Abstract
Background
Few studies have validated bioelectrical impedance analysis (BIA) following bariatric surgery.
Methods
We examined agreement of BIA (Tanita 310) measures of total body water (TBW) and percent body fat (%fat) before (T0) and 12 months (T12) after bariatric surgery, and change between T0 and T12 with reference measures: deuterium oxide dilution for TBW and three-compartment model (3C) for %fat in a subset of participants (n=50) of the Longitudinal Assessment of Bariatric Surgery-2.
Results
T0 to T12 median (IQR) change in deuterium TBW and 3C %fat was −6.4 L (6.4 L) and −14.8 % (13.4 %), respectively. There were no statistically significant differences between deuterium and BIA determined TBW [median (IQR) difference: T0 −0.1 L (7.1 L), p=0.75; T12 0.2 L (5.7 L), p=0.35; Δ 0.35 L(6.3 L), p=1.0]. Compared with 3C, BIA underestimated %fat at T0 and T12 [T0 −3.3 (5.6), p<0.001; T12 −1.7 (5.2), p=0.04] but not change [0.7 (8.2), p=0.38]. Except for %fat change, Bland-Altman plots indicated no proportional bias. However, 95 % limits of agreement were wide (TBW 15–22 L, %fat 19–20 %).
Conclusions
BIA may be appropriate for evaluating group level response among severely obese adults. However, clinically meaningful differences in the accuracy of BIA between individuals exist.
Keywords: Total body water, Fat mass, Weight loss, Bariatric surgery
Introduction
Bariatric surgery is associated with substantial weight loss; however, the composition of weight loss varies with some losing a greater percentage of fat-free mass than others [1–3]. Determining the composition of weight loss is important for clinical monitoring and research, but assumptions underlying estimates may be invalid in severe obesity. The standard hydration level of fat-free mass may be violated due to excess extracellular water [4, 5]. Body geometry is associated with greater body water in the trunk region (due to greater mass), which contributes to higher impedance relative to total body water (TBW), leading to underestimates of TBW and fat-free mass and overestimates of fat mass [6]. Therefore, estimating body composition in severe obesity and following surgery is challenging and requires validation.
Body composition is typically estimated by dividing the body into compartments. A two-compartment model divides the body into fat and fat-free mass [7], whereas a three-compartment model (3C) partitions the fat-free mass into TBW and jointly bone mineral and protein. Four-compartment models partition bone mineral and protein using dual-energy X-ray absorptiometry (DXA) estimates of bone mineral content. Because severely obese subjects do not always fit within the DXA, a 3C model is considered a gold-standard body composition reference method in this population [8].
Single-frequency bioelectrical impedance analysis (BIA; 50 kHz) is a method accessible in clinical settings that measures impedance (ohms) from which TBW is estimated and fat mass (and percent body fat (%fat)) are derived. BIA TBW estimates have been compared with deuterium (D2O) determined values [9] and with D2O determined TBW change during weight loss [10] but not with BIA pre to postoperative TBW change. One study has compared BIA %fat pre to postsurgery with a 3C model (TBW by D2O, body density by ADP, and body mass) [11]. BIA %fat was lower than 3C %fat for 20 women before and after surgery, but pre to postoperative changes in BIA %fat were not different from 3C estimates [11].
Here, we examine the validity of the single-frequency Tanita 310 BIA to assess changes in TBW and %fat from pre to post bariatric surgery. We evaluated whether BIA validity varied by initial BMI and the magnitude of weight and fat loss following surgery.
Methods
Between November 2006 and February 2009, participants (n=64) enrolled in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) at Weill Cornell Medical College and University of Pittsburgh Medical Center sites were enrolled in this ancillary study [12–14]. Due to recruiting delays, 41 additional participants were enrolled through December 2009 for a total of 105 participants, 53 from Weill Cornell and 52 from Pittsburgh.
Only Weill Cornell subjects were included in this analysis because BIA and reference measurements were not collected concomitantly in Pittsburgh. Three participants did not undergo bariatric surgery and were excluded, leaving an analysis sample of 50. All studies were approved by the Institutional Review Board of St. Luke’s-Roosevelt Hospital, and written informed consent was obtained.
