ | ||||
| HAART(time dependent) | |||||
| No | |||||
| No.§ | 451 | 403 | |||
| Person-years | 957 | 895 | |||
| Rate | 15.77 | 5.70 | 1.00 | ||
| Yes | |||||
| No. | 242 | ||||
| Person-years | 508 | ||||
| Rate | 1.18 | 0.04 | <0.001 | ||
| Dual antiretroviral therapy | |||||
| No | |||||
| No. | 449 | 374 | 242 | ||
| Person-years | 933 | 716 | 508 | ||
| Rate | 15.97 | 6.14 | 1.18 | 1.00 | |
| Yes | |||||
| No. | 79 | 182 | |||
| Person-years | 44 | 200 | |||
| Rate | 13.36 | 4.00 | 0.65 | 0.112 | |
| Single antiretroviral medication | |||||
| No | |||||
| No. | 402 | 394 | 242 | ||
| Person-years | 600 | 803 | 508 | ||
| Rate | 14.49 | 5.60 | 1.18 | 1.00 | |
| Yes | |||||
| No. | 309 | 131 | |||
| Person-years | 377 | 114 | |||
| Rate | 18.05 | 6.16 | 1.09 | 0.559 | |
| Anti-PCP* | |||||
| No | |||||
| No. | 422 | 316 | 112 | ||
| Person-years | 720 | 527 | 140 | ||
| Rate | 13.61 | 4.18 | 2.14 | 1.00 | |
| Yes | |||||
| No. | 234 | 276 | 205 | ||
| Person-years | 257 | 390 | 367 | ||
| Rate | 22.16 | 7.70 | 0.82 | 1.24 | 0.149 |
| Anti-opportunistic infection¶ | |||||
| No | |||||
| No. | 404 | 341 | 180 | ||
| Person-years | 611 | 653 | 272 | ||
| Rate | 10.63 | 4.75 | 0.73 | 1.00 | |
| Yes | |||||
| No. | 325 | 253 | 177 | ||
| Person-years | 366 | 264 | 236 | ||
| Rate | 24.60 | 7.95 | 1.70 | 1.90 | <0.001 |
| Anti-MAI* | |||||
| No | |||||
| No. | 452 | 406 | 237 | ||
| Person-years | 942 | 886 | 480 | ||
| Rate | 14.97 | 5.53 | 0.83 | 1.00 | |
| Yes | |||||
| No. | 48 | 43 | 40 | ||
| Person-years | 35 | 31 | 28 | ||
| Rate | 39.90 | 9.73 | 7.23 | 2.26 | <0.001 |
| Antifungal | |||||
| No | |||||
| No. | 435 | 383 | 222 | ||
| Person-years | 793 | 820 | 430 | ||
| Rate | 10.35 | 4.51 | 0.93 | 1.00 | |
| Yes | |||||
| No. | 188 | 121 | 90 | ||
| Person-years | 185 | 97 | 77 | ||
| Rate | 39.52 | 15.54 | 2.59 | 3.18 | <0.001 |
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences; PCP, Pneumocystis carinii pneumonia; MAI, Mycobacterium avium, M. intracellulare.
Relative hazards are for overall associations, adjusted for lead time (see text).
“Treatment” and “no treatment” refer to receipt of the HAART regimen (see text).
Number of participants in each category for the fixed factors and the number contributing information to each category of time-dependent factors.
MAI, cytomegalovirus, fungus, tuberculosis.
Table 6 shows the results of the final multivariable Cox model incorporating period (pre-HAART and HAART eras), use of HAART and other medications, and clinical, socio-demographic, and drug use factors while adjusting for lead time. Mortality was lower for persons receiving HAARTand persons in the HAART era who did not receive HAART (adjusted relative hazard = 0.06 and 0.33, respectively) than in the pre-HAART era. Within the HAART era only, the adjusted relative hazard for the HAART treated versus those not treated was 0.16. Of note, after consideration of the time period and use of HAART, both combination antiretroviral therapy and monotherapy were modestly protective (relative hazard = 0.59 and 0.80, respectively). Likewise, after adjustment, a modest protective effect of P. carinii prophylaxis was observed (relative hazard = 0.81), but not for other anti-opportunistic infection medicines. Anemia, thrush, and hospitalization were associated with a higher probability of death (table 6). Notably, mortality was lower for women than men (relative hazard = 0.74). Recent injection drug use was not associated with death, nor was frequency of injection (data not shown). Snorting cocaine was associated with lower mortality (relative hazard = 0.27). Formal tests of in teraction of inhaled cocaine by use of HAART showed no significant associations (i.e., separately for snort, sniff, and smoke and then combined as “any inhaled”). Interactions of HAART and anti-opportunistic infections were not signifi-cant (data not shown). Analyses repeated using HAART as a fixed variable showed similar results to those of models treating HAART as time dependent (data not shown).