Baseline measures were obtained on average 1.3 week before surgery (T0; range 0–11 week) and postoperative measures were approximately 12 months later (T12; range 10.6–17.7 months) at St. Luke’s-Roosevelt Hospital. A self-report medication log was obtained and checked against pill bottles and medical records when available. Exclusion criteria included pregnancy, abnormal thyroid or cortisol levels, and self-report of medications known to influence body composition (e.g., diuretics and corticosteroids). After chart abstraction, four subjects were identified to be on hydrochlorothiazide, a diuretic, and at baseline.
Weight (Weight Tronix, New York, NY; and Scale-Tronix, Wheaton, IL), height (Holtain; Crosswell, Wales-New York), BIA (Model TBF-310, Tanita Inc., Arlington Heights, IL), and body density (Bod Pod; (Cosmed, Chicago, IL; software version 2.3) [15, 16] measurements were obtained [17]. TBW was measured using a D2O (~1 g/kg) oral dose administered after a venous blood sample was taken from an antecubital vein. After 3 h, a second blood sample was obtained (see online supplementary materials for details). A 3C model was used to estimate fat mass [18]: fat (kg)=2.122×(BW/d) −0.779×TBW −1.356×BW, where BW is the body weight in kilograms, d is the body density derived from BodPod, and TBW is the total body water in kilograms.
Statistical Analysis
Wilcoxon rank-sum tests compared body composition and anthropometrics of those with data at both time points with those with data at T0 or T12 only. Spearman correlation coefficients, Wilcoxon signed-rank tests, and Bland-Altman plots [19] evaluated the relationship between (1) D2O and BIA TBW, (2) 3C and BIA %fat, at T1, T12, and change. Spearman correlation coefficients and linear regression evaluated whether the difference between BIA and reference values varied by initial BMI, weight loss (kg), or fat loss (kg by 3C) from T1 to T12. Stata 12.0 (College Station, TX) was used for all analyses with an α-level of 0.05.
Results
Characteristics of subjects are reported in Table 1. Some subjects were on hydrochlorothiazide, a diuretic medication, at T0 (n=4, 10 %) and T12 (n=3, 7 %). Of the 50 subjects, 32 had complete data; the remaining had data only at T0 (n=9) or T12 (n=9). Body composition characteristics of subjects with complete data were not different than those with data only at T0 and T12 (data not shown). Table 2 shows the body composition of subjects. While impedance increased from T0 to T12, all other values decreased (all p values<0.001).
Table 1.
Characteristics of study participants (n=50)
| Age, y | 45 (17) |
| Race, n (%) | |
| White | 22 (44) |
| African–American | 12 (24) |
| Hispanic | 15 (30) |
| Other | 1 (2) |
| Female, n (%) | 36 (72) |
| Surgery, n (%) | |
| LAPBAND | 4 (8) |
| BPD/DS | 4 (8) |
| Roux-en-Y gastric bypass | 30 (60) |
| Gastric sleeve | 12 (24) |
| Height, cm | 168.1 (12.7) |
| Baseline weight, kg | 128.2 (30.9) |
| Weight at 1 yeara, kg | 87.0 (27.1) |
| Baseline body mass index, kg/m2 | 44.1 (7.3) |
| Body mass index at 1 year2, kg/m2 | 30.7 (8.8) |
32 participants have complete data for analysis at baseline and 1 year. Nine participants with baseline data are missing data at 1 year, and nine participants with 1-year data are missing data at baseline
Median (IQR) unless otherwise noted
LAPBAND laparoscopic adjustable gastric band, BPD/DS biliopancreatic diversion with duodenal switch
7 subjects are missing data
Table 2.
Body composition at baseline, 1 year following bariatric surgery, and change between baseline and 1 year (n=50)
| Baseline n=41 | 1 year n=41 | Δa n=32 | |
|---|---|---|---|
| BIA | |||
| Fat-free mass, kg | 64.6 (16.6) | 55.8 (19.0) | −8.9 (6.2) |
| Fat mass, kg | 60.0 (17.4) | 30.2 (17.2) | −32.2 (11.9) |
| Percent body fat, % | 49.9 (6.8) | 35.6 (12.5) | −14.5 (10.2) |
| Impedance, Ω | 377.0 (71.0) | 427.0 (99.0) | 43.0 (53.5) |
| Total body water, L | 47.2 (12.2) | 40.8 (14.0) | −6.5(4.5) |
| Deuterium (D2O) | |||
| Total body water, L | 45.8 (11.8) | 40.8 (15.2) | −6.4 (6.4) |
| 3-compartment (Silva) | |||
| Fat-free mass, kg | 59.4 (16.4) | 54.9 (17.5) | −6.8 (5.6) |
| Fat mass, kg | 65.3 (18.0) | 33.1 (18.4) | −34.6 (17.5) |
| Percentage body fat, % | 51.4 (9.6) | 35.0 (14.8) | −14.8 (13.4) |
BIA bioelectrical impedance analysis, %fat percent body fat, D2O deuterium, TBW total body water, 3C 3-compartment
Of 50 subjects, nine are missing baseline data and nine are missing 1-year data so baseline to 1-year change cannot be determined in 18 subjects
Median (IQR), all such values
Calculated as 1 year baseline
Total Body Water
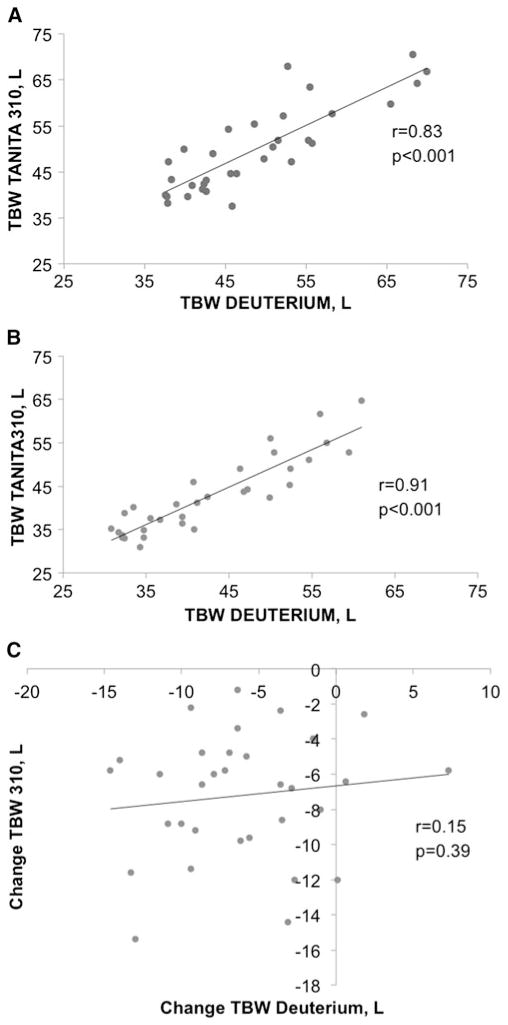
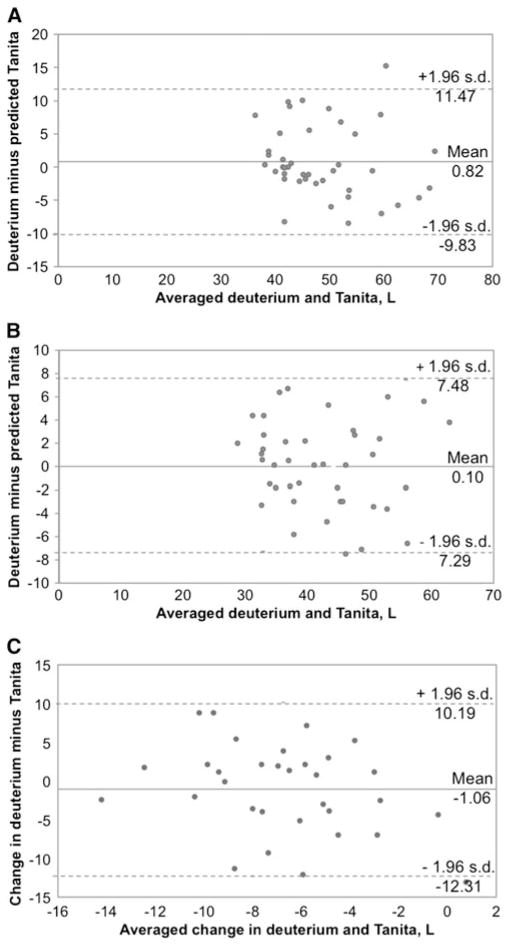
D2O TBW was correlated with BIA TBW at T0 (r=0.79, p<0.001) and T12 (r=0.91, p<0.001) but not TBW change from T0 to T12 (r=0.09, p=0.60; Fig. 1). There was no difference between BIA and D2O TBW values at T0, T12, or change (Table 3). Post hoc power calculations indicate that with 32 subjects, this study was powered to detect very large TBW change differences (i.e., we had 80 % power to detect a difference ≥2.5 L (see online supplementary materials for power calculations). Bland-Altman plots 95 % limits of agreement with D2O TBW (indicating how far apart TBW by BIA and D2O are likely to be for most individuals) were relatively wide (Fig. 2). Difference between TBW measurements was not correlated with initial BMI (T0 r=0.15, p=0.36; T12 r=−0.10, p=0.54; and Δ r=−0.10, p=0.59), weight loss from T0 to T12 (T12 r=−0.09, p=0.58 and Δ r=0.31, p=0.08), or fat loss from T0 to T12 at T12 (r=0.03, p=0.88). However, difference between TBW change measurements was associated with fat loss (Δ r=−0.58, p<0.001). Based on a linear regression model, a 1-kg greater loss of fat was associated with a 0.21-L greater difference between measurements of TBW change (p=0.004) (e.g., underestimate of TBW by BIA).