TABLE 6.
Final multivariate survival regression model by HAART* availability and utilization, adjusting for time-dependent clinical, behavioral, and demographic factors, ALIVE* Study, 1988–2002
| Factors | Relative hazard† | 95% confidence interval† |
|---|---|---|
| Pre-HAART era | 1.00 | |
| HAART era | ||
| Not treated‡ | 0.33 | 0.22, 0.49 |
| Treated‡ | 0.06 | 0.02, 0.14 |
| Dual antiretroviral therapy | ||
| No | 1.00 | |
| Yes | 0.59 | 0.34, 1.02 |
| Single antiretroviral medication | ||
| No | 1.00 | |
| Yes | 0.80 | 0.59, 1.09 |
| Anti-PCP* | ||
| No | 1.00 | |
| Yes | 0.81 | 0.60, 1.09 |
| CD4 count at entry | ||
| Increase of 50 cells | 0.63 | 0.55, 0.73 |
| Anemia | ||
| No | 1.00 | |
| Yes | 3.18 | 2.27, 4.43 |
| Thrush | ||
| No | 1.00 | |
| Yes | 1.89 | 1.40, 2.56 |
| Inpatient visits | ||
| No | 1.00 | |
| Yes | 1.90 | 1.41, 2.56 |
| Any injection during past 6 months | ||
| No | 1.00 | |
| Yes | 0.92 | 0.68, 1.26 |
| Snort cocaine | ||
| No | 1.00 | |
| Yes | 0.27 | 0.10, 0.73 |
| Alcohol | ||
| No | 1.00 | |
| Yes | 0.84 | 0.62, 1.13 |
| Gender | ||
| Male | 1.00 | |
| Female | 0.74 | 0.54, 1.02 |
HAART, highly active antiretroviral therapy; ALIVE, Acquired Immunodeficiency Syndrome Link to Intravenous Experiences; PCP, Pneumocystis carinii pneumonia.
Relative hazards and confidence intervals are for overall associations, adjusted for lead time (see text).
“Treated” and “not treated” refer to receipt of the HAART regimen after 1996 (see text).
In analyses that incorporated HIV load, the sample size was reduced (n = 460); the median viral load was lowest for the HAART-treated group but was similar for the other two groups. Adding viral load to the final model showed a positive association with mortality but no change in survival estimates for the three treatment groups (data not shown).
We next considered whether the improved survival in the HAARTera might be due to selection into treatment. Assuming that the pre-HAART-era group combines patients who might or might not have received HAARTif it were available, that CD4+ cell count is a marker of health, and that sicker patients would receive HAART, then those not receiving HAARTafter 1996 should have higher CD4+ cell counts than those in the pre-HAART group. With longitudinal data analysis methods, the average annual CD4+ cell declines for the pre-HAART, HAART-untreated, and HAART-treated groups were —36.6 (95 percent CI: —42.0, —31.2), —10.1 (95 percent CI: —4.2, 11.5), respectively. The rates/100 person-years for outpatient/emergency room visits for the three groups were 1.51, 1.24, and 1.67, respectively. Both untreated and treated HAART-era groups were less likely than the pre-HAART-era group to be active injectors or homeless, but they were more likely to be in methadone treatment (data not shown).
DISCUSSION
Because availability and utilization of HAART in predominantly minority injection drug users have lagged behind those in other populations (14), data have been sparse on the effectiveness of HAART in this population. Earlier studies in other populations have focused on either the survival in periods before versus after the introduction of HAART or comparisons restricted to the HAART era between those receiving or not receiving treatment (5–7, 23–25). Our study utilized both approaches, and each demonstrated results similar to those of these previous studies, suggesting that the benefits reported for HAART extend to this population.