Fig. 1.
Association between total body water (TBW) estimates from deuterium and Tanita 310 at baseline (n=41) (a), 1 year after surgery (n=41) (b) and change between baseline at 1 year in participants with complete data (n=32) (c)
Table 3.
Pair wise median differences in percentage fat and total body water between bioelectrical impedance analysis and reference values at baseline, 1 year, and change between baseline and 1 year (n=50)
| Baseline (n=41)
|
1 year (n=41)
|
Δ (n=32)
|
||||
|---|---|---|---|---|---|---|
| Difference (IQR) | p valuea | Difference (IQR) | p valuea | Difference (IQR) | p valuea | |
| Total body waterb, L | −0.10 (7.09) | 0.75 | 0.20 (5.70) | 0.35 | 0.35 (6.30) | 1.0 |
| Percentage fatc | −3.30 (5.64) | <0.001 | −1.66 (5.15) | 0.03 | 0.65 (8.16) | 0.38 |
Of 50 subjects, nine are missing baseline data and nine are missing 1-year data so baseline to 1-year change cannot be determined in 18 subjects Power calculations for these analyses are provided in the supplementary material
TBW total body water
p values from Wilcoxon signed-rank test
Compared with Silva 3C model, difference calculated as bioelectrical impedance analysis %Fat–Silva %Fat
Compared with deuterium oxide, difference calculated as bioelectrical impedance analysis TBW–deuterium oxide TBW
Fig. 2.
Bland-Altman plot comparing measured and predicted total body water at baseline (n=41) (a), 1 year after surgery (n=41) (b), and change between baseline and 1 year in participants with complete data (n=32) (c)
Percentage Fat
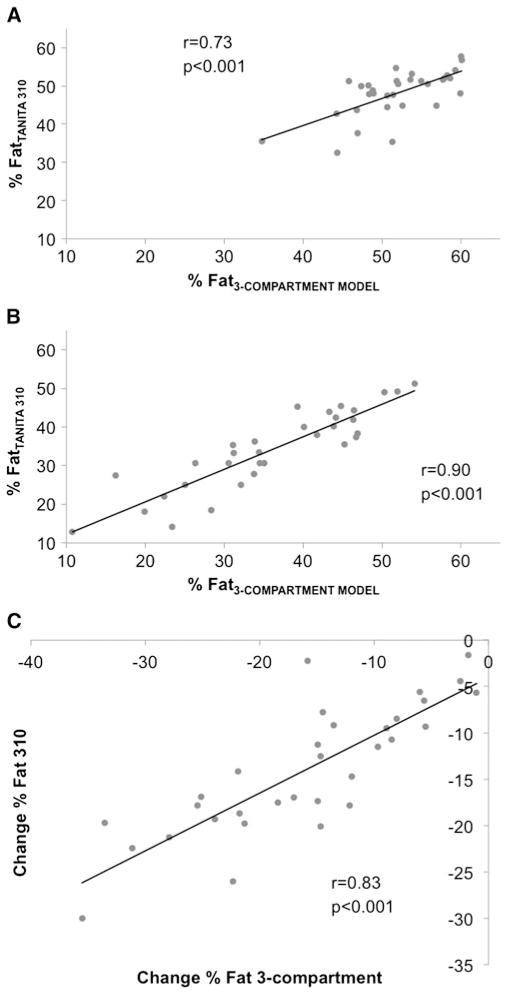
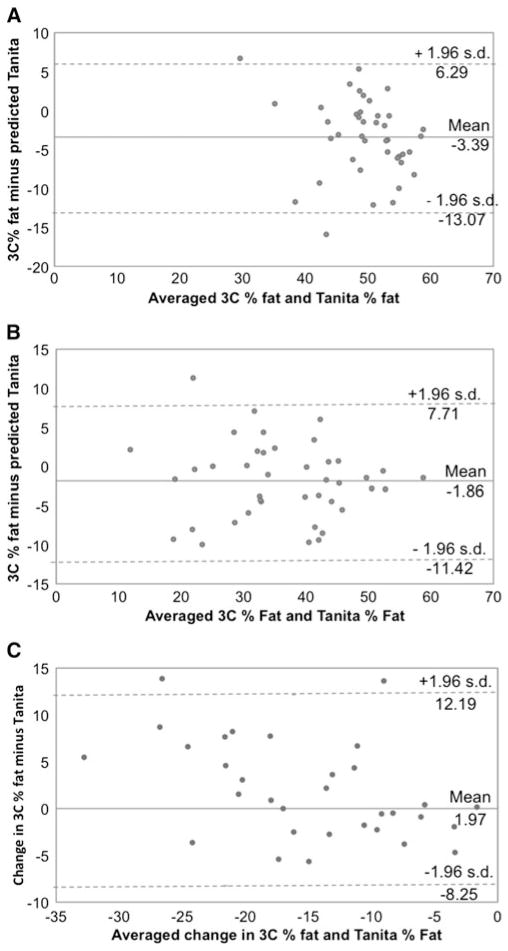
3C %fat had high correlations with BIA %fat of 0.71 (p<0.001), 0.88 (p<0.001), and 0.81 (p<0.001) at T0, T12, and change, respectively (Fig. 3). Compared with 3C, BIA underestimated %fat at T0 and T12, but there was no difference between measures of %fat change (Table 3). Bland-Altman plots 95 % limits of agreement with 3C %fat were relatively wide (Fig. 4). For %fat change, some bias was present (Δ F (1.30)=7.8, p=0.009); based on a linear regression model, a 1 % greater change in %fat from T1 to T12 was associated with a 0.31 % smaller difference between BIA and 3C measurements. Difference in %fat was not correlated with initial BMI (T0 r=−0.19, p=0.22; T12 r=−0.01, p=0.93; and Δ r=0.11, p=0.53), weight loss from T0 to T12 (T12 r=−0.04, p=0.81 and Δ r=−0.35, p=0.06), or fat loss from T0 to T12 at T12 (r=−0.08, p=0.64). However, difference between %fat change measurements was related to fat loss (Δ r=−0.58, p<0.001); based on a linear regression model, a 1-kg greater loss of fat was associated with 0.20 % smaller difference in %fat change (p=0.002) (e.g., overestimate of %fat by BIA).
Fig. 3.
Association between percent body fat determined with bioelectrical impedance analysis and the referent 3-compartment model at baseline (n=41) (a), 1 year after surgery (n=41) (b), and change between baseline and 1 year in participants with complete data (n=32) (c)
Fig. 4.
Bland-Altman plot comparing 3-compartment measured percent fat and predicted percent fat at baseline (n=41) (a), 1 year after surgery (n=41) (b), and change between baseline and 1 year in participants with complete data (n=32) (c)
Examination of Outliers
Some outliers were identified in Bland-Altman plots. Each TBW plot had one outlier. The %fat plots had two outliers at T0 for %fat change, and one outlier for T12. At T0, one of the %fat outliers was also a TBW outlier, which was attributable to the overestimate of TBW in this subject, but this subject was not on a diuretic. The second %fat outlier at T0 was on hydrochlorothiazide. At T12, the %fat outlier was the subject who lost the largest amount of weight in the sample but was not on a diuretic. The TBW outlier at T12 was not on a diuretic. It is unclear why these subjects had divergent results. One of the outliers for %fat change had a BMI of 42 kg/m2 with 44 % fat at T0, and after surgery, this subject began an endurance exercise regimen and at T12 was 11 % fat. Thus, the divergent results for %fat change may be attributable to the marked fat loss coupled with changes in lean mass from the exercise. The second outlier for %fat change was on hydrochlorothiazide at baseline and T12. The one outlier for TBW change was on hydrochlorothiazide at T12.