While the dramatic improvement in survival from the pre-HAART era to the HAART era suggests a potent effect of HAART, we also noted a dramatic improvement in survival between the pre-HAART-era and the HAART-era participants who did not receive potent antiretroviral therapy. This finding suggests that factors other than HAART therapy itself might influence survival. Such factors might include lead-time bias, frailty or survival bias, exposure to other antiretrovirals, and selection for treatment.
A possible consideration for the improved survival among the post-1996 participants not receiving HAART is lead-time bias, since the study started with prevalent cases of first CD4+ cell counts below 200/μl. However, neither excluding prevalent cases nor controlling for lead time affected the inferences. Another possible consideration is that persons with CD4+ cell counts below 200/μl during the earlier part of the study were more frail than those during the later part of the study, so that the results might have reflected a survivor bias. When analyses were restricted to those whose first CD4+ cell count of less than 200 cells/μl was in 1993 (3 years prior to the introduction of HAART) or later, survival comparisons were unchanged. A related consideration is that a host genetic variant might be contributing to a survivor bias. However, a comparison of the established geno-type frequencies for HIV progression (26–34) by period revealed no difference in survival for any genotype, arguing against host genetics as a major factor in the improved survival of those in the HAART era (data not shown).
We then considered whether utilization of antiretroviral therapy other than HAART or other HIV medications, such as anti-P. carinii, anti-M. avium complex, or antifungal medications, might have influenced the observed associations. We noted that survival among individuals on non-HAART antiretroviral treatment or on prophylactic medicines improved during the HAART era, suggesting possible changes in indications or clinical care over time. However, even after accounting for other antiretroviral treatment or other HIV- related medicines, in models that also adjusted for CD4+ cell count and clinical status, we still showed similar associations of protection using HAART or being in the HAART era with or without treatment.
A separate consideration to tie together the discrepancy in results is the process of selection into treatment. As earlier studies during the era of antiretroviral monotherapy suggest, sicker patients are more likely to receive therapy (35). The remarkable effectiveness of HAART (1–5) could clearly predominate over the fact that sicker individuals receive the treatment. However, for treatment of modest potency (but proven in randomized controlled trials) such as monotherapy, it is difficult to know how much one factor (treatment associated with poorer prognosis) is masking the effect of the other (i.e., treatment effectiveness). As noted in the Results, in the pre-HAART era, “selection for treatment” (i.e., for sicker patients) appears to trump the effectiveness of anti- retroviral therapy (with mono- more so than dual therapy). However, in the HAARTera, combination non-HAART therapy is associated with a protective effect. Multiple mechanisms may be at play including increased access (14), which we accounted for in this study, and evolving readiness for HIV treatment. Also, in the pre-HAART era, combination therapy was the best available and used for the sickest patients. However, in the HAART era, particularly early on before the ramifications of resistance were fully appreciated, combination therapy was used as a prelude to HAARTand at earlier HIV stages, while HAART was reserved for the sickest patients. Thus, the improved survival of the HAART-era group not treated with HAART compared with the pre-HAART group may reflect evolving considerations for anti-retroviral therapies. The longitudinal data analysis of CD4+ cell counts supports this conclusion of treatment selection. The observation that the CD4+ cell count decreased less in the HAART-untreated patients than in the pre-HAART-era patients suggests that this group was less ill (given the modest effectiveness of antiretroviral therapies short of HAART), leaving the HAART group as those who had more advanced disease. However, the increase in CD4+ cell counts among those receiving HAART indicates the potency of these medications as noted by others. Therefore, with a treatment selection process operating, the effect of treatment in the HAART era (using the comparison of those in same era but not receiving HAART) would be underestimated.
Given that the cohort was injection drug users, we considered the possible effect of illicit drugs on the impact of HAART on survival. While injection drug use frequency was not significant, we considered other routes of adminis tration. Inhaled cocaine was associated with survival before 1996, but this putative benefit dissipated during the HAART era. The mechanism underlying these observations is unclear. Considering recent data showing that use of methamphetamines may have an adverse impact on survival for those on HAART (36), we find that the absence of an effect for illicit drugs observed here is not reassuring.