Discussion
Measuring body composition changes in severe obesity is challenged by the variability in fat-free mass hydration [11], body water distribution [4, 5], and the composition of weight loss following surgical interventions [20]. Given these conditions coupled with the body composition changes following surgery, we expected to observe divergence between BIA and reference measures for TBW and %fat pre to post bariatric surgery.
BIA TBW estimates were not different from D2O measures; thus, a fixed bias was not detected. However, as noted in the results, this study was only powered to detect a very large difference between BIA and D2O measures of TBW change. BIA and D2O determined that TBW change was not correlated. Bland-Altman plots demonstrated that there was no proportional bias, but the limits of agreement spanned a wide range (i.e., T0 21.3 L, T12 14.8 L, and Δ 22.5 L), indicating that individuals’ BIA TBW estimates could differ from their D2O TBW values by as much as 22.5 L.
A fixed bias was detected with BIA estimates of %fat, such that compared with 3C, BIA underestimated %fat both prior to and following surgery. While the underestimation was slightly greater prior to surgery, there was no difference between 3C and BIA estimates of pre to postoperative %fat change. Bland-Altman plots demonstrated that while there was no proportional bias at baseline or 1 year, a greater change in %fat was associated with a smaller difference between BIA and 3C measures, such that for every 1 % greater change in %fat, the difference between measures was 0.3 % smaller. Additionally, the 95 % limits of agreement with 3C were wide (%fat T0 19.3 %, T12 19.1 %, and Δ 20.4 %), suggesting that clinically meaningful differences are present between BIA and 3C %fat. Together, these findings suggest that BIA (Tanita 310) estimates of TBW and %fat are not appropriate to guide individual clinical treatment but may be suitable for monitoring group level response.
Differences between reference and BIA estimates for %fat and TBW did not vary by initial BMI or the magnitude of weight loss after surgery. Greater fat loss following surgery (by 3C) was associated with an underestimate of TBW change and an overestimate of %fat change by BIA but was not associated with TBW and %fat estimates postsurgery. Given the violation of assumptions underlying BIA TBW estimates from which %fat estimates are derived (e.g., no contribution of water from adipose tissue, assumed constant hydration of fat-free mass (0.73)), the association between greater fat loss and divergence between BIA and reference estimates may be attributable to meaningful (i.e., nonnegligible) contributions from adipose tissue to TBW that may vary by adiposity and amount of fat loss following surgery [11, 21].
Our baseline TBW findings are similar to a previous study in severely obese adults (n=42; mean (SD) BMI 50.2±8.8 kg/ m2), where TBW values, estimated by Tanita 310, were highly correlated (r=0.92) with D2O TBW [9]. The 95 % limits of agreement were also relatively wide (10.3–6.7 L) but narrower than our observed limits (10.2–12.3 L). Similar to our findings, one outlier was identified whose divergent results could not be fully explained [9].
Previously, %fat BIA estimates (Morel 103B, RJL Systems, Mt. Clemens, MI) were lower than 3C values in 20 severely obese women before surgery (−5.7±0.6 %) and14 months after surgery (−5.1±1.1 %). Since the fixed bias was similar between time points, estimates of %fat changes were not different from 3C values [11]. In contrast, in our study, BIA underestimated %fat to a larger degree prior to versus following surgery (i.e., T0 −3.4±4.9 and T12 −1.9± 4.9), leading to an overestimation of %fat change. The 95 % limits of agreement in our study were larger preoperatively, even though they were comparable postoperatively and for %fat change [11]. While mean differences from 3C values in our study were smaller pre and postoperatively, standard deviations were larger, indicating more variability between subjects. This may be due to differences in the type of BIA device (standing vs supine) or device-specific equations.