Several study limitations should be acknowledged. The extent to which this study population represents minority injection drug users with advanced HIV infection in Baltimore, Maryland, or in other cities is difficult to determine, although comparisons of our overall study with samples drawn in other ways in the same city at the same time (37, 38) are similar. Likewise, our results may differ from clinic-derived studies because of differences in who receives no treatment. The results are based on a sample of injection drug users who had CD4+ cell counts below 200/μl; this limited inferences to the observed impact of HAART on those with advanced immunosuppression. While this was done to preserve comparability over the length of the study period, so as to incorporate observations when treatment was recommended primarily for those with advanced immunosuppression, data from our cohort on those with CD4+ cell counts above 200/μl have been published elsewhere (39). Another possible limitation involves the use of self-reports and the concern over responding in a socially desirable manner. In the absence of reward or sanctions for responses, most studies have shown that self reports from drug users are reliable and valid (40). Future studies will need to more closely examine the possible interactions with HAART and illicit drugs or treatments for drug abuse, in terms of pharmacologic effects as well as frequency and types of toxicities that might affect clinical outcomes including survival. While adherence to medications is a consideration for response to medications, measures were not used until late in the study; assuming that adherence was imperfect, the results observed likely represent a conservative estimate of survival, a fact that should not be minimized in considerations of antiretroviral use in international settings. Finally, while comparisons of “before and after” studies or historical cohort studies and those involving non-randomized treatment designs each have well known biases, we have adjusted for several known biases in this analysis to limit their impact on the results. In addition, a randomized controlled clinical trial, which would have fewer biases, is unlikely to be completed in the near future. This highlights the importance of carefully conducted and analyzed cohort studies.
In conclusion, clinicians have been somewhat reluctant to provide treatment to this population because of concerns about adherence and evolution of resistance that might be transmitted to others. While this analysis cannot directly address these important concerns, the data on improved survival for those who received therapy suggest that treatment can work in this and other populations internationally where reduced access has occurred from reluctance to provide treatment over concerns of adherence and resistance. Approaches are needed to reach the intravenous drug-using population and then to offer and monitor effective antiretroviral treatment.
ACKNOWLEDGMENTS
Supported by National Institute on Drug Abuse grant DA 04334.
The authors acknowledge Dr. Joseph B. Margolick for performing T-cell subset studies; Drs. Tom C. Quinn and Homayoon Farzadegan for performing HIV viral load assays; Dr. Liza Solomon, Lisette Johnson, Lisa Purvis, and Lisa McCall for project direction; and Richard Lane for his entree to this population.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ALIVE
AIDS Link to Intravenous Experiences
- CD4+
CD4-positive T lymphocytes
- CI
confidence interval
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
REFERENCES
- 1.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 2.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 3.Survival after introduction of HAART in people with known duration of HIV-1 infection. The CASCADE Collaboration. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1158–9. [PubMed] [Google Scholar]
- 4.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Anastos K, Barron Y, Miotti P, et al. Risk of progression to AIDS and death in women infected with HIV-1 initiating highly active antiretroviral treatment at different stages of disease. Arch Intern Med. 2002;162:1973–80. doi: 10.1001/archinte.162.17.1973. [DOI] [PubMed] [Google Scholar]
- 6.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 7.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 8.Poundstone K, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15:1115–23. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Whitman S, Murphy J, Cohen M, et al. Marked declines in human immunodeficiency virus related mortality in Chicago in women, African Americans, and injection drug users 1995–1997. Arch Intern Med. 2000;160:365–9. doi: 10.1001/archinte.160.3.365. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Estimated incidence of AIDS and deaths of persons with AIDS adjusted for delays in reporting, by quarter-year of diagnosis/death, United States, 1985–1987. HIV/AIDS Surveill Rep. 1997;9:1–44. [Google Scholar]