Despite the difficulty in using DXA in severely obese adults, one study found that single-frequency BIA estimates (BIA 101 RJL, Akern Bioresearch, Firenze, Italy) were highly correlated (all r≥0.89, p<0.001) with DXA (Hologic QDR 4500A, Bedford, MA) derived estimates of %fat before and 6/12 months after surgery among 45 adults with a baseline BMI of 42.1 kg/m2 (range 34.5 to 48.7 kg/m2) [22]. Prior to surgery, the range in limits of agreement (18 %) were comparable with our observed limits (19 %); but 12 months after surgery, the range in limits was narrower than our observed limits (10.7 % vs 19 %) [22]. This difference may reflect differences in weight loss, as our subjects had a higher mean baseline BMI (46.1 vs 42.1 kg/m2) and lower mean BMI after surgery (31.9 vs 33.4 kg/m2), or use of different BIA systems and reference methods [22].
This study has limitations. First, outliers were identified in each Bland-Altman plot, including individuals on diuretics, but we were unable to evaluate whether the diuretic dosage influenced TBW estimates. Second, this study was underpowered to detect TBW change. This study has several strengths. This is the first study to report on the validity of the Tanita 310 BIA for estimating TBW and %fat in severely obese adults pre to post bariatric surgery, using a 3C model as the reference method for %fat. These findings are likely generalizable to other severely obese adults and postsurgical intervention, as our population had exhibited a wide magnitude of weight change and was racially diverse.
Conclusion
The results of this study suggest that clinically meaningful differences exist between BIA estimates of TBW and %fat and reference values. BIA estimates may be more appropriate for use in measuring change over time at the group level rather than at the individual level.
Supplementary Material
Acknowledgments
Dr Goodpaster and Dr Gallagher report grants from the National Institutes of Health during the conduct of the study. Dr Courcoulas reports other from J&J Ethicon Scientific, personal fees from J&J Ethicon Scientific, grants from the NIH-NIDDK, grants from Covidien, grants from EndoGastric Solutions, and grants from Nutrisystem, outside the submitted work. Dr Pomp reports personal fees from Covidien, and personal fees from WL Gore & Associates, outside the submitted work. Grant support RO1-DK-72507, P30-DK-26687, UL1 TR000040, and T32 DK091227 (E. Widen)
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11695-014-1182-5) contains supplementary material, which is available to authorized users.
Conflict of Interest Dr Widen, Dr Strain, Dr King, Mr Yu, Dr Lin, and Dr Thornton report no conflict of interest.
Contributor Information
Elizabeth M. Widen, Email: ew2435@columbia.edu, Institute of Human Nutrition and Department of Epidemiology, Columbia University, New York Obesity Nutrition Research Center, St. Luke’s-Roosevelt Hospital, 1111 Amsterdam Avenue, Scrymser Bsmt, New York, NY 10025, USA
Gladys Strain, Email: gls2010@med.cornell.edu, Department of Surgery, Weill Cornell Medical College, 525 East 68th Street, Starr 8, New York, NY 10065, USA.
Wendy C. King, Email: kingw@edc.pitt.edu, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 517 Parran Hall, Pittsburgh, PA 15261, USA
Wenwen Yu, Email: wyu@chpnet.org, New York Obesity Nutrition Research Center, St. Luke’s-Roosevelt Hospital, 1111 Amsterdam Avenue, Scrymser Bsmt, New York, NY 10025, USA.
Susan Lin, Email: xl18@columbia.edu, Center for Family and Community Medicine, Columbia University, New York Obesity Nutrition Research Center, St. Luke’s-Roosevelt Hospital, 1111 Amsterdam Avenue, Scrymser Bsmt, New York, NY 10025, USA.
Bret Goodpaster, Email: bgoodpaster@sanfordburnham.org, Diabetes and Obesity Research Center, Sanford Burnham Medical Research Institute, 6400 Sanger Road, Orlando, FL 32827, USA.
John Thornton, Email: JCTatSTLBCU@aol.com, New York Obesity Nutrition Research Center, St. Luke’s-Roosevelt Hospital, 1111 Amsterdam Avenue, Scrymser Bsmt, New York, NY 10025, USA.
Anita Courcoulas, Email: courcoulasap@upmc.edu, Division of Surgery, University of Pittsburgh Medical Center, 200 Lothrop St., Pittsburgh, PA 15213, USA.
Alfons Pomp, Email: alfonspomp@gmail.com, Department of Surgery, Weill Cornell Medical College, 525 East 68th Street, Starr 8, New York, NY 10065, USA.
Dympna Gallagher, Email: dg108@columbia.edu, New York Obesity Nutrition Research Center, Body Composition Unit, St. Luke’s-Roosevelt Hospital, Institute of Human Nutrition, Columbia University, 1111 Amsterdam Avenue, Scrymser Basement, New York, NY 10025, USA.
References
- 1.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31(5):743–50. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 2.Strain GW, Gagner M, Pomp A, et al. Comparison of weight loss and body composition changes with four surgical procedures. Surg Obes Relat Dis. 2009;5(5):582–7. doi: 10.1016/j.soard.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Strain GW, Gagner M, Pomp A, et al. Comparison of fat-free mass in super obesity (BMI≥50 kg/m2) and morbid obesity (BMI<50 kg/m2) in response to different weight loss surgeries. Surg Obes Relat Dis. 2012;8(3):255–9. doi: 10.1016/j.soard.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Mazariegos M, Kral JG, Wang J, et al. Body composition and surgical treatment of obesity. Effects of weight loss on fluid distribution. Ann Surg. 1992;216(1):69–73. doi: 10.1097/00000658-199207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waki M, Kral JG, Mazariegos M, et al. Relative expansion of extracellular fluid in obese vs. nonobese women. Am J Physiol. 1991;261(2 Pt 1):E199–203. doi: 10.1152/ajpendo.1991.261.2.E199. [DOI] [PubMed] [Google Scholar]
- 6.Deurenberg P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr. 1996;64(3 Suppl):449S–52S. doi: 10.1093/ajcn/64.3.449S. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Heshka S, Wang J, et al. Magnitude and variation of fat-free mass density: a cellular-level body composition modeling study. Am J Physiol Endocrinol Metab. 2003;284(2):E267–73. doi: 10.1152/ajpendo.00151.2002. [DOI] [PubMed] [Google Scholar]
- 8.Levitt DG, Beckman LM, Mager JR, et al. Comparison of DXA and water measurements of body fat following gastric bypass surgery and a physiological model of body water, fat, and muscle composition. J Appl Physiol (1985) 2010;109(3):786–95. doi: 10.1152/japplphysiol.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strain GW, Wang J, Gagner M, et al. Bioimpedance for severe obesity: comparing research methods for total body water and resting energy expenditure. Obesity (Silver Spring) 2008;16(8):1953–6. doi: 10.1038/oby.2008.321. [DOI] [PubMed] [Google Scholar]
- 10.Kushner RF, Kunigk A, Alspaugh M, et al. Validation of bioelectrical-impedance analysis as a measurement of change in body composition in obesity. Am J Clin Nutr. 1990;52(2):219–23. doi: 10.1093/ajcn/52.2.219. [DOI] [PubMed] [Google Scholar]
- 11.Das SK, Roberts SB, Kehayias JJ, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab. 2003;284(6):E1080–8. doi: 10.1152/ajpendo.00185.2002. [DOI] [PubMed] [Google Scholar]
- 12.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–35. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surgery Obes Relat Dis. 2007;3(2):116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belle SH, Chapman W, Courcoulas AP, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS) Surg Obes Relat Dis. 2008;4(4):474–80. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27(12):1692–7. [PubMed] [Google Scholar]
- 16.McCrory MA, Gomez TD, Bernauer EM, et al. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27(12):1686–91. [PubMed] [Google Scholar]
- 17.Pietrobelli A, Rubiano F, St-Onge MP, et al. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr. 2004;58(11):1479–84. doi: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- 18.Silva AM, Shen W, Wang Z, et al. Three-compartment model: critical evaluation based on neutron activation analysis. Am J Physiol Endocrinol Metab. 2004;287(5):E962–9. doi: 10.1152/ajpendo.00104.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 20.Palazuelos-Genis T, Mosti M, Sanchez-Leenheer S, et al. Weight loss and body composition during the first postoperative year of a laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18(1):1–4. doi: 10.1007/s11695-007-9311-z. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Deurenberg P, Wang W, et al. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol. 1999;276(6 Pt 1):E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]
- 22.Savastano S, Belfiore A, Di Somma C, et al. Validity of bioelectrical impedance analysis to estimate body composition changes after bariatric surgery in premenopausal morbidly women. Obes Surg. 2010;20(3):332–9. doi: 10.1007/s11695-009-0006-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.