- 11.Chaisson MA, Berenson L, Li W, et al. Declining AIDS mortality in New York City. J Acquir Immune Defic Syndr. 1999;21:59–64. doi: 10.1097/00126334-199905010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Celentano DD, Vlahov D, Cohn S, et al. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280:544–6. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 13.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–9. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 14.Celentano DD, Galai N, Sehti AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–15. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 15.Vlahov D, Wang CL, Galai N, et al. Mortality risk among new onset injection drug users. Addiction. 2004;99:946–54. doi: 10.1111/j.1360-0443.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Vol. 14. US Department of Health and Human Services; Washington, DC: Jul, 2003. ( http://www.aidsinfo.nih.gov/guidelines) [Google Scholar]
- 17.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection. 2004 recommendations of the International AIDS Society–USA panel. JAMA. 2004;292:251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 18.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE Study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 19.Cohen MH, French AL, Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–8. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 21.Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Ann Intern Med. 1996;124:999–1005. doi: 10.7326/0003-4819-124-11-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–23. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 23.Sterling TR, Chaisson RE, Moore RD. HIV-1 RNA, CD4 T-lymphocytes, and clinical response to highly active antiretroviral therapy. AIDS. 2001;15:2251–7. doi: 10.1097/00002030-200111230-00006. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson LP, Li R, Phair J, et al. Evaluation of the effectiveness of highly active antiretroviral therapy in persons with human immunodeficiency virus using biomarker-based equivalence of disease progression. Am J Epidemiol. 2002;155:760–70. doi: 10.1093/aje/155.8.760. [DOI] [PubMed] [Google Scholar]
- 25.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Smith MW, Dean M, Carrington M, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277:959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 27.Kostrikis LG, Huang Y, Moore JP, et al. A chemokine receptor CCR2 allele delays HIV-1 disease progression and is associated with a CCR2 promoter mutation. Nat Med. 1998;4:350–3. doi: 10.1038/nm0398-350. [DOI] [PubMed] [Google Scholar]
- 28.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 29.Smith MW, Dean M, Carrington M, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science. 1997;277:959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 30.Martin MP, Dean M, Smith MW, et al. Genetic acceleration by a promoter variant of CCR5. Science. 1998;282:1907–11. doi: 10.1126/science.282.5395.1907. [DOI] [PubMed] [Google Scholar]
- 31.Winkler CA, Modi W, Smith MW, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998;279:389–93. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 32.Shin HD, Winkler C, Stephens JC, et al. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc Natl Acad Sci U S A. 2000;97:14467–72. doi: 10.1073/pnas.97.26.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An P, Nelson GW, Wang L, et al. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc Natl Acad Sci U S A. 2002;99:10002–7. doi: 10.1073/pnas.142313799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duggal P, An P, Beaty TH, et al. Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African Americans. Genes Immun. 2003;4:245–50. doi: 10.1038/sj.gene.6363950. [DOI] [PubMed] [Google Scholar]
- 35.Graham NM, Zeger SL, Kuo V, et al. Zidovudine use in AIDS-free HIV-1 seropositive homosexual men in the Multicenter AIDS Cohort Study (MACS), 1987–1989. J Acquir Immune Defic Syndr. 1991;4:267–76. [PubMed] [Google Scholar]
- 36.Ellis RJ, Childers ME, Cherner M, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–6. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 37.Lange WR, Snyder FR, Lozovsky D, et al. Geographic distribution of human immunodeficiency virus markers in parenteral drug abusers. Am J Public Health. 1988;78:443–6. doi: 10.2105/ajph.78.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahov D, Brewer F, Munoz A, et al. Temporal trends of human immunodeficiency virus type 1 (HIV-1) infection among inmates entering a statewide prison system, 1985–1987. J Aquir Immune Defic Syndr. 1989;2:283–90. [PubMed] [Google Scholar]
- 39.Wang CL, Vlahov D, Galai N, et al. Mortality among HIV-seropositive vs. seronegative persons in the HAART era: implications for when to initiate therapy. J Infect Dis. 2004;190:1046–54. doi: 10.1086/422848. [DOI] [PubMed] [Google Scholar]
- 40.Samuels JF, Vlahov D, Anthony JC, et al. Measurement of HIV risk behaviors among intravenous drug users. Br J Addict. 1992;87:417–28. doi: 10.1111/j.1360-0443.1992.tb01942.x. [DOI] [PubMed] [Google Scholar]